Abstract
Background and purpose:
The metabolic syndrome, characterized by obesity, insulin resistance and dyslipidemia, is a major cause of cardiovascular disease. The origins of the syndrome have been hypothesized to lie in continuous availability of energy dense foods in modern societies. In contrast, human physiology has evolved in an environment of sporadic food supply and frequent food deprivation. Intermittent food restriction in rats has previously been shown to lead to reduction of cardiovascular risk and a greater life span. The non-metabolizable glucose analogue, 2-deoxy-D-glucose (2-DG) is taken up by cells and induces pharmacological inhibition of metabolism of glucose. We hypothesized that intermittent inhibition of glucose metabolism, a metabolic deprivation, may mimic intermittent food deprivation and ameliorate metabolic and pathophysiological aspects of the metabolic syndrome.
Experimental approach:
Insulin resistant, atherosclerosis-prone JCR:LA-cp rats were treated with 2-DG (0.3% w/w in chow) on an intermittent schedule (2 days treated, one day non-treated, two days treated and two days non-treated) or continuously at a dose to give an equivalent averaged intake.
Key results:
Intermittent 2-DG-treatment improved insulin sensitivity, which correlated with increased adiponectin concentrations. Further, intermittent treatment (but not continuous treatment) reduced plasma levels of leptin and the inflammatory cytokine IL-1β. Both 2-DG treatments reduced micro-vascular glomerular sclerosis, but only the intermittent schedule improved macro-vascular dysfunction.
Conclusions and implications:
Our findings are consistent with reduction in severity of the metabolic syndrome and protection against end stage micro- and macro-vascular disease through intermittent metabolic deprivation at a cellular level by inhibition of glucose oxidation with 2-DG.
Keywords: metabolic syndrome, atherosclerosis, 2-deoxy-D-glucose, glucose metabolism, vascular dysfunction, glomerular sclerosis, JCR:LA-cp rat
Introduction
The metabolic syndrome is a major and increasing cause of cardiovascular morbidity and mortality worldwide (Gotto et al., 2006; Haffner, 2006). A central feature of this disorder is the development of insulin resistance and consequent hyperinsulinemia. The hyperinsulinemia appears to be a major determinant of the vasculopathy, atherosclerosis and ischemic cardiovascular disease that are strongly associated with the metabolic syndrome (Uusitupa et al., 1990; Després et al., 1996). Consequently, the reduction of insulin resistance and hyperinsulinemia offers an important target for reduction of cardiovascular disease and mortality. Treatments at a clinical level have included changes in diet, food intake and physical activity, but these have proven difficult to implement and maintain in the human population, especially when the metabolic syndrome is well established. A number of pharmacological agents have been developed that have insulin-sensitizing action, including benfluorex, D-fenfluramine and S15261 (Brindley and Russell, 1995; Russell et al., 2000). However, these have been withdrawn from clinical use or development for various reasons, including suggestions of cardiovascular risk (Ryan, 2000; Colman, 2005). Peroxisome proliferator-activated receptor agonists, particularly the thiazolidinediones, continue to be considered promising insulin-sensitizing agents (Blaschke et al., 2006; Takahashi et al., 2006). However, troglitazone has been withdrawn due to apparent toxicity, possibly related to inhibition of hepatic bile salt transport, which may be a class effect (Snow and Moseley, 2007). Rosiglitazone and pioglitazone appear to be superior to the other thiazolidinediones and continue in active clinical use, notwithstanding ongoing concerns regarding efficacy and toxicity (Fernando et al., 2006; Masubuchi, 2006; Richter et al., 2006). In the absence of alternatives, there remains a paucity of effective treatments (Colman, 2005) and renewed interest in extreme measures, such as gastric bypass surgery (Coppini et al., 2006).
The causative origins of the developing epidemic of obesity and metabolic syndrome remain unclear. The availability of inexpensive energy-dense and palatable foods, leading to excessive consumption, is an obvious and attractive explanation. However, phylogenetically, humans evolved in an environment of insecure and episodic food availability and metabolic deprivation was frequent. There is evidence to suggest that the continuous well-fed status of modern populations may play a role in metabolic dysfunction. Consistent with this hypothesis, chronic food restriction extends life span and reduces disease in both animals and humans (Weindruch and Sohal, 1997). Intermittent feeding has been shown to protect metabolically normal rats against induced ischemic myocardial injury and stroke (Wan et al., 2004; Ahmet et al., 2005; Mattson and Wan, 2005). In a natural extension of this concept, we propose that equivalent or greater metabolic effects may be possible in animals that are insulin resistant and spontaneously develop end-stage disease.
The JCR:LA-cp rat is a unique strain in which animals homozygous for the autosomal recessive cp gene (cp/cp) spontaneously develop the range of the dysfunction and pathophysiology associated with the metabolic syndrome in humans (O'Brien and Russell, 1997; Richardson et al., 1998; Russell et al., 2007). These include obesity, a profound insulin resistance, atherosclerosis, vascular dysfunction, spontaneous myocardial infarcts and glomerular sclerosis (Richardson et al., 1998; Russell et al., 1998a; Russell et al., 2007). We have shown that a number of interventions reduce the insulin resistance and associated hyperinsulinemia in this animal model (Russell et al., 1995, 1998b, 2000, 2004), and this is accompanied by reduction of end-stage cardiovascular disease (Russell et al., 1998b, 2000, 2004; Proctor et al., 2005). Insulin itself acts a cytokine at sufficiently high concentrations and this may underlie much of the vascular damage and dysfunction seen in the cp/cp rat (Absher et al., 1997, 1999). The cp mutation causes a stop codon in the extracellular domain of the ObR leptin receptor (Wu-Peng et al., 1997) leading an absence of all of the isoforms of the receptor in the cp/cp rat and an extreme hyperleptinemia. Leptin, a peptide hormone released by adipose tissue in a mass-dependent manner, has complex and not fully understood metabolic actions. Most importantly, it reaches hypothalamic receptors through a complex saturable and regulated transport system (Banks, 2001) and inhibits release of neuropeptide Y (NPY), the most powerful mediator of eating and drinking (Dube et al., 1999). The absence of hypothalamic leptin action in the cp/cp rat results in elevated NPY levels (Williams et al., 1990) and a marked hyperphagia and obesity (Russell et al., 1995). The complexity of the regulatory actions of leptin is increasingly obvious, if not entirely understood (Covey et al., 2006; Eikelis et al., 2007; Stepanyan et al., 2007).
