Abstract
Background and purpose:
Cannabinoids have analgesic and anti-inflammatory properties but their use is limited by psychotropic activity at CNS receptors. Restricting cannabinoid delivery to peripheral tissues at systemically inactive doses offers a potential solution to this problem.
Experimental approach:
WIN 55,212-2 was continuously delivered to the site of a partial ligation injury to the sciatic nerve via a perineural catheter connected to a mini-osmotic pump implanted at the time of injury. Bilateral reflex limb withdrawal behaviour was measured in adult male Wistar rats in response to mechanical and cooling stimulation of the hind paw.
Key results:
Compared with vehicle treatment, WIN 55,212-2 (1.4μg μl−1 hr−1) reduced hypersensitivity to stimuli applied to the injured limb at 2, 4 and 6 days after injury. The effects of WIN 55,212-2 (0.6-2.8μg μl−1 hr−1) were dose-dependent. Estimated EC50 values for reduction in mean responses to mechanical and cooling stimulation (day 4 post-surgery) were 1.55 (95% C.I, [1.11-2.16]) μg μl−1 hr−1 and 1.52 (95% C.I, [1.07-2.18]) μg μl−1 hr−1, respectively. When delivered to the contralateral side to injury, WIN 55,212-2 (1.4 or 2.8μg μl−1 hr−1) did not significantly affect nerve injury-associated hypersensitivity. Co-perineural application of a CB1 receptor antagonist SR141716a and WIN 55,212-2 prevented the effects of WIN 55,212-2 on hypersensitivity. Co-application of CB2 receptor antagonist SR144528 reversed WIN 55,212-2's effect on mechanical hypersensitivity on day 2 only.
Conclusions and implications:
These data support a peripheral antihyperalgesic effect of WIN 55,212-2 when delivered directly to the site of a nerve injury at systemically inactive doses.
Keywords: Cannabinoid receptor; nerve injury; neuropathic pain; WIN 55,212–2
Introduction
Peripheral neuropathic pain may be defined as pain initiated or caused by a primary lesion in the peripheral nervous system, and is, for example, a feature of some peripheral neuropathies. Some of the sensory abnormalities sometimes associated with neuropathic pain have been reproduced in rodent models by producing a unilateral injury to the sciatic nerve innervating the hind limb (Seltzer et al., 1990; Kim and Chung, 1992). The behavioural hypersensitivity to sensory stimuli that develops following the injury can be measured in rodents as a reduction in the threshold of sensory stimulation required for reflex limb withdrawal behaviour.
A substantial body of literature supports the analgesic properties of cannabinoids in rodent pain models, effects that are mediated by signalling at both cannabinoid receptor type 1 (CB1) and type 2 (CB2) (Pertwee, 2001; Ibrahim et al., 2003; Scott et al., 2004; Rice, 2005; Lever and Rice, 2006). Cannabinoids are also effective in reducing pain-related behaviours when tested in rodent models of neuropathic pain (Herzberg et al., 1997; Mao et al., 2000; Ibrahim et al., 2003; Elmes et al., 2004; Scott et al., 2004; Guindon and Beaulieu, 2006). Synthetic cannabinoids, such as the aminoalkylindole compound WIN 55,212–2 (Bell et al., 1991), are potent activators of cannabinoid receptors and have demonstrable efficacy at reducing hypersensitivity developing after nerve injury in rodents (Herzberg et al., 1997; Bridges et al., 2001; Fox et al., 2001; Lim et al., 2003; Costa et al., 2004). Analgesic effects are similarly reported in models of diabetic and chemotherapy-induced peripheral nerve injury (Ulugol et al., 2004; Pascual et al., 2005) as well as in models of nerve injury induced by viral or chemical agents (Wallace et al., 2005; Hasnie et al., 2006).
