Abstract
Background and purpose:
The D2/D3 receptor antagonist, D4 receptor partial agonist, and high efficacy 5-HT1A receptor agonist F15063 was shown to be highly efficacious and potent in rodent models of activity against positive symptoms of schizophrenia. However F15063 induced neither catalepsy nor the ‘serotonin syndrome'. Here, we evaluated its profile in rat models predictive of efficacy against negative symptoms/cognitive deficits of schizophrenia.
Experimental approach:
F15063, given i.p., was assessed in models of behavioural deficits induced by interference with the NMDA/glutamatergic (phencyclidine: PCP) or cholinergic (scopolamine) systems.
Key results:
Through 5-HT1A activation, F15063 partially alleviated (MED: 0.04 mg kg−1) PCP-induced social interaction deficit between two adult rats, without effect by itself, underlining its potential to combat negative symptoms. At doses above 0.16 mg kg−1, F15063 reduced interaction by itself. F15063 (0.16 mg kg−1) selectively re-established PCP-impaired ‘cognitive flexibility' in a reversal learning task, suggesting potential against adaptability deficits. F15063 (0.04–0.63 mg kg−1) also reversed scopolamine-induced amnesia in a juvenile-adult rat social recognition test, indicative of a pro-cholinergic influence. Activity in this latter test is consistent with its D4 partial agonism, as it was blocked by the D4 antagonist L745,870. Finally, F15063 up to 40 mg kg−1 did not disrupt basal prepulse inhibition of startle reflex in rats, a marker of sensorimotor gating.
Conclusions and implications:
The balance of D2/D3, D4 and 5-HT1A receptor interactions of F15063 yields a promising profile of activity in models of cognitive deficits and negative symptoms of schizophrenia.
Keywords: 5-HT1A agonist, atypical antipsychotic, cognitive deficit, D2 antagonist, D4 partial agonist, schizophrenia
Introduction
Currently available antipsychotics control the so-called positive symptoms of schizophrenia (hallucination, delusions, etc) in about two-thirds of patients treated, reflecting their antagonist properties at dopamine D2 receptors (Kapur and Remington, 2001). Other aspects of the pathology, such as negative symptoms (flat affect, avolition, anergia, etc) and cognitive dysfunction (including working memory impairment, perseveration and attention deficits) are also alleviated but to a more limited extent (Tandon et al., 1993, Breier et al., 1994, Buchanan et al., 1998, Rosenheck et al., 1999). These cognitive deficits are increasingly considered to be part of the core symptoms of the pathology and the limited efficacy of antipsychotics to combat them hampers proper societal functioning of patients and their reinsertion (Freedman, 2003; Green et al., 2004), and constitutes a real challenge to clinicians.
The search for more effective antipsychotics (i.e. with beneficial effects on negative symptomatology and cognitive deficits) has resulted in the development of numerous compounds with a wide variety of target receptors (Sanger, 2004). One approach involves the association of DA D2 receptor antagonist and 5-HT1A receptor agonist activities. Such a combination has been demonstrated to prevent catalepsy – a preclinical marker of extrapyramidal syndrome (EPS) – that results from blockade of DA D2 receptors. It has also been proposed to bring additional benefits against negative symptoms and cognitive dysfunction associated with schizophrenia (see Millan, 2000; Bantick et al., 2001; Ichikawa et al., 2001; Bardin et al., 2006a; Depoortere et al., 2007, for more detailed discussion). Indeed, several new compounds in development conform to this profile: bifeprunox (Feenstra et al., 2001; Wolf, 2003), SSR181507 (Claustre et al., 2003; Depoortere et al., 2003; Boulay et al., 2004, Terranova et al., 2005), SLV313 (Feenstra et al., 2002; McCreary et al., 2002) and sarizotan (now developed as an anti-dyskinetic: Bibbiani et al., 2001; Rabiner et al., 2002; Bartoszyk et al., 2004).
We have previously reported on the binding, neurochemical (Newman-Tancredi et al., 2006) and behavioral profile in tests predictive of antipsychotic activity (Depoortere et al., 2006) of F15063 (N-[(2,2-dimethyl-2,3-dihydro-benzofuran-7-yloxy)ethyl]-3-(cyclopent-1-enyl)-benzylamine; mono-tartrate), synthesized by the Medicinal Chemistry department of Pierre Fabre Recherche (Vacher et al., 2002). This compound interacts potently with human DA D2L (pKi=9.44), D2S (pKi=9.25), D3 (pKi=8.95) and D4 (pKi=8.81) receptors, and has somewhat lesser affinity for 5-HT1A receptors (pKi=8.37). It presents low affinity at a multitude of other receptors, including 5-HT2A and 5-HT2C, α1 and α2 adrenoceptors, muscarine M1 and histamine H1. In functional in vitro tests, it behaves as an antagonist at DA D2 receptors (contrary to other preferential D2/5-HT1A antipsychotics such as bifeprunox and SSR181507 that act as partial agonists at these receptors: Bruins Slot et al., 2006; Cosi et al., 2006), and as a partial agonist at D4 and an high efficacy agonist at 5-HT1A receptors (Newman-Tancredi et al., 2006). F15063 was potently (effective doses for 50% response (ED50s) from 0.23 to 1.10 mg kg−1 intraperitoneal (i.p.)) active in rodent models that detect activity against positive symptoms of schizophrenia: methylphenidate-induced stereotyped behaviors, D-amphetamine- or ketamine-induced hyperlocomotion, apomorphine-induced prepulse inhibition (PPI) deficits, conditioned avoidance test and apomorphine-induced climbing. However, because of its 5-HT1A agonism, F15063 had no cataleptogenic potential (ED50>40 mg kg−1, i.p.), suggesting that it should not produce EPS. Finally, F15063 did not induce the ‘serotonin syndrome' in rats (ED50>32 mg kg−1 i.p.). In conclusion, F15063 has the preclinical profile of a potent atypical antipsychotic (Depoortere et al., 2006, 2007).
However, as emphasized above, controlling positive symptomatology is not sufficient to enable schizophrenic patients to return to normal social activity and there is a considerable unmet need for relieving negative symptoms and memory/cognitive defects associated with the pathology. It is therefore essential to characterize potential antipsychotics in a battery of preclinical tests purported to mimic negative symptoms and cognitive deficits of schizophrenia.
Based on the hypothesis that interference with glutamatergic transmission via the NMDA receptor underlies aspects of schizophrenia (Javitt and Zukin, 1991; Olney and Farber, 1995), we undertook the assessment of F15063 in two models of negative symptoms and deficits of cognition/memory produced by the noncompetitive antagonist of NMDA receptors, phencyclidine (PCP): (1) social interaction between a dyad of adult rats and (2) a reversal learning task (RLT) using a two-lever food-reinforced operant schedule.
Additionally, alterations of cholinergic neurotransmission have been shown to occur in schizophrenia (Bymaster et al., 1999; Hyde and Crook, 2001). For that reason, we assessed the influence of F15063 on memory disruption consecutive to administration of the anticholinergic agent scopolamine, in a model of social recognition between an adult and a juvenile rat. Further, because F15063 is also a partial agonist at the dopamine D4 receptor, we also conducted antagonist studies with the D4 receptor blocker L745,870 in this model.
Lastly, because of its 5-HT1A agonist activity, F15063 was evaluated in the PPI of the startle reflex in rats. In effect, activation of 5-HT1A receptors by agonists such as 8-OH-DPAT and buspirone (Nanry and Tilson, 1989; Rigdon and Weatherspoon, 1992) diminishes basal PPI in rats. Such a disruption could be of concern, since schizophrenic patients already suffer from reduced basal PPI levels (Braff et al., 1978; Swerdlow et al., 2000 for review), and it would thus be desirable for an antipsychotic not to further affect basal PPI.
Data presented in this paper have been for the most part presented in abstract forms (Auclair et al., 2006b; Bardin et al., 2006b, Depoortere et al., 2006).
Materials and methods
Animals
Male Sprague–Dawley rats (180–200 g for adults, 70–80 g for juveniles, at the start of the experiments) were supplied by Iffa-Credo (Les Oncins, France). Animals were kept in temperature- and humidity-controlled rooms (21±1°C, relative humidity: 55±5%) on a 12:12 h light:dark cycle (lights on at 0700 h). Food (standard A04 rodent chow, Animal Food and Engineering, Epinay sur Orge, France) and filtered water (0.22 μm pores) were available ad libitum (except when specified otherwise below). Animals were handled and cared for in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, USA) and the European Directive 86/609. In addition, the protocols were carried out in compliance with French regulations and the local ethical committee guidelines for animal research.