There are a number of recognized cytokines that are related to leptin, cardiovascular disease and obesity, including the adipocyte-related peptide adiponectin and the inflammatory marker interleukin-1β (IL-1β) (Dinarello, 1998; Whitehead et al., 2005; Huypens, 2007). We have shown that treatment that reduces insulin levels and vascular dysfunction in the cp/cp rat also raises plasma adiponectin (Proctor et al., 2006a), consistent with observations, by others, that adiponectin correlates inversely with obesity and is a marker of inflammation (Whitehead et al., 2005; Brooks et al., 2006). Similarly, IL-1β is a mediator of systemic pro-inflammatory pathways and may provide an index of the inflammatory processes that are known to accompany atherosclerosis.
The glucose analogue, 2-deoxy-D-glucose (2-DG), is non-oxidisable, but taken up by the cellular glucose transporters interchangeably with D-glucose. As with glucose, 2-DG is phosphorylated to 2-deoxy-glucose-6 phosphate, but this is not further metabolized. These properties have lead to an extensive use of 2-DG as a tool to measure rates of cellular glucose uptake for over 50 years (e.g. see Sachs et al., 1965; Sweet et al., 1996). Recently, 2-DG at pharmacological concentrations has been shown to be a useful experimental modulator of glucose-6-phosphate dehydrogenase regulation of inducible nitric oxide synthase (iNOS) (Won et al., 2003). In addition, Keenan et al. (2004) have shown 2-DG, at very high concentrations (6–10 mM), to have anti-metabolite activity in a human carcinoma cell line through glycolytic inhibition. Consistent with these reports, 2-DG has been shown to reduce plasma insulin concentrations and blood pressure in the metabolically normal Sprague–Dawley rat, without reduction in food intake (Wan et al., 2004). The metabolic effects of 2-DG may thus mimic those of caloric restriction, induced through continuous restriction of food intake or through intermittent feeding (Mattson and Wan, 2005). The current project was designed to explore the benefits of 2-DG treatment in the insulin-resistant JCR:LA-cp rat, on the premise that continuous and/or intermittent metabolic inhibition may have significant ameliorative effects on the metabolic syndrome.
Methods
Animals
Male JCR:LA-cp rats, cp/cp (obese, insulin resistant) and +/? (lean, metabolically normal controls) were bred in our established colony at the University of Alberta, as described previously (Russell et al., 1995).
Treatment of animals
All animals were placed on a reversed light cycle in the experimental room at 6 weeks of age, allowing metabolic studies to be conducted under subdued light during the active (dark) phase of their diurnal cycle, and acclimatized over a 2-week period. Rats were housed in an individually ventilated caging environment (Techniplast, Milano, Italy). The rats were fed Lab Diet 5001 (PMI Nutrition International, Brentwood, MO, USA) ad libitum, and food consumption and body weights were measured twice weekly. Rats were treated chronically from 8 weeks of age (young adult animals in which the insulin resistance syndrome is fully established) to 13 weeks of age with 2-DG incorporated into the feed (Russell et al., 2000). Animals on 2-DG intermittent treatment were given non-treated food and 2-DG containing food at 0.3% (w/w) on an intermittent weekly schedule (2 days treated, 1 day non-treated; 2 days treated and 2 days non-treated), as in the procedure of Wan et al. (2004). Continuously treated rats were fed food containing 2-DG, at a concentration calculated to match total intake, over each week of the experiment, consumed by the intermittently treated animals, which was approximately 150 mg kg l−1 day l−1 (concentrations were in the range of 0.17% w/w). At 12 weeks of age, rats were fasted over the light (non-active) phase with 2DG-treated rats being treated on the preceding day (including intermittently treated animals). A fasting blood sample was obtained from the tail, a standardized meal tolerance test performed using control rat chow (Russell et al., 1999) and the rats immediately returned to the treatment schedule. At 13 weeks of age, the rats were killed under anaesthesia with isoflurane in oxygen in the fed state and samples of fluids and tissue removed.
All procedures on animals were in accordance with the guidelines of the Canadian Council on Animal Care and with prior approval of the Animal Policy and Welfare Committee of the Faculty of Agriculture Forestry and Home Economics, University of Alberta.
Biochemical assays
Plasma glucose was determined using a glucose oxidase assay procedure (Diagnostic Chemicals Ltd, Charlottetown, PEI, Canada). Insulin was assayed by rat enzyme-linked immunosorbent assay (ELISA) assay (Mercodia AB, Uppsala, Sweden). Urine albumin and creatinine measurements were performed on a Beckman Coulter LX20i analyser using immunoturbidimetric and Jaffé methods, respectively. Plasma triglyceride (L-type TG H), total cholesterol (Cholesterol E) and low-density lipoprotein (LDL) Cholesterol (L-type LDL-C) assays were obtained from Wako Pure Chemicals USA, Inc. (Richmond, VA, USA). High-density lipoprotein (HDL) cholesterol was assayed using direct HDL assay (Diagnostic Chemicals). Adiponectin was measured by radio immunoassay (# MADP-60HK) and IL-1β by ELISA assay using LINCOplex plates (Linco Research, St Charles, MO, USA).
Histology
Kidneys were fixed in formalin, and subjected to conventional processing and sectioning, followed by hematoxylin and eosin staining. The extent of glomerular sclerosis was determined using a procedure similar to that of Schäfer et al. (2004). Ten fields of view of the right kidney of each rat were recorded at 100 × magnification on a digital camera system (Nikon E600 with DMX 1200 camera and ACT-1 software, Nikon Corporation, Tokyo, Japan). The images were visualized using Photoshop (V5.5, Adobe Systems Inc., San Jose, CA, USA), examined without knowledge of the treatments and all glomeruli rated as normal or sclerotic. Results were expressed as the percent of glomeruli that exhibited sclerosis.
Vascular function
The vascular function of aortic rings, with intact endothelium, was assessed using established methods (Russell et al., 2001). The contractile response of endothelium-intact rings of aortae to phenylephrine (PE) was assessed through concentration–response curves for PE (1 nmol−1 to 300 mmol−1). The basal NO-mediated relaxation of aortic rings (pre-contracted with PE to 80% of maximal contraction) was assessed by determining the concentration response to the endothelial NO-releasing agent, ACh and the nitric oxide donor sodium nitroprusside (SNP). Direct assessment of NO-mediated effects was also determined through addition of NG-nitro-L-arginine methyl ester (L-NAME), at 100 μM, to inhibit NOS activity (Radomski and Salas, 1995).