In spite of evidence for the analgesic properties of cannabinoids, their development into useful analgesic drugs has been hampered by their narrow therapeutic index and an association of cannabis use with psychosis risk (Henquet et al., 2005). Because the majority of cannabinoid-induced adverse effects are attributable to central nervous system (CNS)-mediated actions, targeting their peripheral action is a strategy that may enhance the therapeutic index. Delivery of systemically ineffective doses of cannabinoids directly to the site of peripheral nerve injury is one strategy for achieving this goal. Local injections of cannabinoids into rat skin tissue can reduce the injury-associated behavioural hypersensitivity occurring after an inflammatory injury and can also produce direct anti-inflammatory effects, such as a reduction in tissue oedema (Calignano et al., 1998; Jaggar et al., 1998; Richardson et al., 1998b; Sokal et al., 2003). Peripheral administration of cannabinoids to hind paw skin can also reduce behavioural hypersensitivity in nerve injury models (Fox et al., 2001; Elmes et al., 2004; Ulugol et al., 2004; Guindon and Beaulieu, 2006). These analgesic effects can then be reversed by local application of cannabinoid receptor antagonists, implying that the analgesic mechanisms involve peripheral CB receptors activated independently from receptors in the CNS.
Anatomical and functional studies provide evidence for both CB1 and CB2 receptors in peripheral tissues. In skin, both receptors have been localized to nerve fibres and keratinocytes (Ibrahim et al., 2005; Ständer et al., 2005). CB1 mRNA is found in sensory neurones from lumbar 4 and 5 (L4/5) dorsal root ganglia innervating the hind limb, where it is predominantly located on cell populations subtending large-myelinated afferent fibres (Bridges et al., 2003). Functional CB1 receptors have also been demonstrated on peripheral sensory neurones in culture (Ross et al., 2001; Khasabova et al., 2002). The activation of these receptors inhibits excitatory calcium responses and reduces exocytosis of neuropeptides from both the cell bodies of these neurones (Tognetto et al., 2001; Ahluwalia et al., 2003) as well as their peripheral terminals stimulated in skin (Richardson et al., 1998b; Ellington et al., 2002). Similarly, topical application of cannabinoids to skin has a suppressive effect on the pro-inflammatory and pro-nociceptive efferent functions of C-fibre sensory neurones (Dovrak et al., 2003; Rukwied et al., 2003). CB2 mRNA has also been detected in L4/5 DRG tissue (Beltramo et al., 2006) and CB2 protein was immunolocalized to fibres in injured sciatic nerve tissue (Wotherspoon et al., 2005) and in cultured neonatal rat DRG cells analysed by flow cytometry (Ross et al., 2001). Activation of CB1 and CB2 receptors inhibited the calcium responses of cultured DRG neurones that were derived from both sham-operated and nerve-injured rats (Sagar et al., 2005). An antinociceptive action for cannabinoids injected directly into the hind paw skin of uninjured rats has also been demonstrated (Elmes et al., 2004; Nackley et al., 2004).
In studies reporting the peripheral antihypersensitivity effects of WIN 55,212–2 injected into the paw after nerve injury, the hind paw site was also used to deliver sensory stimuli for testing reflex behaviours (Fox et al., 2001; Ulugol et al., 2004). This introduces difficulties in distinguishing between a possible antinocieptive action of cannabinoids on peripheral nerve endings receiving the test stimuli in hind paw skin (inhibiting excitatory responses in nociceptor terminals) and effects on behavioural hypersensitivity. Although some of the peripheral analgesic effects are likely to be mediated at a cutaneous level (by inhibiting nociceptor firing), there are also inflammatory and neuronal responses operating at the nerve injury site that contribute to the sensitization of nociceptor responses (Lindenlaub and Sommer, 2000). The extent to which peripheral cannabinoids modulate these processes is currently unknown. Cannabinoid receptors are present on cells that are functionally involved in the peripheral immune responses to tissue injury (Walter and Stella, 2004) and the local application of WIN 55,212–2 has been associated with anti-inflammatory effects (Pozzi et al., 2003; Oka et al., 2006). Similarly, neurodegenerative processes that occur as a consequence of glutamate and calcium-mediated excitotoxicity, are also reduced by exogenous cannabinoids to produce the reported neuroprotective effects of cannabinoid receptor activation (Baker et al., 2003; Molina-Holgado et al., 2003; Pryce et al., 2003).