PCP-induced social interaction deficit between a dyad of adult rats
The social interaction procedure was adapted from that developed by Sams-Dodd (1995) and described in detail by Bruins Slot et al. (2005). F15063 or vehicle was administered i.p. daily for 3 days in combination with a subcutaneous (s.c.) injection of either vehicle or 2.5 mg kg−1 PCP. Social interaction was measured on the last (3rd) day of drug treatment, 45 min after the last injection. For the antagonism study, rats received a supplementary s.c. injection of either vehicle or 0.63 mg kg−1 WAY100,635 90 min before the behavioral observations. Pairs of unfamiliar rats having received the same treatment were placed for 10 min in a wooden open arena (150 × 100 × 40 cm high) painted in black. The total number of the following behavioral items was scored (one point for each occurrence of behavior in either rat, with no lower cutoff time limit) by an investigator blind to the treatment: investigative sniffing behavior (sniffing the conspecific's snout or parts of the body including the anogenital region), following (moving towards and following the conspecific around the arena), climbing over or under (climbing over the conspecific's back or pushing the head and forepart of the body beneath the conspecific), and aggression (including biting, fighting, mounting and threats). Scores for each behavioural item were added to yield a total score for a given pair of rats (i.e., total number of events).
Data (total scores) were first analyzed by a two-way ANOVA with the dose of F15063 and pretreatment (saline or PCP) as the between-subjects factors, followed by one-way ANOVA on the dose of F15063, and post hoc Bonferroni's test. For the antagonism study with WAY100,635, data were analyzed with a one-way ANOVA, followed by a post hoc Bonferroni's test.
PCP-induced deficit of reference memory and task reacquisition in a RLT in rats
All rats were tested in operant Skinner boxes (29 × 25 × 32 cm, W × L × H, Coulbourn Instruments, Lehigh Valley, PA, USA) enclosed in ventilated and sound-attenuating cubicles (54 × 40 × 45 cm, W × L × H). Each box was fitted with two retractable levers (3 × 2 cm deep) on either side of the magazine where 45 mg food pellets were delivered. A white cue lamp and a buzzer (85 dB, 2 s tone: a high-tone (10 kHz), and a low-tone (2 kHz), associated with presentation of the right and left retractable lever, respectively) served as stimulus cues.
First, rats were shaped (daily sessions of 30 min) to lever-press to receive a reinforcement (45 mg pellet) on a fixed-ratio 1 schedule. Initially, one lever was randomly presented: if the rat pressed this lever, one pellet was delivered, the lever was retracted, and another lever was immediately randomly (left or right) presented. If the rat failed to press within 30 s of lever presentation, the lever was retracted, and a lever was again immediately randomly presented. Each lever was presented in concomitance with its associated cue light and tone combination (see above). This pretraining period lasted between 5 and 11 days. Rats progressed to the learning task 1 (LT1) schedule once they pressed each lever at least 20 times during two consecutive pretraining sessions.
This LT1 schedule lasted for 5 days: each daily session (40 min) started with the introduction of both levers, only one being active, that is delivering a pellet when pressed. The active lever was signalled by the concomitant presentation of the associated cue light and tone. If the rat pressed the active lever within 10 s of presentation, a pellet was delivered, both levers were retracted and reintroduced 10 s later. The active lever was randomly reassigned. If the rat did not press within 10 s or pressed the inactive lever, the house light was turned off, both levers were retracted for 10 s, after which a new trial of presentation was initiated, the active lever remaining the same as in the preceding ‘failed' trial. Rats were required at least 70% correct responding on three consecutive sessions to be trained in the learning task 2 (LT2) schedule.
The LT2 schedule was in all points similar to that of the LT1, except that the rules were reversed, that is the active lever was the one not associated with its cue-light and tone. Rats were required at least 70% correct responding on three consecutive sessions to enter the phase of pharmacological treatment. This schedule lasted up to 20 days.
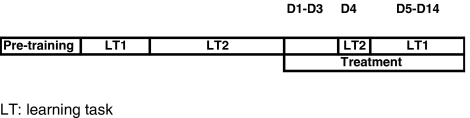
This phase lasted for 14 sessions: during the first three sessions (D1–D3), rats were treated with vehicle or F15063 (i.p.) 45 min before vehicle or PCP (2.5 mg kg−1, s.c.). During these 3 days, rats were not tested: this period of treatment was intended to induce tolerance to the motor-disrupting side-effects of PCP, which could interfere with operant responding during tests sessions. On the 4th day (D4), rats were treated as usual and tested under the LT2 schedule 45 min after the second injection: this test was introduced to assess the impact of pharmacological treatment (D1–D4) on the retention of LT2 (reference memory component). From D5–D14, rats were tested under the LT1 schedule: this phase served to assess the influence of pharmacological treatment on the reacquisition of LT1 (second reversal) (Scheme 1).
Scheme 1.
Synopsis of training schedules and pharmacological treatment phase for the RLT experiment.
The percentage of correct responses and the latency time to respond on any lever (in 1/10 s) were recorded during each LT1 and LT2 schedule session. Control (vehicle–vehicle) and vehicle-PCP-treated rats included rats injected with saline i.p. (with and without Tween 80) and with saline s.c. This is because a whole series of reference antipsychotics (data to be published) have been tested in parallel with F15063, with different routes of administration and excipient. Data were analyzed by means of a one-way ANOVA for comparing, across treatment conditions, basal data (average of the last 3 days before starting the pharmacological treatment) and for comparing data obtained during D4. It was followed when appropriate by a post hoc Bonferroni's test. Data collected during D5–D14 were collated across all 10 sessions, and were analyzed with a one-way ANOVA, followed when appropriate by post hoc Dunnett's test.
Scopolamine-induced deficit of social recognition between an adult and a juvenile rat
The procedure was adapted from that described by Perio et al. (1989). Briefly, on the test day, an adult was placed alone in an observation arena for 5 min, after which a juvenile rat was introduced for a first 5 min session (T1). The same juvenile was re-exposed to the adult rat 30 min later, for a second session of social interaction (T2). The time (in s) spent by the adult interacting socially with the juvenile (sniffing, following, climbing over or going under, or aggression) was manually recorded during T1 and T2 by an investigator blind to the treatment. The adult rat was treated with vehicle or F15063 i.p. 45 min before the first observation session (T1), followed 15 min later by scopolamine (0.63 mg kg−1, s.c.).
An additional control experiment aimed at assessing the specificity of reversing effects against scopolamine-induced amnesia was undertaken. To that end, the adult rat was exposed to an unfamiliar juvenile during T2, after being injected with F15063 alone (without scopolamine). If F15063 produces a retention score close to that seen in vehicle-treated rats, it will mean that the adult spent as much time investigating the unfamiliar juvenile during T2 as investigating the first juvenile during T1. This would indicate that the reversing effects seen against scopolamine are not secondary to a lack of interest for the juvenile or to aspecific motor effects produced by F15063, which would somehow interfere with proper social interaction. Finally, in order to assess the amnesic activity of F15063 on its own, the compound was also injected alone, 30 min before T1, and the adult rat was presented with the same juvenile during T2.
In a separate experiment, adult rats received an i.p. injection of vehicle or 0.63 mg kg−1 of the DA D4 receptor antagonist L745,870, 15 min before administration of F15063, which was followed by an injection of scopolamine (see above).
Retention scores (difference in the time spent in active social behavior during the second encounter minus the time spent in active social behavior during the first encounter: T2–T1, in second) were analyzed by a one-way ANOVA followed by post hoc Bonferroni's test.
Effects on basal PPI of the startle reflex in rats
A detailed description is given in Auclair et al. (2006a). Rats were pretested in startle chambers (SR LAB, San Diego Instruments, San Diego, CA, USA) 1 h 45 min before the pharmacological challenge (test) session. This pretest session was used to accustom rats to the procedure. Three different trial types were presented against a continuous 70 dB background noise: no pulse (NP), 118 dB pulse (pulse alone: PA) and 78 dB prepulse (pp) followed by a 118 dB pulse (prepulse-pulse: ppP). The PA duration was 40 ms, the pp duration 20 ms, and the interval between the end of the pp and the onset of the PA 80 ms. Sessions started with a 5 min adaptation period after which the animals were exposed to 10 PA (included to induce habituation to startle, such that habituation during the following PPI assessment would be minimized: these trials were not used for data analysis). These 10 PA trials were followed by 10 PA, 10 ppP and three NP trials presented in a pseudo-random order. The interval between trials was variable but with a median of 15 s.