Statistical analysis
Results are expressed as mean±s.e.m. and were analysed using SigmaStat (Jandel Scientific, San Rafael, CA, USA) and plotted using SigmaPlot (SPSS Inc., Chicago, IL, USA) and Prism (Graphpad, San Diego, CA, USA). Data were compared using one-way analysis of variance followed by multiple comparison tests. Concentration–response curves were analysed using the program ALLFIT (De Lean et al., 1978), which fits the complete data set to the logistic equation and permits independent testing of differences between individual parameters. A value of P<0.05 was taken as being statistically significant.
Materials
Reagents and chemicals, including ACh, bradykinin, PE and SNP, were obtained from Sigma Chemical (Oakville, ON, Canada). 2-Deoxy-D-glucose was supplied by Threshold Pharmaceuticals Inc. (Redwood City, CA, USA).
Results
Body weight and food intake
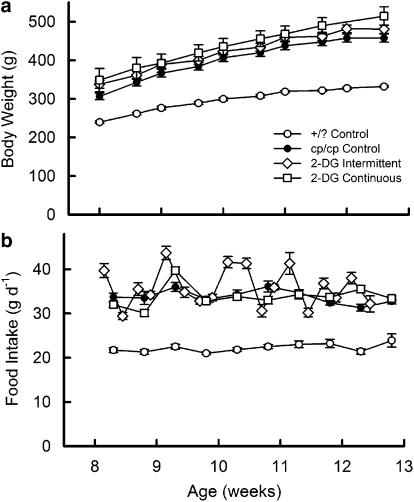
Body weight and food intake of 2DG-treated and -control rats are shown in Figure 1. Both groups treated with 2-DG had slightly higher body weights throughout, whereas continuously treated rats had significantly greater body weight than the cp/cp control rats at death (Figure 1a). Interestingly, rats treated with 2-DG intermittently had higher food consumption on days they received dietary 2-DG than on the days they were fed control food (Figure 1b).
Figure 1.
Body weight (a) and food intake (b) of 2-DG-treated and control JCR:LA-cp rats. Data are mean±s.e.m., 10 rats in each group. The continuously treated group had significantly greater body weight than the cp/cp control rats at the end of the experiment (P<0.001). In (b), rats treated with 2-DG intermittently consumed more food on the days when they were given dietary 2-DG (P<0.001).
Insulin and glucose metabolism
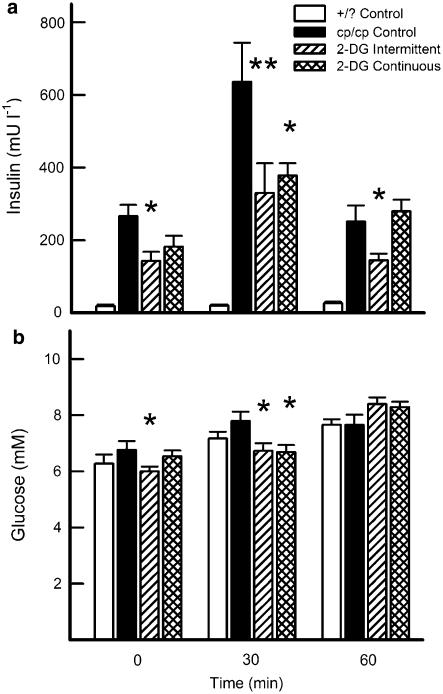
Treatment with 2-DG on an intermittent, but not on a continuing, basis caused a significant reduction in the fasting insulin concentration compared with cp/cp control animals (Figure 2a). At 30 min post-tolerance test-meal, both treated groups showed approximately 50% reduction in plasma insulin response. At 60 min, when the insulin response had largely abated, only the intermittent group continued to show reduced insulin levels.
Figure 2.
Effects of 2-DG treatment on plasma insulin (a) and glucose (b) levels in JCR:LA-cp rats, fasted and in response to a meal challenge. Data are mean±s.e.m., 10 rats in each group. *P<0.05; **P<0.01 vs cp/cp control.
Under normal circumstances, plasma glucose levels in JCR: LA-cp rats do not vary significantly, with the cp/cp animals maintaining euglycemia, even postprandially, but at the expense of very high insulin levels. Fasting and postprandial glucose concentrations (following tolerance-test meal) are shown for each group in Figure 2b. In the fasting state, only rats being treated intermittently showed a reduction in plasma glucose. At 30 min after meal, both groups treated with 2-DG showed a reduced glucose response, which had diminished by the 60-min time point.
Plasma lipids
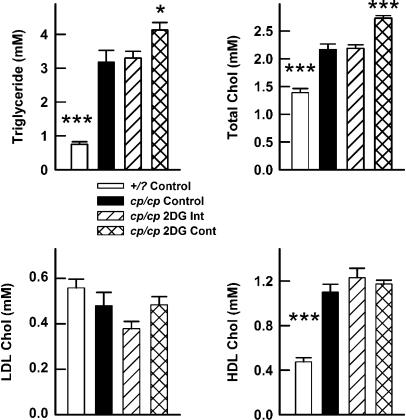
Consistent with previous studies, concentrations of total and HDL cholesterol were significantly lower in +/? control rats compared with cp/cp controls (Figure 3). Importantly, continuous but not intermittent, treatment with 2-DG significantly raised plasma concentrations of triglyceride and total cholesterol, whereas values for LDL and HDL cholesterol were not modified.
Figure 3.
Fasting plasma lipid concentrations in JCR:LA-cp rats treated with 2-DG. *P<0.05; ***P<0.001 vs cp/cp control.