The aim of this study was to deliver cannabinoids directly to the site of a sciatic nerve injury, away from the sensory testing site in the hind paw at systemically inactive doses and to examine effects on hypersensitivity by measuring hind limb reflex withdrawal behaviour. Continuous solution delivery to the sciatic nerve was previously achieved using mini-osmotic pumps connected to a supply catheter (Boyd and Gordon, 2002). An adaptation of this system was used to deliver WIN 55,212–2 perineurally to the site of a partial ligation injury to the sciatic nerve. CB receptor antagonists were also co-applied using this system, to determine the involvement of CB1 or CB2 receptor signalling systems.
Materials and methods
All experiments were conducted according to UK Home Office regulations. Adult male Wistar rats 250–300 g (n=124) were housed three per cage at constant temperature under a 14:10 h light–dark cycle, with free and continuous access to food and water.
Sciatic nerve surgery and perineural catheter implantation
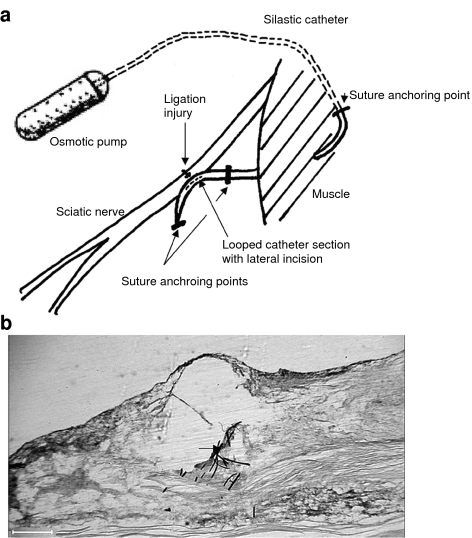
Rats were anaesthetized (3% isoflurane) and surgery was performed using standard aseptic procedures. Mini-osmotic pumps (Model 2001 Alzet, Cupertino, CA, USA) were attached to silastic catheter tubing (0.012 ID × 0.07 wall: SF medical) and pre-filled with drug or vehicle solutions in sterile conditions, followed by a 12-h pre-implantation incubation at 37°C. The pumps were implanted subcutaneously in the thoraco-lumbar region and secured with 4.0 sutures to the underlying muscle layer. The catheter was then tunnelled transmuscularly to the area overlying the sciatic nerve, externalized, and anchored with a suture. The sciatic nerve was exposed in the mid-thigh region and the catheter was internalized back through the muscle towards the exposed nerve site. The catheter was then looped over the nerve and secured at both ends of the loop by sutures into the underlying muscle layer (see Figure 1a). A section of nerve, approximately 1 cm proximal to the bifurcation point, was tightly ligated across half of the nerve trunk with a 7.0 suture (Seltzer et al., 1990). A small lateral incision across the overlying perineurium membrane was incorporated at the ligation site. The loop of the catheter was positioned directly over the injury site and secured by tightening the distal suture anchoring it. This occluded the catheter lumen. A longitudinal incision (∼5 mm) was then made in the loop of the catheter overlying the nerve injury site before the wound was sutured. This experimental model is referred to as partial nerve ligation (PNL).
Figure 1.
Schematic of the system for continuous delivery to the site of a partial nerve ligation (PNL) injury to the sciatic nerve. (a) Diagram representing the positions of the implanted mini-osmotic pump, the perineural delivery catheter and the site of partial ligation injury to the rat sciatic nerve. (b) Micrograph image of a longitudinal, paraformaldehyde-fixed section from a rat sciatic nerve, removed 7 days after a partial ligation injury. Black arrow indicates the position of the suture material from the ligature across the sciatic nerve trunk – forming the partial ligation injury; red stain, eosin counterstain; blue tissue stain, thionin dye applied via the implanted perineural catheter. Scale bar, 400 μm.