At the end of the pretest session, animals were injected i.p. with F15063 or its vehicle and 45 min later with vehicle s.c. This second injection was meant to be under conditions similar to those used in the reversion of apomorphine deficits by antipsychotics, where vehicle was replaced by apomorphine (Auclair et al., 2006a). They were then (15 min later) subjected to a test session, in all respects similar to the pretest session (vide supra).
For each test session, the median of the amplitude of the startle responses for the last 10 PA trials and for the 10 ppP was calculated. The percentage PPI was calculated as follows:
Data (percentage PPI and amplitude of PA) were analyzed with a one-way ANOVA with the treatment as the between-subjects factor, followed by a Dunnett's post hoc test.
Statistical analyses
Results were analyzed by ANOVA (one- or two-way) with appropriate post hoc tests (Dunnet's, Bonferroni's or Newman–Keul's). The details of the statistical treatment of each data set are given at the end of the description of the methods used to generate the data.
Drugs
F15063 ((N-[(2,2-dimethyl-2,3-dihydro-benzofuran-7-yloxy)ethyl]-3-(cyclopent-1-enyl)-benzylamine; mono-tartrate) and WAY100,635 were synthesized by Bernard Vacher/Stephane Cuisat and Jean Louis Maurel (Medicinal Chemistry, Centre de Recherche Pierre Fabre, Castres, France). All other compounds were obtained commercially: scopolamine (Sigma RBI, St Quentin Fallavier, France), L745,870 (Tocris, Illkirch, France) and PCP hydrochloride (Francopia, Paris, France), and like WAY100,635, were dissolved in distilled water and administered s.c. at a volume of 10 ml kg−1. F15063 was administered i.p. in a volume of 10 ml kg−1 in distilled water + Tween 80 (0.1% v v−1). Doses refer to the weight of the free base.
Results
Attenuation of PCP-induced social interaction deficit between a dyad of adult rats
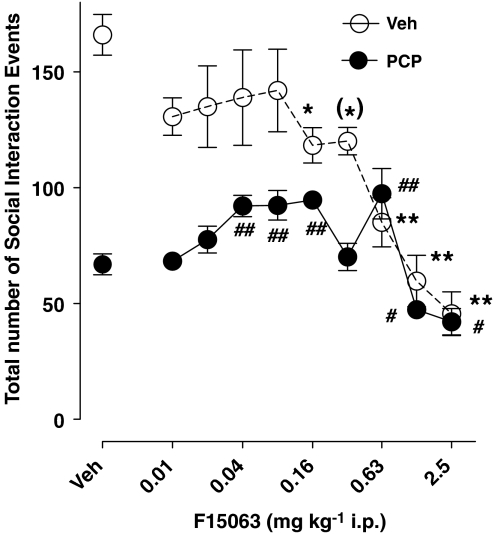
Treatment with 2.5 mg kg−1 s.c. PCP produced a robust reduction of the number of social interaction events, representing a 60% decrease versus control values (compare the two most leftward symbols, Figure 1). F15063, from 0.04 to 0.16, and at 0.63 mg kg−1, significantly attenuated PCP-induced reduction of social interaction (filled circles). At these doses, it had no significant effect of its own (0.04 and 0.08 mg kg−1) or significantly reduced (0.16 and 0.63 mg kg−1) social interaction in the vehicle-pretreated animals (Figure 1).
Figure 1.
F15063 attenuated a deficit of social interaction in rats produced by PCP. Each symbol/bar represents the mean (±s.e.m.) number of social interaction events between a dyad of rats. F15063 or vehicle was administered daily for 3 days in combination with a s.c. injection of either vehicle or 2.5 mg kg−1 PCP. Social interaction (10 min observation period) was measured on the last (3rd) day of drug treatment, 45 min after the last injection. Statistical analysis: two-way ANOVA: F(9,132)=14.1, P<0.001, F(1,132)=815, P<0.001 and F(9,132)=6.7, P<0.001, for the F15063 dose, PCP treatment and F15063 dose × PCP treatment interaction, respectively. This two-way ANOVA was followed by separate one-way ANOVAs. (*)P=0.05, *P<0.05, **P<0.01, versus vehicle for vehicle-treated rats, Bonferroni's post hoc test, following significant one-way ANOVA: F(9,69)=10.3, P<0.001. #P<0.05, ##P<0.01, versus vehicle for PCP-treated rats, Bonferroni's post hoc test, following significant one-way ANOVA: F(9,69)=11.4, P<0.001. N=7–9 pairs of rats per group.
Attenuation by F15063 of PCP-induced social interaction deficit is antagonized by the 5-HT1A receptor antagonist WAY100,635
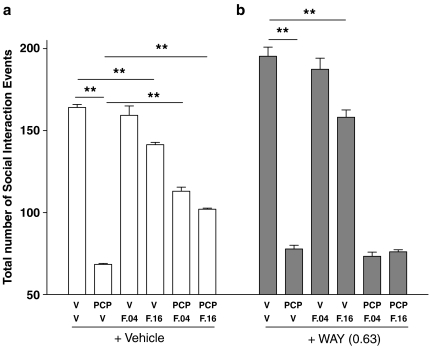
F15063 (0.04. and 0.16 mg kg−1 i.p.) significantly attenuated PCP-induced deficit of social interaction (compare 2nd with 5th and 6th white bars, starting from the left, Figure 2, left panel). By itself, at these doses, F15063 produced a significant reduction of social interaction at 0.16 mg kg−1 only (compare 1st with 4th white bars, starting from the left).
Figure 2.
The attenuating effects of F15063 against PCP-induced social interaction deficit (a) were antagonized by the 5-HT1A receptor antagonist WAY100,635 (b). See legend of Figure 1 for details. Rats were given saline (a) or WAY100,635 (0.63 mg kg−1, s.c.) (b), 90 min before the observation period. **P<0.01, Bonferroni's post hoc test, following significant one-way ANOVA; for data in a, (F(5,38)=210.0, P<0.001; data in b, F(5,38)=188.1, P<0.001). N=7 pairs of rats per group.
Pretreatment with WAY100,635 (0.63 mg kg−1, s.c.) prevented the attenuating effects of F15063 on disruption of social interaction produced by PCP (compare the 2nd with 5th and 6th black bars, starting from the left, Figure 2, right panel). In combination with WAY100,635, F15063 had a significant effect by itself at 0.16 mg kg−1 only (compare 1st with 4th black bars, starting from the left).
Attenuation of PCP-induced deficit of choice accuracy and augmentation of lever-pressing latency times during a test session for reference memory in the RLT in rats
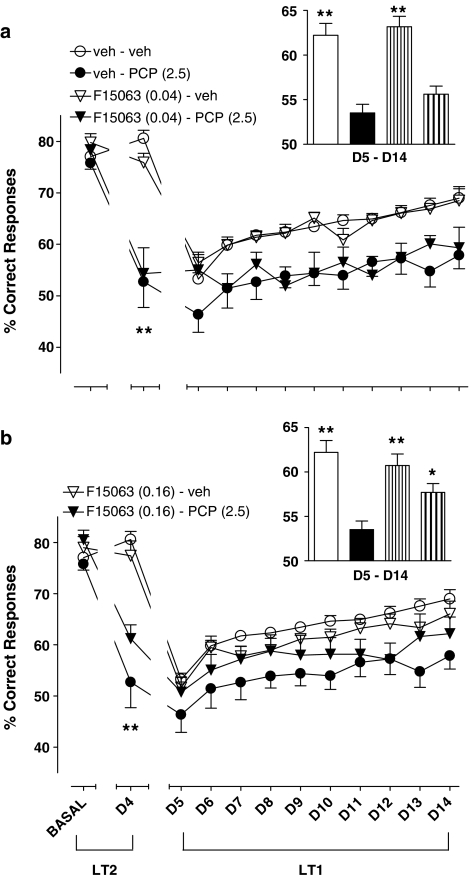
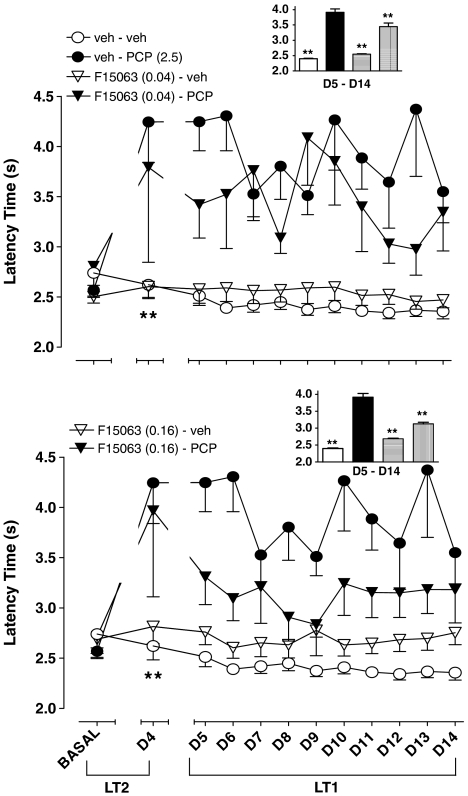
Basal (before starting pharmacological treatment) values did not significantly differ between groups of rats (compare leftmost cluster of symbols, Figure 3a and b). On the 4th day of treatment (D4), rats treated with 2.5 mg kg−1, s.c. PCP produced a marked and significant decrease of percentage of correct responses in comparison with vehicle–vehicle control rats (compare circles, second cluster of symbols, starting from the left, Figure 3a and b). More precisely, the level of choice accuracy dropped from 80.6±1.6 (n=15) for controls to 50.9±5.1 (n=11) for PCP, a value very close to the chance level. This suggests that reference memory for responding governed by LT2 was greatly affected by PCP. By itself, F15063, at both doses, did not produce changes (compare open symbols). In combination with PCP, F15063 at 0.16 mg kg−1 tended to attenuate the deleterious effect of PCP (compare filled symbols).