Cytokines and inflammatory markers
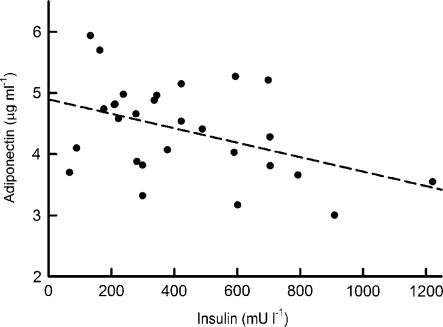
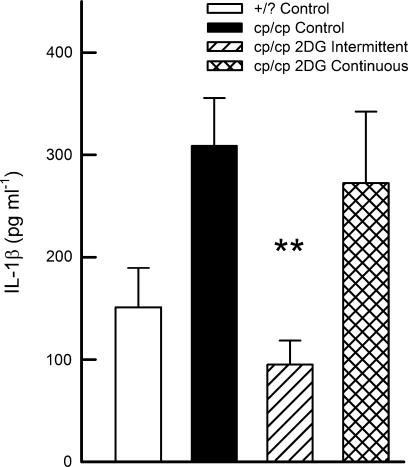
Plasma leptin concentrations were highly elevated in the cp/cp rats compared with the +/? controls (57.7±4.3 vs 2.9±0.6 ng ml−1). 2DG treatment did not alter the plasma leptin levels compared with the cp/cp controls (49.1±3.3 and 64.3±9.5 ng ml−1, for intermittent and continuous treatment, respectively). 2-DG treatment, on either dosing schedule, significantly raised the plasma adiponectin concentrations in cp/cp rats (3.60±0.12, 4.97±0.19 and 4.51±0.14 μg ml−1, for control, intermittently and continuously treated, respectively; P<0.0001 for both schedules). In addition, we observed a significant negative correlation between adiponectin and plasma insulin concentrations during the meal tolerance test (Figure 4) and in the fasting state (P<0.05, data not shown). As shown in Figure 5, the intermittent treatment normalized the elevated plasma concentration of IL-1β in the cp/cp rat, whereas the continuous treatment had no effect.
Figure 4.
Regression analysis of plasma concentrations of adiponectin and 30 min insulin response to test meal of cp/cp rats. The slope of the regression line is significant (P<0.025).
Figure 5.
Plasma concentrations of IL-1β of JCR:LA-cp rats, treated with 2-DG. **P<0.005 vs cp/cp control.
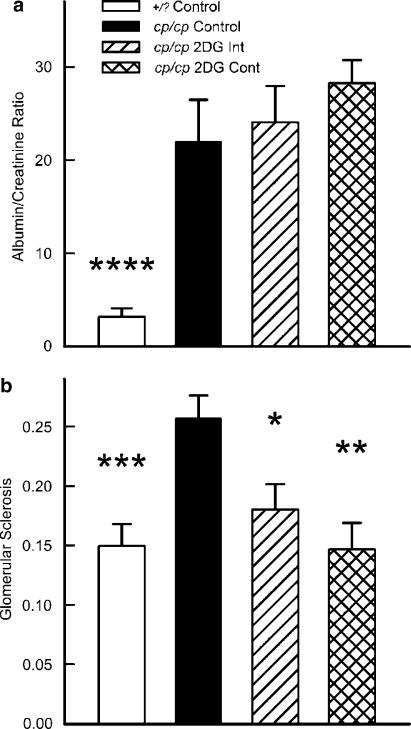
Renal microvascular effects
Figure 6a shows that 2-DG treatment, on either schedule, did not alter the urinary albumin/creatinine ratio. However, 2-DG treatment (regardless of schedule) reduced the glomerular sclerosis to a severity comparable to that of +/? control rats.
Figure 6.
Urine albumin/creatinine ratio (a) and fraction of glomeruli exhibiting sclerosis (b) in 2-DG–treated rats. *P<0.02; **P<0.01; ***P<0.005; P<0.0001 vs cp/cp control animals.
Macrovascular function
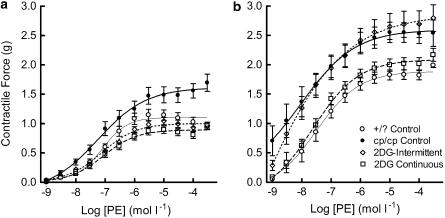
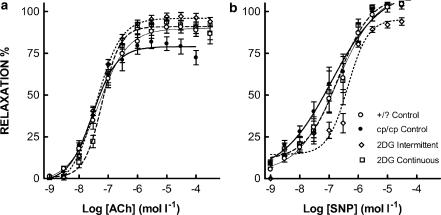
There was a significant reduction in the hyper contractile response to PE of the cp/cp rat aortae from animals treated with 2-DG, on either schedule, with maximum forces equivalent to +/? controls (Figure 7a). In the presence of L-NAME (Figure 7b), the continuously treated (but not the intermittent) group showed equivalence in maximum contractility to +/? rats. Figure 8a shows that aortae from animals treated with 2-DG, on either schedule, showed a significant increase in the maximum relaxation induced by the NO releasing agent ACh. There was no difference in the response to the direct NO donor SNP between any of the groups (Figure 8b).
Figure 7.
Contractile dose response to PE of aortic rings from cp/cp and +/? rats. (a), in the absence of L-NAME; (b), in the presence of L-NAME. Data are mean±s.e.m., 10 rats in each group. There are significant differences in maximum contractile response to PE between cp/cp control and 2-DG-treated rats (P<0.0001), as detailed in the text.
Figure 8.
Relaxant dose response to ACh (a) and SNP (b), of aortic rings, precontracted with PE, from cp/cp and +/? rats. Data are mean±s.e.m., 10 rats in each group. The 2-DG-treated aortae showed significantly improved ACh-mediated relaxation compared with cp/cp controls (P<0.0001 and P<0.001 for intermittently and continuously treated, respectively). There was no significant difference between groups in the response to SNP.
Discussion
Metabolic effects
Administration of 2-DG to the cp/cp rat has significant effects on metabolism and pathophysiology. It is most striking that some of these effects of 2-DG are dependent upon the schedule of administration. Notably, continuous treatment with 2-DG did not alter food intake per se, but resulted in a modest, yet significant, increase in body weight. We speculate that this effect could be the result of a reduced rate of glucose oxidation. It is interesting that on the first cycle of treatment (2 days), the food intake of the intermittent treatment group was 18% higher than the cp/cp control rats (39.7±1.6 g d−1 compared with 33.7±0.9 g d−1 for the cp/cp control rats and 21.7±0.5 g d−1 for +/? controls). Thus, the rats are able, over a period of 1 day, to detect the metabolic effects of the 2-DG and compensate. It is possible that the 2-DG food was more attractive (sweeter) than the control food, but this is not consistent with addition of 0.3% 2-DG to a diet containing 5.88% mono and disaccharides by weight. We acknowledge that the intermittent treatment involved somewhat greater concentrations of 2-DG and it is possible that the metabolic effects are due to acute exposure to this substrate, which could be further explored through a dose–response study.