Controls
Sham surgery was performed by exposure of the sciatic nerve (and in some cases implanting the catheter) in the absence of a ligation injury. To control for a possible systemic analgesic action of WIN 55,212–2 , both the efficacious doses of WIN 55,212–2 (1.4 and 2.8 μg μl−1) were loaded into mini-osmotic pumps and delivered by a perineural catheter to the opposite, contralateral side to nerve injury. For all experiments, the correct positioning of the catheter at the injury site was verified at the end of the testing period by post-mortem examination. To verify correct solution delivery, the mini-osmotic pumps were removed and checked along with the pump connection and patency of the delivery catheter.
Sensory testing
Sensory testing was conducted on PNL-injured animals as described previously (Bridges et al., 2001). Behavioural tests were employed to measure behavioural hypersensitivity to sensory stimuli developing after a PNL injury in rats (Seltzer et al., 1990). This involved timing limb withdrawal responses to a punctate mechanical stimulus and a cooling stimulus applied to the plantar surface of the hind paw, of both the injured and the uninjured limb, by an investigator who was unaware of the treatments. At the beginning of the experiment, rats were randomly assigned to treatment groups then habituated to the behavioural testing environment (a Plexiglass box (23 × 18 × 14 cm) with 0.8 cm diameter mesh flooring) for 1 h on two separate days before baseline testing. Baseline measurements of hind limb withdrawal thresholds to both stimuli were collected on two separate days. A box-acclimatization period of 15–30 min was allowed at the start of each testing session. For the measurement of cold hypersensitivity, an acetone drop was applied to the hind paw from the tip of a 1 ml syringe, with a paw withdrawal scored as a positive response (technique modified from Carlton et al., 1994). The stimulus was applied five times at intervals of >3 min and recorded as a percentage response. For the measurement of mechanical hypersensitivity, mechanical force was applied to the hind paw using an electronic Von Frey device (probe tip diameter: 0.5 mm2, type 735, Somedic, Sweden). The probe was manually applied to the hind paw surface at a rate of 8–15 g s−1 and the mean force eliciting a withdrawal response was calculated from five separate tests, each >3 min apart.
Immunohistochemistry
Rats were anaesthetized with sodium pentobarbital (60 mg kg−1) and perfused through the ascending aorta with 100 ml 0.9% saline, then 300 ml 4% paraformaldehyde in 0.1 M phosphate buffer (PB). To verify delivery to the sciatic nerve injury site via an implanted perineural catheter, the pump was removed and dye (1.3% thionin) was injected down the catheter until it reached the nerve site. The sciatic nerve was then removed and post-fixed for 1–2 h at 4°C, cryoprotected in 15%, then 30% sucrose in 0.1 M PB for 12 h at 4°C and embedded in mounting medium. Longitudinal cryosections proximal and distal to the injury site were cut and thaw-mounted onto Superfrost slides at 15 μm thickness. These were counterstained with eosin, then dehydrated through ascending alcohol solutions, cleared in xylene and mounted using dipex mounting medium. Micrograph images were viewed under a Leica DMR microscope and captured on a Hamamatsu camera using QWIN V3 image processing software (Leica, Milton Keynes, UK).
Data analysis
Mean±(s.e.m) paw withdrawal responses for mechanical and cooling stimuli, measured ipsi- and contralateral to PNL injury, were calculated for animals in each treatment group on each testing day before and after surgery. Statistical comparisons were made between withdrawal responses – on different testing days or between different treatment groups on the same testing day – using a one-way analysis of variance (ANOVA), followed by the appropriate post hoc multiple comparison procedure. This was an ANOVA, then Tukey or Dunn's test or Kruskal–Wallis one-way ANOVA on ranks using the Student–Newman–Keuls method. For mechanical dose–response data, the mean paw withdrawal response values for each testing time point were normalized to the pre-surgery baseline of each animal. Estimated EC50 values were calculated using Graph Pad Prism software (San Diego, CA, USA).