Figure 3.
Reduction by F15063 of a deficit of choice accuracy induced by chronic treatment with PCP in the RLT in rats. Each symbol represents the mean (±s.e.m.) percentage of correct responses (i.e. responses on the appropriate lever) obtained during the three sessions immediately preceding the start of the pharmacological treatment (BASAL), the 4th day of treatment (D4) and during the 5th to 14th day of treatment (D5–D14). Basal values (lefthand cluster of points) did not differ between groups of rats (one-way ANOVA: F(3,36)=1.2, P>0.05 and F (3,39)=1.9, P>0.05, for 0.04 and 0.16 mg kg−1 of F15063, respectively). *P<0.05, **P<0.01, compared with vehicle–vehicle value at D4, for the corresponding treatment, Bonferroni's post hoc tests following significant one-way ANOVA (F(3,32)=18.5, P<0.001 and F(3,33)=18.6, P<0.001, for 0.04 and 0.16 mg kg−1 of F15063, respectively). N=5–9 rats per group. Inset: each bar represents the average (±s.e.m.) percentage of correct responses collated across the 5th to the 14th day. *P<0.05, **P<0.01, compared with vehicle/PCP treated rats, Dunnett's post hoc test following significant one-way ANOVA. The order of treatments is the same as that in the legend on the left.
During the basal phase, the latency time to lever-press on any of the two levers did not differ significantly between groups (compare leftmost cluster of symbols, Figure 4a and b). During D4, rats treated with 2.5 mg kg−1 PCP took nearly twice as long before initiating lever-pressing, a significant effect.
Figure 4.
Reduction by F15063 of the increase in the latency time to the first response induced by chronic treatment with PCP in the RLT in rats. Each symbol represents the mean (±s.e.m.) latency time to the first response on any lever, obtained during the three sessions immediately preceding the start of the pharmacological treatment (BASAL), the 4th day of treatment (D4) and during the 5th to 14th day of treatment. Basal values did not differ between groups (one-way ANOVAs: F (3,36)=1.0, P>0.05 and F (3,39)=0.5, P>0.05, for 0.04 and 0.16 mg kg−1 of F15063, respectively) For day 4, *P<0.05, **P<0.01, compared with vehicle/vehicle value at D4, for the corresponding treatment, Bonferroni's post hoc tests following significant one-way ANOVA (F (3,27)=7.1, P<0.001 and F (3,31)=5.7, P<0.05, for 0.04 and 0.16 mg kg−1 of F15063, respectively). N=5–9 rats per group. Inset: each bar represents the average (±s.e.m.) latency time collated across the 5th to the 14th day. At the lower dose, F15063 did not reverse PCP-induced effects (Dunnett's post hoc test, following significant one-way ANOVA: F(3,36)=18.6, P<0.0001). At the higher dose (0.16 mg kg−1), **P<0.01, compared to vehicle/PCP-treated rats, Dunnett's post hoc test following significant one-way ANOVA (F (3,36)=10.9, P<0.0001). The order of treatments is the same as that in the legend on the left.
Attenuation of PCP-induced deficit of choice accuracy and augmentation of lever-pressing latency times during the reacquisition phase for LT1 (D5–D14) in the RLT in rats
During the reacquisition phase for LT1 (D5–D14 phase, right-hand side of Figure 3a), PCP-injected rats (filled circles) performed less well than their control counterparts. However, the performance of both groups progressed over time, albeit with an apparent steeper slope (faster learning curve) for the vehicle-treated group. Complementary analysis performed on data collated across all 10 sessions showed that F15063 at 0.16 mg kg−1 partially but significantly reversed the deficit produced by PCP (compare 2nd and 4th bars, starting from the left, inset of Figure 3b). At 0.04 mg kg−1, F15063 did not significantly reverse PCP-induced disruption (compare 2nd and 4th bars, starting from the left, inset of Figure 3a).
During this phase, the latency time to lever-pressing remained elevated under PCP treatment, (filled circles, right-hand side of Figure 4a and b). Complementary analysis performed on data collated across all 10 trials showed that F15063 at 0.16 mg kg−1 partially but significantly reversed the increase in latency produced by PCP (compare 2nd and 4th bars, starting from the left, inset of Figure 4b). However, contrary to what was observed with the percentage of correct responses (vide supra), F15063 at 0.04 mg kg−1 also significantly attenuated PCP-induced effects on latency (compare 2nd and 4th bars, starting from the left, inset of Figure 4a).
Reversal of scopolamine-induced deficit of social recognition between an adult and a juvenile rat
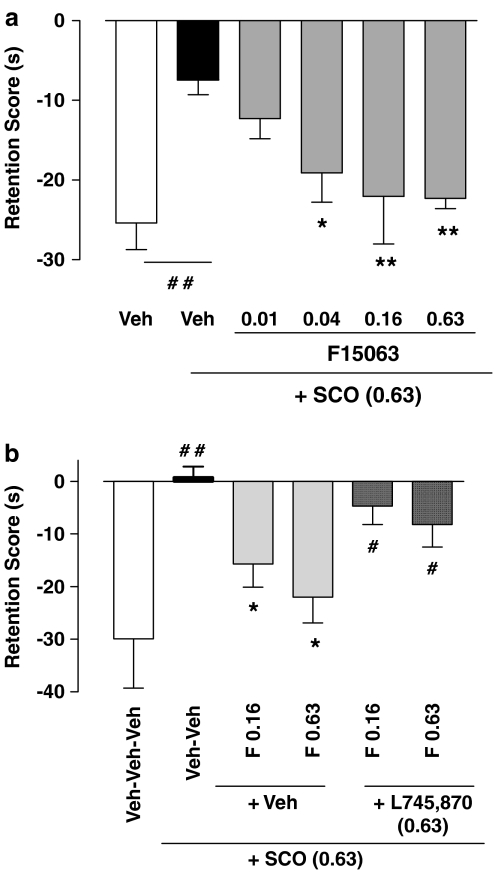
With respect to those of the control (vehicle/vehicle-injected) group, rats pretreated with 0.63 mg kg−1 s.c. of scopolamine presented a highly significant reduction of the retention score (compare first pair of bars, starting from the left, Figure 5a). In other words, under scopolamine, they spent almost as much time interacting with the juvenile during T2 than during T1 (hence a retention score, T2–T1, close to 0), consistent with an amnesic effect of scopolamine. F15063 dose-dependently reversed this deleterious effect of scopolamine, with values from 0.04 mg kg−1 i.p. not significantly different from those of controls (Bonferroni's post hoc test).
Figure 5.
(a) F15063 reversed the decrease of retention score induced by scopolamine. (b) This reversal is antagonized by the dopamine D4 receptor antagonist L745,870. Each bar represents the mean (±s.e.m.). difference in the time spent in social interaction during the second encounter (T2) minus the time spent in social interaction during the first encounter (T1). (a) Vehicle or F15063 were administered i.p. to the adult rat 45 min before testing, and scopolamine (Sco: 0.63 mg kg−1, s.c.) or vehicle were administered 30 min before the observation session. Scopolamine caused a reduction of the score (significant one-way ANOVA: F(5,112)=6.84, P<0.001) which was totally reversed by F15063. N=9 adult rats per dose. (b) L745,870 or vehicle were administered i.p. 15 min before vehicle or F15063. The D4 antagonist prevented the effects of F15063 against scopolamine induced amnesia N=11 adult rats per dose. For both panels: *P<0.05, **P<0.01, compared with the scopolamine-injected group; #P<0.05, ##P<0.01, compared with the vehicle-injected group using Bonferroni's test, following significant ANOVA (one-way, F(5,58)=4.62, P<0.001).