The cp/cp rats divert diet-derived glucose to hepatic triglyceride production and conversion to very low-density lipoprotein (VLDL) (Brindley and Russell, 2002), with VLDL hyperlipidemia and secondary modest elevation of HDL and total cholesterol (Vance and Russell, 1990). Although intermittent 2-DG treatment did not change the plasma concentrations of any of the lipids measured, continuously treated rats had significantly greater levels of both triglyceride and total cholesterol, consistent with diversion of substrate to hepatic triglyceride synthesis, uptake in peripheral fat and increased body weight as noted above (Brindley and Russell, 2002). This is a potentially deleterious change, especially in light of delayed clearance of chylomicron remnants and VLDL in the cp/cp rat (Vine et al., 2007), yet highlights the sensitivity of the lipid response to acute 2-DG exposure.
Intermittent treatment with 2-DG caused an approximate 50% decrease in the fasting hyperinsulinemia of the cp/cp rats, whereas continuous treatment resulted in a 30% (non-significant) decrease. At 30 min, after meal in the tolerance test, there was a corresponding 50% reduction in the insulin response, in the intermittent group, compared to a 40% decrease in the continuously treated group. The substantial reduction in the postprandial insulin and accompanying small, but significant, reductions in plasma glucose indicate a reduction in insulin resistance in the intermittently treated rats, with a weaker effect in the continuously treated rats. This response is similar to that seen on intermittent treatment of Sprague–Dawley rats by Wan et al. (2004), but in a state of extreme insulin resistance (Russell et al., 1999). We have reported previously that equivalent reductions in plasma insulin levels and increase in insulin sensitivity, in the cp/cp rat, are accompanied by major improvement in vascular function and reduction in both atherosclerotic lesions and ischemic myocardial lesions (Russell et al., 1998b, 2000, 2004; Proctor et al., 2005).
Cytokines and other markers
Adiponectin is an adipocyte-derived cytokine that is inversely related to both insulin resistance and inflammatory processes (Fasshause et al., 2004) with lower plasma concentrations reported in both obese humans and some rodent models of obesity/insulin resistance (Altomonte et al., 2003; Alemzadeh and Tushaus, 2004; Fredersdorf et al., 2004). For example, Alemzadeh and Tushaus (2004) reported lower plasma adiponectin concentrations in obese fa/fa ZDF rats than in the lean phenotype. Adiponectin levels in cp/cp rats were increased by 2-DG treatment on both schedules, with the intermittent treatment giving higher (but marginally non-significantly, P=0.06) levels than the continuous treatment. Adiponectin levels were inversely related to insulin concentration, consistent with the changes in insulin metabolism. In the absence of changes in body weight, the significant increases in plasma adiponectin are unusual, given the negative association with obesity (Alemzadeh and Tushaus, 2004). Thus, the effect of 2-DG treatment must be unrelated to adipose tissue mass and may reflect underlying changes in the sensitivity of the adipocyte to improvements in fasting and postprandial insulin.
IL-1β is a powerful mediator of systemic pro-inflammatory pathways (Dinarello, 1998a, 1998b) and the elevated levels seen in the cp/cp rat are consistent with the concept that atherosclerosis is an inflammatory process (Zhou et al., 2002). The normalization of circulating IL-1β by the intermittent 2-DG treatment is consistent with a reduction in the pro-inflammatory state and corresponding improvements to macrovascular dysfunctions (Russell et al., 1998b, 2000, 2004). These results are consistent with the observations of others that intermittent metabolic inhibition, through fasting, has beneficial effects on the cardiovascular system of non-obese non-insulin-resistant animals (Ahmet et al., 2005; Mattson and Wan, 2005).
Effects on vascular function
The aortic vascular function results indicate a major reduction in the macrovascular dysfunction that is a prominent component of the pathophysiology of the metabolic syndrome. The hypercontractile response of the arterial system of the cp/cp rat is not confined to major conductance vessels, such as the aorta, but is seen in mesenteric resistance vessels and the coronary and glomerular circulation (O'Brien et al., 1998; Russell et al., 2004). In this respect, the cp/cp rat strongly resembles insulin-resistant and type 2 diabetic humans (Sing et al., 2003; O'Neill et al., 2005). Such vascular dysfunction has recently become recognized as a significant contributor to the enhanced risk of acute coronary events in diabetics associated with exposure to airborne fine particulate matter (Sing et al., 2003; Mills et al., 2005; O'Neill et al., 2005; Proctor et al., 2006b), illustrating the complex multifactoral nature of cardiovascular disease. The cp/cp rat also is prone to spontaneous ischemic lesions secondary to myocardial infarct (O'Brien and Russell, 1997; Richardson et al., 1998). Thus, the reduction in the contractile response to the noradrenergic agonist PE, induced by 2-DG treatment, may be expected to be reflected in reduced susceptibility to spontaneous vasospasm and myocardial injury. The vascular dysfunction is related to impaired endothelial NO-dependent relaxation (Russell et al., 2001). Improved ACh-mediated relaxation in the 2-DG-treated rats is consistent with improved endothelial function and NO metabolism. This observation is confirmed by the absence of any differences in SNP-mediated relaxation, indicating normal vascular smooth muscle relaxant function.
Effects on microvascular dysfunction
Micro albuminuria is a microvascular reflection of the vasculopathy and damage accompanying the hyperinsulinemic state and is associated with glomerular capillary damage leading to glomerular sclerosis (Marshall and Flyvjerg, 2006). As Figure 8a shows, the urinary albumin levels are markedly elevated in the cp/cp rat and 2-DG treatment did not reduce the levels. This may reflect the young age (12 weeks) of the rats. Alternatively, albuminuria while strongly associated with glomerular damage, is not simply a marker, but may play a role in the development of renal pathophysiology, independent of hyperinsulinemia. In any event, the continuously treated rats had an incidence of glomerular sclerosis comparable to +/? control rats (P<0.01 vs cp/cp control) and the intermittently treated rats had a somewhat lesser, but also significant, reduction in incidence (P=0.016). The reduction in glomerular sclerosis thus reflects a significant amelioration of the microvascular damage and dysfunction by 2-DG treatment, consistent with reduction in hyperinsulinemia and IL-1β (Russell et al., 2004).
Effects of treatment schedule and mechanism of cardioprotection
Treatment with 2-DG had effects that were highly dependent upon the schedule which, at first consideration, is apparently paradoxical. However, we have found that the cp/cp rat shows significant physiological and metabolic changes when subjected to a simple pair feeding regimen, in which the animal is daily provided with the same amount of food that an unmanipulated control rat ate on the same experimental day (Russell and Proctor, unpublished observations). The rats eat the food allocation immediately and are then deprived of food for the balance of the diurnal period, inducing an intermittent metabolic deprivation analogous to that of the intermittent 2-DG treatment, albeit with a shorter time cycle. Wan et al. (2004) also used an intermittent treatment 2-DG schedule, as an analogue of intermittent fasting, which they have shown to have beneficial effects on the cardiovascular system (Mattson and Wan, 2005). The dose of 2-DG used is low and had no toxic effects in either the cp/cp rat or Sprague–Dawley rats (Wan et al., 2004). The continuous treatment may be less effective due to intracellular accumulation of 2-deoxy-glucose-6-phosphate, or it may be, as suggested by Wan et al. (2004), that intermittent stress is beneficial.