On its own, F15063, administered 30 min pretest (before T1) did not modify the retention score (−24.6±3.4, −27.2 5.5, −16.3±11.7, −21.8±5.4 and −29.5±4.4, for vehicle, 0.01, 0.04, 0.16 and 0.63 mg kg−1, respectively; one-way ANOVA: F(4,49)=0.56, P>0.05).
When confronted with an unfamiliar juvenile during T2, rats treated with F15063 did not show retention scores different from those of vehicle-treated rats (−13.8±2.2, −13.0±4.7, −19.9±6.9, −9.3±8.0 and −29.8±6.6, for vehicle, 0.01, 0.04, 0.16 and 0.63 mg kg−1, respectively; one-way ANOVA: F(4,40)=1.79, P>0.05). This demonstrates that under these conditions, rats treated with F15063 retain their ability to interact when confronted with an unfamiliar juvenile, and that the reversion observed in combination with scopolamine with a familiar juvenile is not the consequence of nonspecific motor, sedating or attention deficit side-effects produced by F15063.
Co-administration of the DA D4 receptor antagonist L745,870 (0.63 mg kg−1) almost completely reversed the beneficial effects of F15063 against scopolamine-induced amnesia (compare the middle and right pairs of bars, Figure 5b). Thus the rats given vehicle/vehicle/scopolamine or L745,870/F15063(0.16)/scopolamine or L745,870/F15063(0.63)/scopolamine behaved differently from those treated with vehicle/vehicle/vehicle. By itself, L745,870 had no effect on scopolamine-induced amnesia (−25.2+3.0, −6.6±1.8, −6.0±5.0, −12.4±4.2, −8.7±10.6, for vehicle, scopolamine, 0.04, 0.16 and 0.63 mg kg−1 of L745,870, respectively).
Absence of effect on basal PPI of the startle reflex in rats
Over a wide range of doses (0.01. to 10 mg kg−1 i.p.), F15063 did not significantly modify basal PPI levels (Table 1). F15063 significantly affected PA amplitude, with post hoc test revealing that the effects were restricted to the 0.04 and 10 mg kg−1 doses (Table 1).
Table 1.
Effects of F15063 on the percentage of PPI and on the pulse alone amplitude of the startle reflex in rats
| Dose | Vehicle | 0.0025 | 0.01 | 0.04 | 0.16 | 0.63 | 2.5 | 10 |
|---|---|---|---|---|---|---|---|---|
| % PPI | 48.1±3.7 | 45.4±4.2 | 48.1±9.6 | 50.1±7.3 | 50.1±15.2 | 62.3±6.8 | 52.4±8.4 | 51.8±7.3 |
| Pulse amplitude | 96.6±10.7 | 104.1±9.5 | 91.6±15.4 | 238.7±101.4* | 119.8±33.1 | 78.6±14.9 | 79.6±10.2 | 248.1±55.3* |
Abbreviatons: ANOVA, analysis of variance; i.p., intraperitoneal; PPI, prepulse inhibition; s.e.m., standard error of the mean.
Data shown in the Table are the means±s.e.m.; doses of F15063 are expressed in mg kg−1 i.p. There was no effect of F15063 on % PPI values (one-way ANOVA, F(7,62)=0.4, P>0.05). For the pulse alone amplitude, *P<0.05, compared with the vehicle-injected group using Dunnett's post hoc test, following significant one-way ANOVA (F(7.62)=3, P<0.01). N=7 rats per group, except for vehicle (n=30).
Table 2 summarizes the pharmacological activity of F15063 in tests predictive of activity against memory/cognitive deficits associated with schizophrenia. F15063 did not disrupt (ED50>10 mg kg−1) basal PPI of the startle reflex, suggesting that at therapeutically meaningful doses, F15063 should be free from this potentially troublesome side effect.
Table 2.
Summary of pharmacological activity of F15063 in models predictive of activity against negative symptoms and cognitive deficits of schizophrenia
| Model | Response | Active dose(s) (mg kg−1 i.p.) |
|---|---|---|
| PCP-induced social interaction impairment | Augments interaction | 0.04–0.16 & 0.63 |
| PCP-induced impairment in reversal learning task | Ameliorates correct choice | 0.16 |
| Diminishes latency times to respond | 0.04 & 0.16 | |
| Scopolamine-induced social recognition deficit | Blocks amnesia | 0.04–0.63 |
Discussion
The present data underline the following points: (1) F15063 reversed or attenuated memory and social-interaction deficits produced by blockade of glutamate/NMDA or muscarinic receptors; (2) Beneficial activity against the latter was mediated by its partial agonist activity at DA D4 receptors; (3) F15063 had no detrimental effect by itself in these memory/cognition models, except at high doses in the social interaction model where it reduced the score and (4) F15063 did not disrupt basal PPI of the startle reflex.
F15063 is active in models predictive of activity against memory and cognitive deficits associated with schizophrenia
F15063 was, among several reference antipsychotics including clozapine, the only compound with activity in each of two different models of cognitive/memory impairment (present results, Auclair et al., 2006b; Bardin et al., 2006b): (1) PCP-induced memory reacquisition impairment in a ‘RLT' operant conditioning paradigm, and (2) scopolamine-induced social memory deficits in the adult/juvenile social recognition model.
In the RLT experiment, semi-chronic treatment with PCP markedly impaired performance on day 4, when rats were tested under the LT2 schedule. This test provides a measure of ‘reference' memory, because rats were required to lever-press using a rule that had previously been learned with a high level of accuracy (cf. the 80% or so accuracy score recorded during the basal phase). Furthermore, in the same model, PCP produced a slowing of reacquisition of the first LT1, that is the animals responded at random (i.e. at chance level). Overall, the effects of PCP in this model are reminiscent of the impairment of reference memory and behavioral inflexibility presented by schizophrenic patients (Crider, 1997). This last aspect is clinically exemplified in the Wisconsin Card Sorting Test, where patients show an impaired ability to learn the new rule and adopt a winning strategy (Fey, 1951). Performance in the WCST is thought to rely heavily on the prefrontal cortex and the poor level of performance in this task displayed by schizophrenic patients is thought to reflect hypofrontality (Deicken et al., 1995; Ragland et al., 1998).
F15063 ameliorated the level of performance in the RLT test. More precisely, it attenuated the deficit of correct responding produced by PCP during day 4. F15063 by itself did not modify this parameter and the latency time to lever-press. During the reversal phase (D5–D14), F15063 ameliorated the level of accuracy and decreased latency times, again with no or very limited effects of its own. In a comparative study (Auclair et al., 2006b) of the effects of reference antipsychotics on deficits of RLT produced by PCP, SSR181507 showed a profile of activity similar to that of F15063. Clozapine also attenuated the deficit, albeit with a longer onset of action: its effects were observable from day 8 of treatment only. Concerning haloperidol and aripiprazole, although both compounds also seemed to reverse the deleterious effects of PCP on task reacquisition, their effects were attributable to deleterious perseveration of responding governed by the initial (LT1) rule. Furthermore, during the retention phase, SSR181507 on its own diminished performance, while aripiprazole and haloperidol caused further deterioration of the PCP-induced impairment. There are very few examples in the literature of pharmacological studies on RLT: Ziprasidone, but not haloperidol or clozapine, attenuated a deficit of RLT in an operant conditioning schedule induced by an acute challenge with PCP (Abdul-Monim et al., 2003; Idris et al., 2005).
F15063 attenuates PCP-induced social interaction impairment between a dyad of adult rats, owing to its 5-HT1A agonist activity
F15063 alleviated, through activation of 5-HT1A receptors, PCP-induced deficit of social interaction between a pair of adult rats. In a preceding paper (Bruins Slot et al., 2005), we reported that among eight antipsychotics tested under similar experimental conditions, only the mixed DA D2/5-HT1A compounds SSR181507 and aripiprazole, and the DA D2 receptor antagonist remoxipride, were able to attenuate this type of deficit. One possible alternative explanation for the beneficial effect of F15063 in this model would be that the compound attenuated hyperlocomotion and motor stereotypies produced by 2.5 mg kg−1 PCP. However, this is improbable, as hyperactivity induced by this dose of PCP was not modified by 0.04 mg kg−1 i.p. F15063 (the most active dose in the social interaction test: cf. Figures 1 and 2). However, when tested in actimeters used for the psychotomimetic-induced hyperlocomotor activity experiments described in the companion paper (Depoortere et al., 2007), motor scores over 15 min were unchanged 67.4±6.1 and 65.0±14.3, for veh/PCP and F15063/PCP-treated rats, respectively (t=0.16, NS).
It should be noted that for all compounds active in this model, the reversal of deterioration of performance following PCP, although significant, was only partial (present data, Bruins Slot et al., 2005). It is possible that under slightly different experimental conditions (such as a lower dose of PCP and/or by habituating rats to the apparatus and to a different companion over several days and/or by measuring the time spent in social contact instead of scoring the total number of social events), a more robust effect might have been observed, as has been reported for SSR181507 by Boulay et al. (2004). Also, at doses of 0.16 mg kg−1 and above, F15063 diminished social interaction on its own: a similar response was observed for all antipsychotics that were tested under similar experimental conditions, whether they did (SSR181507 and aripiprazole) or did not (clozapine) attenuate PCP-induced social interaction deficit (Bruins Slot et al., 2005). These effects, which may have indeed limited the extent to which they could reverse PCP-induced deterioration of social behavior (see above) are probably related to sedation: however, patients can develop tachyphylaxis to the sedative effects of antipsychotics. Further, under the present experimental conditions, both rats were treated with an antipsychotic: if only one rat had been treated, interaction with a ‘normal' (i.e. vehicle-treated) companion may have been less sensitive to the effects of antipsychotics given alone. This issue deserves further investigation. Similarly to what was observed here, the 5-HT1A receptor antagonist WAY100,635 blocked the effects of SSR181507 and aripiprazole, showing that activation of 5-HT1A receptors is involved in their activity. Furthermore, results with SSR181507, in a variant of the PCP-induced deficit of social interaction used in this laboratory, led Boulay et al. (2004) to formulate the same type of conclusion. The difficulty encountered by schizophrenic patients in establishing social contacts is a major aspect of a cluster of negative symptoms (Dickerson et al., 1996). Activity of F15063 in this present social interaction model suggests potential amelioration of social functioning of patients. This result, in conjunction with the increase in DA in prefrontal cortex of rats (Newman-Tancredi et al., 2006), a surrogate putative marker of activity against negative symptoms of schizophrenia (Kapur and Remington, 1996), additionally underlie the potential of F15063 to combat negative symptomatology.
Efficacy of F15063 in a model of scopolamine-induced memory disruption is mediated by activation of DA D4 receptors
F15063 fully reversed the deleterious effects of scopolamine in an adult-juvenile social recognition paradigm. Schizophrenic patients show deficit of short-term memory as well as a marked impairment in their ability to initiate and maintain social links (Dickerson et al., 1996); in addition, deficient cholinergic neurotransmission is implicated in schizophrenia (Bymaster et al., 1999, Hyde and Crook, 2001). Both these elements justify the use of this model to probe the pro-mnesic/pro-cognitive activity of a potential antipsychotic. In the case of F15063, its activity against memory perturbation consequent to a pharmacologically induced hypocholinergic state lends credence to the notion that compounds displaying such profile could be useful in pathologies characterized both by a deficient cholinergic system and a high incidence of psychosis. Such pathologies include Alzheimer's disease (AD) and Lewy body dementia. Indeed, up to 41% of AD patients, and 70% of Lewy body dementia patients, present psychosis with hallucinations, delirious thoughts and delusions (Ropacki and Jeste, 2005, Perry and Perry, 1995), and the therapeutic treatment of these patients poses a great challenge to clinicians (Sultzer, 2004).
The beneficial effect of F15063 against scopolamine-induced deficit was dependent on DA D4 receptor activation, as shown by the blockade of this effect following co-treatment with the DA D4 antagonist L745,870. In a parallel study, in which we assessed a whole range of antipsychotics in this model, we found that clozapine, olanzapine and SLV313 (another potential antipsychotic with combined DA D2 antagonist and 5-HT1A agonist and DA D4 partial agonist activities, with a level of efficacy at the latter receptor somewhat less than that of F15063: (Cussac et al., 2006) reversed scopolamine-induced disruption of social interaction. Further, the positive activity of SLV313 was also antagonized by L745,870 (Bardin et al., 2006b).
In a slightly different version of the paradigm, where social recognition deficits were induced by increasing the time interval between the two presentations of the juvenile to the adult, the DA D4 receptor agonist A-412997 had a similar beneficial effect (Browman et al., 2005). Other agonists at D4 receptors have shown positive effects in mice memory models: PD168077 facilitated memory consolidation in a passive avoidance model (Bernaerts and Tirelli, 2003), and RO-10-5824 increased novel object exploration placed in the center of a familiar open field (Powell et al., 2003). Nonetheless, one should note that there are also indications that DA D4 receptors antagonists, such as NGD 94-1 and L745,870 can have beneficial impact on cognition: Hence, NGD 94-1 reversed PCP-induced cognition deficit in monkeys (Jentsch et al., 1999), and in a delayed alternation task, L745,870 at high doses disrupted working memory in rats with good baseline performance, but improved performance at low dose in poorly performing subjects (Zhang et al., 2004). However, both these ligands have been shown to act as partial agonists under some in vitro conditions (Gazi et al., 2000, Zawilska et al., 2003). Nevertheless, under our experimental conditions ([35S]GTPγS binding in CHO cells transfected with hD4.4 receptors, manuscript in preparation), in which F15063 acts as a partial agonist, L745,870 behaves as a silent antagonist (NGD94–1 was not tested). In addition, in the delayed alternation task study, results led Zhang and co-workers to conclude that ‘Optimal working memory requires an intermediate level of DA D4 receptor stimulation'. Such a conclusion is consistent with our data suggesting that partial agonism might be the most appropriate means of enhancing memory performance through DA D4 receptors. It is interesting to note that D4 receptors are preferentially expressed in the frontal cortex and hippocampus (Tarazi et al., 2004), two brain regions particularly associated with control of memory/cognition.
F15063 does not produce deficit of basal PPI of the startle reflex
In a preceding study, F15063, like haloperidol, risperidone, clozapine and olanzapine (Auclair et al., 2006a), attenuated apomorphine-induced PPI disruption, a model of gating deficits observed in schizophrenic patients, and considered to predict antipsychotic activity (Geyer et al., 2001). Other selective DA D2/5-HT1A compounds such as sarizotan, SSR181507 and SLV313, failed to reverse apomorphine-induced PPI disruption, due to their excessive 5-HT1A agonist actions (Auclair et al., 2006a). Of interest was the finding that sarizotan, SSR181507, bifeprunox, and to a lesser extent SLV313, disrupted PPI when given alone, probably because of this marked 5-HT1A receptor agonist activity (Auclair et al., 2006b). Such a disruption is potentially problematic, considering that schizophrenic patients present diminished basal PPI levels (Braff et al., 1978; for review, Swerdlow et al., 2000). It would thus be desirable for an antipsychotic not to further affect basal PPI. Interestingly though, F15063, over a wide dose-range (0.01–10 mg kg−1 i.p.), did not modify basal PPI level. This is thought to be related to the preferential affinity of F15063 for DA D2 over 5-HT1A receptors, as well as to an antagonistic activity at the former. This emphasizes the importance of the precise balance of DA D2 antagonism versus 5-HT1A agonism for optimal pharmacological activity of new generation antipsychotics targeting both of these receptors (see Newman-Tancredi et al. (2007) and Depoortere et al. (2007) for further discussion).
Conclusions
F15063 was potently active in preclinical models of negative symptoms and memory-cognitive dysfunction of schizophrenia, at dose-ranges overlapping with those efficacious in tests detecting activity against positive symptoms (Depoortere et al., 2007). Its partial agonist activity at DA D4 receptors distinguishes F15063 from most other antipsychotics and was shown to be involved in its activity in the scopolamine-induced deficit of social recognition between an adult and a juvenile rat. Furthermore, F15063 does not produce deficits of basal PPI of the startle reflex, an advantage for a potential antipsychotic. These data also provide compelling evidence that F15063 possesses a profile that distinguishes it from currently commercialized antipsychotic drugs, with a favorable balance of affinity/activity at D2 and 5-HT1A receptors, and an additional partial agonist profile at DA D4 receptors. Taken together, the present data suggest that a pharmacological profile exemplified by that of F15063 should be beneficial in alleviating a wide array of manifestations of schizophrenia, ultimately leading to improvement in patients' outcome and rehabilitation.