The mechanism underlying the beneficial action of 2-DG in the cp/cp rat is not clear. The literature suggests that protection against experimentally induced myocardial and cerebral ischemia, and prolongation of life span, is related to antioxidant effects and/or antiapoptotic mechanisms (Wan et al., 2004; Ahmet et al., 2005; Mattson and Wan, 2005). Although such mechanisms may play a role in the metabolically normal animals used in those studies, evidence to date indicates that hyperinsulinemia is a central underlying cause of pathophysiology in the pre-diabetic cp/cp rat (O'Brien and Russell, 1997; Absher et al., 1997, 1999; Richardson et al., 1998). 2-DG treatment in the cp/cp rat resulted in significant reduction in the hyperinsulinemia, analogous to that seen by Wan et al. (2004) in metabolically normal Sprague–Dawley rats. Similar reduction in hyperinsulinemia induced by other treatments was associated with antiatherogenic and/or cardioprotective effects in the cp/cp rat (Russell et al., 1998b, 2000, 2004; Proctor et al., 2005). The association between glucose and glucose-6-phosphate/glucose-6-phosphate dehydrogenase metabolism and iNOS activity, identified by Won et al. (2003), also raises the interesting possibility that 2-DG affects vascular function through these pathways.
Summary
Treatment with 2-DG has a number of highly beneficial effects in the cp/cp rat model of pre-diabetes and the metabolic syndrome. It is evident that intermittent treatment is physiologically superior and that continuous treatment of the rats was less efficacious in inducing beneficial effects and had some deleterious effects not seen with intermittent treatment. The improvements in insulin/glucose metabolism, increase in adiponectin, reduction in IL-1β, reduction in renal damage and reduced vascular dysfunction are all substantial and consistent with reduced severity of the metabolic syndrome. These results provide the first demonstration of protection against end-stage micro- and macrovascular disease in a pre-diabetic or type 2 diabetic animal model by intermittent 2-DG treatment. Our findings also suggest that it may be possible to develop other, more effective, metabolic modulators of glucose metabolism to treat the metabolic syndrome and to prevent the associated cardiovascular complications.
Acknowledgments
This work was supported financially by a grant from Threshold Pharmaceuticals Inc., Redwood City, CA, USA. SD Proctor was also supported by an NSERC Discovery Grant. We thank Kristina MacNaughton and Sharon Sokolik for providing their expert technical assistance.
Abbreviations
- 2-DG
2-deoxy-D-glucose
- IL-1β
interleukin-1β
- L-NAME
NG-nitro-L-arginine methyl ester
- SNP
sodium nitroprusside
Conflict of interest
The authors state no conflict of interest.
References
- Absher PM, Schneider DJ, Baldor LC, Russell JC, Sobel BE. The retardation of vasculopathy induced by attenuation of insulin resistance in the corpulent JCR: LA-cp rat is reflected by decreased vascular smooth muscle cell proliferation in vivo. Atherosclerosis. 1999;143:245–251. doi: 10.1016/s0021-9150(98)00295-0. [DOI] [PubMed] [Google Scholar]
- Absher PM, Schneider DJ, Russell JC, Sobel BE. Increased proliferation of explanted vascular smooth muscle cells: a marker presaging atherogenesis. Atherosclerosis. 1997;131:187–194. doi: 10.1016/s0021-9150(97)06104-2. [DOI] [PubMed] [Google Scholar]
- Ahmet I, Wan R, Mattson MP, Lakatta MD, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- Alemzadeh R, Tushaus KM. Modulation of adipoinsular axis in prediabetic Zucker diabetic fatty rats by diazoxide. Endocrinology. 2004;45:5476–5484. doi: 10.1210/en.2003-1523. [DOI] [PubMed] [Google Scholar]
- Altomonte J, Harbaran S, Richter A, Dong H. Fat depot-specific expression of adiponectin is impaired in Zucker fatty rats. Metabolism. 2003;52:958–963. doi: 10.1016/s0026-0495(03)00092-1. [DOI] [PubMed] [Google Scholar]
- Banks WA. Enhanced leptin transport across the blood–brain barrier by á1-adrenergic agents. Brain Res. 2001;899:209–217. doi: 10.1016/s0006-8993(01)02242-9. [DOI] [PubMed] [Google Scholar]
- Blaschke F, Spanheimer R, Khan M, Law RE. Vascular effects of TZDs: new implications. Vasc Pharmacol. 2006;45:3–18. doi: 10.1016/j.vph.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Brindley DN, Russell JC. Metabolic abnormalities linked to obesity: effects of dexfenfluramine in the corpulent rat. Metabolism. 1995;44 Suppl 2:23–27. doi: 10.1016/0026-0495(95)90205-8. [DOI] [PubMed] [Google Scholar]
- Brindley DN, Russell JC. Animal models of insulin resistance and cardiovascular disease: some therapeutic approaches using the JCR:LA-cp rat. Diabetes Obes Metab. 2002;4:1–10. doi: 10.1046/j.1463-1326.2002.00164.x. [DOI] [PubMed] [Google Scholar]
- Brooks NL, Moore KS, Clark RD, Perfetti MT, Trent CM, Combs TP.Do low levels of circulating adiponectin represent a biomarker or just another risk factor for the metabolic syndrome Diabetes Obes Metab 2006 10.1111/j.1463-1326.2006.00596.xin press, doi: [DOI] [PubMed]
- Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Inter Med. 2005;143:380–385. doi: 10.7326/0003-4819-143-5-200509060-00013. [DOI] [PubMed] [Google Scholar]
- Coppini LZ, Bertevello PL, Gama-Rodrigues J, Waitzberg DL. Changes in insulin sensitivity in morbidly obese patients with or without metabolic syndrome after gastric bypass. Obes Surg. 2006;16:1520–1525. doi: 10.1381/096089206778870030. [DOI] [PubMed] [Google Scholar]
- Covey SD, Wideman RD, McDonald C, Unniappan S, Huynh F, Asadi A, et al. The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab. 2006;4:291–302. doi: 10.1016/j.cmet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- De Lean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Després J-P, Lamarche B, Mauriège P, Cantin B, Dagenais GR, Moorjani S, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1β, interleukin-18 and the interleukin-1β converting enzyme. Ann NY Acad Sci. 1998b;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- Dube MG, Xu B, Kalra PS, Sninsky CA, Kalra SP. Disruption in neuropeptide Y and leptin signaling in obese ventromedial hypothalamic-lesioned rats. Brain Res. 1999;816:38–46. doi: 10.1016/s0006-8993(98)00985-8. [DOI] [PubMed] [Google Scholar]
- Eikelis N, Wiesner G, Lambert G, Esler M. Brain leptin resistance in human obesity revisited. Reg Peptides. 2007;139:45–51. doi: 10.1016/j.regpep.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Fasshause M, Paschke R, Stumvoll M. Adiponectin, obesity, and cardiovascular disease. Biochimie. 2004;86:779–784. doi: 10.1016/j.biochi.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Fernando DJS, Bulugahapitaya U, Prior K. Delayed onset of rosiglitazone-induced pulmonary oedema. Eur J Intern Med. 2006;17:590. doi: 10.1016/j.ejim.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Fredersdorf S, Thumann C, Ulucan C, Griese DP, Luchner A, Riegger GA, et al. Myocardial hypertrophy and enhanced left ventricular contractility in Zucker diabetic fatty rats. Cardiovasc Pathol. 2004;13:11–19. doi: 10.1016/S1054-8807(03)00109-1. [DOI] [PubMed] [Google Scholar]
- Gotto AM, Jr, Blackburn GL, Dailey GE, III, Garber AJ, Grundy SM, Sobel BE, et al. The metabolic syndrome: a call to action [Therapy and Prevention] Coronary Art Dis. 2006;17:77–80. doi: 10.1097/00019501-200602000-00013. [DOI] [PubMed] [Google Scholar]
- Haffner SM. Risk constellations in patients with the metabolic syndrome: epidemiology, diagnosis, and treatment patterns. Am J Med. 2006;119 Suppl 1:S3–S9. doi: 10.1016/j.amjmed.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Huypens P. Leptin controls adiponectin production in the hypothalamus. Med Hypoth. 2007;68:87–90. doi: 10.1016/j.mehy.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Keenan J, Liang Y, Clynes M. Two-deoxyglucose as an anti-metabolite in human carcinoma cell line RPMI-2650 and drug-resistant variants. Anticancer Res. 2004;24:433–440. [PubMed] [Google Scholar]
- Marshall SM, Flyvjerg A. Prevention and early detection of vascular complications of diabetes. Br J Med. 2006;333:475–480. doi: 10.1136/bmj.38922.650521.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebral vascular systems. J Nutr Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y. Metabolic and non-metabolic factors determining troglitazone hepatotoxicity: a review. Drug Metab Pharmacokinet. 2006;21:347–356. doi: 10.2133/dmpk.21.347. [DOI] [PubMed] [Google Scholar]
- Mills NL, Törnqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- O'Brien SF, McKendrick JD, Radomski MW, Davidge ST, Russell JC. Vascular wall reactivity in conductance and resistance arteries: differential effects of insulin resistance. Can J Physiol Pharmacol. 1998;76:72–76. [PubMed] [Google Scholar]
- O'Brien SF, Russell JC. Insulin resistance and vascular wall function: lessons from animal models (Review) Endocrin Metab. 1997;4:155–162. [Google Scholar]
- O'Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2929. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- Proctor SD, Dreher KL, Kelly SE, Russell JC. Hypersensitivity of pre-diabetic JCR:LA-cp rats to fine airborne combustion particle-induced direct and noradrenergic-mediated vascular contraction. Toxicol Sci. 2006b;90:385–391. doi: 10.1093/toxsci/kfj100. [DOI] [PubMed] [Google Scholar]
- Proctor SD, Kelly SE, Russell JC. A novel complex of arginine–silicate improves micro- and macrovascular function and inhibits glomerular sclerosis in insulin-resistant JCR:LA-cp rats. Diabetologia. 2005;48:1925–1932. doi: 10.1007/s00125-005-1862-8. [DOI] [PubMed] [Google Scholar]
- Proctor SD, Kelly SE, Stanhope KL, Havel PJ, Russell JC. Synergistic effects of conjugated linoleic acid and chromium picolinate improve vascular function and renal pathophysiology in the insulin-resistant JCR:LA-cp rat. Diabetes Obes Metab. 2006a;9:87–95. doi: 10.1111/j.1463-1326.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- Radomski MW, Salas E. Nitric oxide – biological mediator, modulator and factor of injury: its role in the pathogenesis of atherosclerosis. Atherosclerosis. 1995;118:S69–S80. [PubMed] [Google Scholar]
- Richardson M, Schmidt AM, Graham SE, Achen B, DeReske M, Russell JC. Vasculopathy and insulin resistance in the JCR: LA-cp rat. Atherosclerosis. 1998;138:135–146. doi: 10.1016/s0021-9150(98)00012-4. [DOI] [PubMed] [Google Scholar]
- Richter B, Bandeira-Echtler E, Bergerhoff K, Clar C, Ebrahim SH.Pioglitazone for type 2 diabetes mellitus Cochrane Database Syst Rev 2006. Issue 4. Art. No.: CD006060. DOI: 10.1002/14651858.CD006060.pub2 [DOI] [PMC free article] [PubMed]
- Russell JC, Amy RM, Graham SE, Dolphin PJ, Wood GO, Bar-Tana J. Inhibition of atherosclerosis and myocardial lesions in the JCR:LA-cp rat by β, β′-tetramethylhexadecanedioic acid (MEDICA 16) Arterioscler Thromb Vasc Biol. 1995;15:918–923. doi: 10.1161/01.atv.15.7.918. [DOI] [PubMed] [Google Scholar]
- Russell JC, Dolphin PJ, Graham SE, Amy RM, Brindley DN. Improvement of insulin sensitivity and cardiovascular outcomes in the JCR:LA-cp rat by D-fenfluramine. Diabetologia. 1998b;41:380–389. doi: 10.1007/s001250050920. [DOI] [PubMed] [Google Scholar]