Acknowledgments
The authors thank M Aliaga, M Barreto, C Barret-Grevoz, J Besnard, A Galinier, and N Malfetes for their expert technical assistance.
Abbreviations
- ED50
effective dose for 50% response
- EPS
extrapyramidal syndrome
- F15063
N-[(2,2-dimethyl-2,3-dihydro-benzofuran-7-yloxy)ethyl]-3-(cyclopent-1-enyl)-benzylamine mono-tartrate
- LT
learning task
- i.p.
intraperitoneal
- NP
no pulse
- PA
pulse alone
- PCP
phencyclidine
- pp
prepulse
- PPI
prepulse inhibition
- ppP
prepulse-pulse
- p.o.
per os
- s.c.
subcutaneous
Conflict of interest
The present study was funded by Pierre Fabre Médicament. All authors are or were employees of the Centre de Recherche Pierre Fabre at the time when the experiments were conducted.
References
- Abdul-Monim Z, Reynolds GP, Neill JC. The atypical antipsychotic ziprasidone, but not haloperidol, improves phencyclidine-induced cognitive deficits in a reversal learning task in the rat. J Psychopharmacol. 2003;17:57–65. doi: 10.1177/0269881103017001700. [DOI] [PubMed] [Google Scholar]
- Auclair A, Kleven MS, Besnard J, Depoortère R, Newman-Tancredi A. Actions of novel antipsychotic agents on apomorphine-induced PPI disruption: influence of combined serotonin 5-HT1A receptor activation and dopamine D2 receptor blockade. Neuropsychopharmacology. 2006a;31:1900–1909. doi: 10.1038/sj.npp.1301015. [DOI] [PubMed] [Google Scholar]
- Auclair A, Newman-Tancredi A, Depoortère R.Comparative analysis of typical, atypical, and novel antipsychotics with preferential D2/D3 and 5-HT1A affinity in rodent models of cognitive flexibility and sensory gating: (II) The reversal learning task and PPI of the startle reflex Int J Neuropsychopharmacol 2006b9Supp 1P01167 [Google Scholar]
- Bantick RA, Deakin JF, Grasby PM. The 5-HT1A receptor in schizophrenia: a promising target for novel atypical neuroleptics. J Psychopharmacol. 2001;15:37–46. doi: 10.1177/026988110101500108. [DOI] [PubMed] [Google Scholar]
- Bardin L, Kleven MS, Barret-Grevoz C, Depoortère R, Newman-Tancredi A. Antipsychotic-like vs cataleptogenic actions in mice of novel antipsychotics having D2 antagonist and 5-HT1A agonist properties. Neuropsychopharmacology. 2006a;31:1869–1879. doi: 10.1038/sj.npp.1300940. [DOI] [PubMed] [Google Scholar]
- Bardin L, Newman-Tancredi A, Depoortère R.Comparative analysis of typical, atypical, and novel antipsychotics with preferential D2/D3 and 5-HT1A affinity in rodent models of cognition and memory deficits: (I) The hole-board and the social recognition tests Int J Neuropsychopharmacol 2006b9Supp 1P01.166 [Google Scholar]
- Bartoszyk GD, Van Amsterdam C, Greiner HE, Rautenberg W, Russ H, Seyfried CA. Sarizotan, a serotonin 5-HT1A receptor agonist and dopamine receptor ligand. 1. Neurochemical profile. J Neural Transmiss. 2004;111:113–126. doi: 10.1007/s00702-003-0094-7. [DOI] [PubMed] [Google Scholar]
- Bernaerts P, Tirelli E. Facilitatory effect of the dopamine D4 receptor agonist PD168, 077 on memory consolidation of an inhibitory avoidance learned response in C57BL/6J mice. Behav Brain Res. 2003;142:41–52. doi: 10.1016/s0166-4328(02)00371-6. [DOI] [PubMed] [Google Scholar]
- Bibbiani F, Oh JD, Chase TN. Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurology. 2001;57:1829–1834. doi: 10.1212/wnl.57.10.1829. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortère R, Louis C, Perrault G, Griebel G, Soubrié P. SSR181507, a putative atypical antipsychotic with dopamine D2 antagonist and 5-HT1A agonist activities: improvement of social interaction deficits induced by phencyclidine in rats. Neuropharmacology. 2004;46:1121–1129. doi: 10.1016/j.neuropharm.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, et al. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151:20–26. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- Browman KE, Curzon P, Pan JB, Molesky AL, Komater VA, Decker MW, et al. A-412997, a selective dopamine D4 agonist, improves cognitive performance in rats. Pharmacol Biochem Behav. 2005;82:148–155. doi: 10.1016/j.pbb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Bruins Slot LA, De Vries L, Newman-Tancredi A, Cussac D. Differential profile of antipsychotics at serotonin 5-HT1A and dopamine D2S receptors coupled to extracellular signal-regulated kinase. Eur J Pharmacol. 2006;534:63–70. doi: 10.1016/j.ejphar.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Bruins Slot LA, Kleven MS, Newman-Tancredi A. Effects of novel antipsychotics with mixed D(2) antagonist/5-HT(1A) agonist properties on PCP-induced social interaction deficits in the rat. Neuropharmacology. 2005;49:996–1006. doi: 10.1016/j.neuropharm.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT., Jr Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry. 1998;155:751–760. doi: 10.1176/ajp.155.6.751. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Shannon HE, Rasmussen K, DeLapp NW, Ward JS, Calligaro DO, et al. Potential role of muscarinic receptors in schizophrenia. Life Sci. 1999;64:527–534. doi: 10.1016/s0024-3205(98)00597-9. [DOI] [PubMed] [Google Scholar]
- Claustre Y, De Peretti D, Brun P, Gueudet C, Allouard N, Alonso R, et al. SSR181507, a dopamine D2 receptor antagonist and 5-HT1A receptor agonist. I: Neurochemical and electrophysiological profile. Neuropsychopharmacology. 2003;28:2064–2076. doi: 10.1038/sj.npp.1300262. [DOI] [PubMed] [Google Scholar]
- Cosi C, Carilla-Durand E, Assié MB, Ormière AM, Maraval M, Leduc N, et al. Partial agonism of the antipsychotics SSR181507, aripiprazole and bifeprunox at D2 receptors: G-protein activation and prolactin release. Eur J Pharmacol. 2006;535:135–144. doi: 10.1016/j.ejphar.2006.01.051. [DOI] [PubMed] [Google Scholar]
- Crider A. Perseveration in schizophrenia. Schizophr Bull. 1997;23:63–74. doi: 10.1093/schbul/23.1.63. [DOI] [PubMed] [Google Scholar]
- Cussac D, Heusler P, Martel JC, Newman-Tancredi A.The new antipsychotics, bifeprunox, F15063 and SLV313, behave as partial agonists at dopamine D4 receptors: comparison with typical and atypical antipsychotics 2006. 36th Congress of Society for Neuroscience, Atlanta, 14–18 October (332.4/E3)
- Deicken RF, Merrin EL, Floyd TC, Weiner MW. Correlation between left frontal phospholipids and Wisconsin Card Sort Test performance in schizophrenia. Schizophr Res. 1995;14:177–181. doi: 10.1016/0920-9964(94)00036-8. [DOI] [PubMed] [Google Scholar]
- Depoortère R, Bardin L, Auclair AL, Bruins-Slot L, Kleven M, Newman-Tancredi A.F15063, an innovative antipsychotic with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: (II) behavioral profile in models of positive, negative symptoms and cognitive deficits of schizophrenia Int J Neuropsychopharmacology 20069Suppl 1P01165 [Google Scholar]
- Depoortère R, Boulay D, Perrault G, Bergis O, Decobert M, Françon D, et al. SSR181507, a dopamine D2 receptor antagonist and 5-HT1A receptor agonist. II: behavioral profile in tests predictive of antipsychotic, anxiolytic and antidepressant activities. Neuropsychopharmacology. 2003;28:1889–1902. doi: 10.1038/sj.npp.1300261. [DOI] [PubMed] [Google Scholar]
- Depoortère R, Bardin L, Auclair A, Kleven M, Prinssen E, Newman-Tancredi A.F15063, a compound with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: (II) activity in models of positive symptoms of schizophrenia Br J Pharmacol 2007 10.1038/sj.bjp.0707159E-pub ahead of print: 20 March 2007doi [DOI] [PMC free article] [PubMed]
- Dickerson F, Boronow JJ, Ringel N, Parente F. Neurocognitive deficits and social functioning in outpatients with schizophrenia. Schizophr Res. 1996;21:75–83. doi: 10.1016/0920-9964(96)00040-0. [DOI] [PubMed] [Google Scholar]
- Feenstra RW, de Moes J, Hofma JJ, Kling H, Kuipers W, Long SK, et al. New 1-aryl-4-(biarylmethylene)piperazines as potential atypical antipsychotics sharing dopamine D2-receptor and serotonin 5-HT1A-receptor affinities. Bioorg Med Chem Lett. 2001;11:2345–2349. doi: 10.1016/s0960-894x(01)00425-5. [DOI] [PubMed] [Google Scholar]
- Feenstra RW, Long SK, Kuipers W, van der Heyden JA, Tulp MT, Kruse CG. New approaches for psychosis treatment: design, synthesis and SAR of ligands binding to dopamine D2 and serotonin 5-HT1A receptors. Drugs of the future. XVIIth International Symposium on Medicinal Chemistry. 2002;27 Suppl A:237. [Google Scholar]
- Fey ET. The performance of young schizophrenics and young normals on the Wisconsin Card Sorting Test. J Consult Psychol. 1951;15:311–319. doi: 10.1037/h0061659. [DOI] [PubMed] [Google Scholar]
- Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738–1749. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- Gazi L, Schoeffter P, Nunn C, Croskery K, Hoyer D, Feuerbach D. Cloning, expression, functional coupling and pharmacological characterization of the rat dopamine D4 receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:555–564. doi: 10.1007/s002100000236. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Crook JM. Cholinergic systems and schizophrenia: primary pathology or epiphenomena. J Chem Neuroanat. 2001;22:53–63. doi: 10.1016/s0891-0618(01)00101-6. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O'Laughlin IA, Meltzer HY. 5-HT2A and D2 receptor blockade increases cortical DA release via 5-HT1A receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Idris NF, Repeto P, Neill JC, Large CH. Investigation of the effects of lamotrigine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and D-amphetamine in the rat. Psychopharmacology. 2005;179:336–348. doi: 10.1007/s00213-004-2058-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Redmond DE, Jr, Elsworth JD, Youngren KD, Roth RH. Dopamine D4 receptor antagonist reversal of subchronic phencyclidine-induced object retrieval/detour deficits in monkeys. Psychopharmacology. 1999;142:78–84. doi: 10.1007/s002130050865. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry. 1996;153:466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Dopamine D2 receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry. 2001;50:873–883. doi: 10.1016/s0006-3223(01)01251-3. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Glennon J, Tuinstra T, Herremans AHJ, Van der Heyden JAM, Feenstra R, et al. SLV313: A novel antipsychotic with additional antidepressant and anxiolytic-like actions. Eur Neuropsychopharmacol. 2002;12:S274. [Google Scholar]
- Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)1A receptors. J Pharmacol Exp Ther. 2000;295:853–861. [PubMed] [Google Scholar]
- Nanry KP, Tilson HA. The role of 5HT1A receptors in the modulation of the acoustic startle reflex in rats. Psychopharmacology. 1989;97:507–513. doi: 10.1007/BF00439556. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Assié M-B, Martel J-C, Cosi C, Bruins Slot L, Palmier C, et al. F15063, a potential antipsychotic with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: (I) in vitro receptor affinity and efficacy profile Br J Pharmacol 2007. in press, as a companion paper [DOI] [PMC free article] [PubMed]
- Newman-Tancredi A, Assié M-B, Martel J-C, Cosi C, Heusler P, Bruins Slot L, et al. F15063, an innovative antipsychotic with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: (I) in vitro, neurochemical and neuroendocrine profiles Int J Neuropsychopharmacol 20069Suppl 1P01.164 [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Pério A, Terranova JP, Worms P, Bluthe RM, Dantzer R, Biziere K. Specific modulation of social memory in rats by cholinomimetic and nootropic drugs, by benzodiazepine inverse agonists, but not by psychostimulants. Psychopharmacology. 1989;97:262–268. doi: 10.1007/BF00442261. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH. Acetylcholine and hallucinations: disease-related compared to drug-induced alterations in human consciousness. Brain Cogn. 1995;28:240–258. doi: 10.1006/brcg.1995.1255. [DOI] [PubMed] [Google Scholar]
- Powell SB, Paulus MP, Hartman DS, Godel T, Geyer MA. RO-10–5824 is a selective dopamine D4 receptor agonist that increases novel object exploration in C57 mice. Neuropharmacology. 2003;44:473–481. doi: 10.1016/s0028-3908(02)00412-4. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Gunn RN, Wilkins MR, Sedman E, Grasby PM. Evaluation of EMD 128 130 occupancy of the 5-HT1A and the D2 receptor: a human PET study with [11C]WAY-100635 and [11C]raclopride. J Psychopharmacol. 2002;16:195–199. doi: 10.1177/026988110201600301. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Glahn DC, Censits DM, Smith RJ, Lazarev MG, et al. Frontotemporal cerebral blood flow change during executive and declarative memory tasks in schizophrenia: a positron emission tomography study. Neuropsychology. 1998;12:399–413. doi: 10.1037//0894-4105.12.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigdon GC, Weatherspoon JK. 5-Hydroxytryptamine 1a receptor agonists block prepulse inhibition of acoustic startle reflex. J Pharmacol Exp Ther. 1992;263:486–493. [PubMed] [Google Scholar]
- Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer's disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005;162:2022–2030. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Dunn L, Peszke M, Cramer J, Xu W, Thomas J, et al. Impact of clozapine on negative symptoms and on the deficit syndrome in refractory schizophrenia. Department of Veterans Affairs Cooperative Study Group on Clozapine in Refractory Schizophrenia. Am J Psychiatry. 1999;156:88–93. doi: 10.1176/ajp.156.1.88. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Distinct effects of D-amphetamine and phencyclidine on the social behaviour of rats. Behav Pharmacol. 1995;6:55–65. [PubMed] [Google Scholar]
- Sanger DJ. The search for novel antipsychotics: pharmacological and molecular targets. Expert Opin Ther Targets. 2004;8:631–641. doi: 10.1517/14728222.8.6.631. [DOI] [PubMed] [Google Scholar]
- Sultzer DL. Psychosis and antipsychotic medications in Alzheimer's disease: clinical management and research perspectives. Dement Geriatr Cogn Disord. 2004;17:78–90. doi: 10.1159/000074279. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Tandon R, Ribeiro SC, DeQuardo JR, Goldman RS, Goodson J, Greden JF. Covariance of positive and negative symptoms during neuroleptic treatment in schizophrenia: a replication. Biol Psychiatry. 1993;34:495–497. doi: 10.1016/0006-3223(93)90242-6. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Zhang K, Baldessarini RJ. Dopamine D4 receptors: beyond schizophrenia. J Recept Signal Transduct Res. 2004;24:131–147. doi: 10.1081/rrs-200032076. [DOI] [PubMed] [Google Scholar]
- Terranova J-P, Chabot C, Barnouin M-C, Perrault G, Depoortère R, Griebel G, et al. SSR181507, a dopamine D2 receptor antagonist and 5-HT1A receptor agonist, alleviates disturbances of novelty discrimination in rats, a model of selective attention deficit. Psychopharmacology. 2005;181:134–144. doi: 10.1007/s00213-005-2268-5. [DOI] [PubMed] [Google Scholar]
- Vacher B, Cuisiat S, Koek W, Colpaert F.3-(Cyclopenten-1-yl)-benzyl-or 3-(cyclopenten-1-yl)-heteroarylmethyl-amine derivatives and use thereof as medicines for treating schizophrenia. Patent # WO 2004/035561 A1 2002. Priority 16 October 2002
- Wolf W. DU-127090 Solvay/H Lundbeck. Curr Opin Investig Drugs. 2003;4:72–76. [PubMed] [Google Scholar]
- Zawilska JB, Rosiak J, Berezinska M, Nowak JZ. L745, 870 suppresses the nighttime serotonin N-acetyltransferase activity in chick retina: in vivo evidence for agonist activity at D4-dopamine receptors. J Neural Transm. 2003;110:219–227. doi: 10.1007/s00702-002-0787-3. [DOI] [PubMed] [Google Scholar]
- Zhang K, Grady CJ, Tsapakis EM, Andersen SL, Tarazi FI, Baldessarini RJ. Regulation of working memory by dopamine D4 receptor in rats. Neuropsychopharmacology. 2004;29:1648–1655. doi: 10.1038/sj.npp.1300491. [DOI] [PubMed] [Google Scholar]