- Russell JC, Graham SE, Dolphin PJ. Glucose tolerance and insulin resistance in the JCR:LA-cp rat: effect of miglitol (Bay m1099) Metabolism. 1999;48:701–706. doi: 10.1016/s0026-0495(99)90168-3. [DOI] [PubMed] [Google Scholar]
- Russell JC, Graham SE, Richardson M. Cardiovascular disease in the JCR:LA-cp rat. Mol Cell Biochem. 1998a;188:113–126. [PubMed] [Google Scholar]
- Russell JC, Kelly SE, Schäfer S. Vasopeptidase inhibition improves insulin sensitivity and endothelial function in the JCR:LA-cp rat. J Cardiovasc Pharmacol. 2004;44:258–265. doi: 10.1097/00005344-200408000-00016. [DOI] [PubMed] [Google Scholar]
- Russell JC, McKendrick JD, Dubé GP, Dolphin PJ, Radomski MW. Effects of LY117018 and the estrogen analogue, 17-ethinylestradiol, on vascular reactivity, platelet aggregation, and lipid metabolism in the insulin-resistant JCR: LA-cp rat: role of nitric oxide. J Cardiovasc Pharmacol. 2001;37:119–128. doi: 10.1097/00005344-200101000-00014. [DOI] [PubMed] [Google Scholar]
- Russell JC, Kelly SE, Proctor SD.The JCR:LA-cp Rat: animal model of the metabolic syndrome exhibiting micro- and macro-vascular disease Animal Models of Diabetes 2007CRC Press: Boca Raton, FL; 157–180.In: Shafrir (ed) [Google Scholar]
- Russell JC, Ravel D, Pégorier J-P, Delrat P, Jochemsen R, O'Brien SF, et al. Beneficial insulin-sensitizing and vascular effects of S15261 in the insulin-resistant JCR:LA-cp rat. J Pharmacol Exp Ther. 2000;295:753–760. [PubMed] [Google Scholar]
- Ryan DH. Recent progress in obesity pharmacology. Curr Opin Gastroenterol. 2000;16:166–172. doi: 10.1097/00001574-200003000-00012. [DOI] [PubMed] [Google Scholar]
- Sachs GR, Shoemaker R, Hirschowitz BI. Action of 2-deoxy-D-glucose on frog gastric mucosa. Am J Physiol. 1965;209:461–466. doi: 10.1152/ajplegacy.1965.209.3.461. [DOI] [PubMed] [Google Scholar]
- Schäfer S, Steioff K, Linz W, Bleich M, Busch AE, Löhn M. Chronic vasopeptidase inhibition normalizes diabetic endothelial dysfunction. Eur J Pharmacol. 2004;484:361–362. doi: 10.1016/j.ejphar.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Sing CF, Stengård JH, Kardia SLR. Genes, environment, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:1190–1196. doi: 10.1161/01.ATV.0000075081.51227.86. [DOI] [PubMed] [Google Scholar]
- Snow KL, Moseley RH. Effect of thiazolidinediones on bile acid transport in rat liver. Life Sci. 2007;80:732–740. doi: 10.1016/j.lfs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Stepanyan Z, Kocharyan A, Behrens M, Koebnick C, Pyrski M, Meyerhof W. Somatostatin, a negative-regulator of central leptin action in the rat hypothalamus. J Neurochem. 2007;100:468–478. doi: 10.1111/j.1471-4159.2006.04219.x. [DOI] [PubMed] [Google Scholar]
- Sweet IR, Peterson L, Kroll K, Goodner CJ, Berry M, Graham MM. Effect of glucose on uptake of radiolabeled glucose, 2-DG, and 3-O-MG by the perfused rat liver. Am J Physiol. 1996;271:E384–E396. doi: 10.1152/ajpendo.1996.271.2.E384. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Tanaka T, Kodama T, Sakai J. Peroxisome proliferators-activated receptor δ (PPARδ), a novel target site for drug discovery in metabolic syndrome. Pharmacol Res. 2006;53:501–507. doi: 10.1016/j.phrs.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Uusitupa MI, Niskanen LK, Siitonen O, Voutilainen E, Pyorala K. 5-year incidence of atherosclerotic vascular disease in relation to general risk factors, insulin level, and abnormalities in lipoprotein composition in noninsulin-dependent diabetic and nondiabetic subjects. Circulation. 1990;82:27–36. doi: 10.1161/01.cir.82.1.27. [DOI] [PubMed] [Google Scholar]
- Vance JE, Russell JC. Hypersecretion of VLDL, but not HDL, by hepatocytes from the JCR:LA-corpulent rat. J Lipid Res. 1990;31:1491–1501. [PubMed] [Google Scholar]
- Vine D, Takechi R, Russell JC, Proctor SD. Impaired postprandial apolipoprotein-B48 metabolism in the obese, insulin-resistant JCR:LA-cp rat: increased atherogenicity for the metabolic syndrome. Atherosclerosis. 2007;190:282–290. doi: 10.1016/j.atherosclerosis.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Dietary supplementation with 2-deoxy-D-glucose improves cardiovascular and neuroendocrine stress adaptation in rats. Am J Physiol Heart Circ Physiol. 2004;287:H1186–H1193. doi: 10.1152/ajpheart.00932.2003. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin – a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2005;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- Williams G, Cardoso H, Domin J, Ghatei MA, Russell JC, Bloom SR. Disturbances of regulatory peptides in the hypothalamus of the JCR:LA-corpulent rat. Diabetes Res. 1990;15:1–7. [PubMed] [Google Scholar]
- Won J-S, Im Y-B, Key L, Singh I, Singh AK. The involvement of glucose metabolism in the regulation of inducible nitric oxide synthase gene expression in glial cells: possible role of glucose-6-phosphate dehydrogenase and CCAAT/enhancing binding protein. J Neurosci. 2003;23:7470–7478. doi: 10.1523/JNEUROSCI.23-20-07470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Peng XS, Chua SC, Jr, Okada N, Liu S-M, Nicolson M, Leibel RL. Phenotype of the obese Koletsky (f) rat due to Tyr763Stop mutation in the extracellular domain of the leptin receptor: evidence for deficient plasma-to-CSF transport of leptin in both the Zucker and Koletsky obese rat. Diabetes. 1997;46:513–518. doi: 10.2337/diab.46.3.513. [DOI] [PubMed] [Google Scholar]
- Zhou X, Engle T, Goepfert C, Erren M, Assmann G, von Eckardstein A. The ATP binding cassette transporter A1 contributes to the secretion of interleukin 1β from macrophages but not from monocytes. Biochem Biophys Res Comm. 2002;291:598–604. doi: 10.1006/bbrc.2002.6473. [DOI] [PubMed] [Google Scholar]