Compounds
The aminoalkylindole cannabinoid compound: R-(+)-WIN 55,212–2 mesylate salt (WIN 55,212–2) was obtained from Sigma-RBI (UK) and dissolved in dimethylsulphoxide (DMSO, Sigma-RBI, Dorset, UK). For pump delivery, further dilutions were made in a vehicle solution containing Tween 80 and a 2 mg ml−1 solution of rat serum albumin (RSA); (Sigma-RBI) in saline. Solutions of WIN 55,212–2 at 2.8 μg μl−1 contained 4% DMSO, 4% Tween 80 and 92% saline RSA. Vehicle solutions were used as the controls for treatment with WIN 55,212–2 instead of its stereoisomer WIN55,212–3. This is on account of the recently reported activity of the WIN 55,212–3 compound at CB2 receptors (Savinaninen et al., 2005). SR141716a (N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) and SR144528 (N-[(1S)-endo-1,3,3-tri-methyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methyl-benzyl)-pyrazole-3-carboxamide) were kindly supplied by the NIMH Chemical Synthesis and Drug Supply Programme. Solutions of SR141716a and SR144528 in DMSO were diluted to a delivery concentration of 6.25 μg μl−1 in a vehicle solution containing 14.5% DMSO, 14.5% Tween 80 and 71% saline RSA.
Results
PNL injury-related behavioural hypersensitivity to mechanical and cooling stimulation
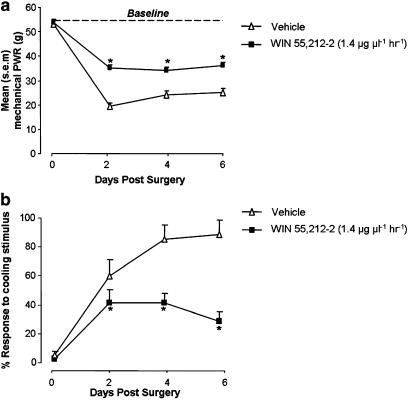
The establishment of a partial ligation injury to the rat sciatic nerve produced an increase in reflex sensitivity to sensory stimulation applied to the hind paw of the injured limb. This was measured as a reduction in the force of punctate mechanical stimulation required to produce a paw withdrawal response, when compared with pre-surgical baseline responses or those measured from the uninjured paw. For animals receiving a PNL injury (from both WIN 55,212–2 and vehicle treatment groups) (Figure 2a), there was a significant reduction in the mean force required to evoke a reflex paw withdrawal response, when compared with the pre-injury baseline responses (P<0.05 ANOVA, post hoc Tukey test). This difference was measurable at 2, 4 and 6 days after injury and was sustained at day 14 (Figure 7a). Similarly, these animals also developed a behavioural sensitivity to cooling stimulation (Figures 2b and 7b); the mean percentage response rate to acetone drop stimulation was significantly increased at days 2, 4 and 6 after PNL injury, compared with pre-injury levels (P<0.05 Kruskal–Wallis ANOVA, Student–Newman–Keuls). These injury-related behavioural changes are representative of mechanical and cold hypersensitivity.
Figure 2.
Mean paw withdrawal responses (PWR) to (a) punctate mechanical stimulation and (b) cooling stimulation; measured 2, 4 and 6 days after PNL injury on the ipsilateral side to injury. Mean baseline ipsilateral paw withdrawal responses are plotted at day 0. (a) Continuous perineural delivery of WIN 55,212–2 (1.4 μg μl−1) solution at a rate of 1 μl h−1 to the nerve injury site (n=13) significantly increased the mean paw withdrawal response force to punctate mechanical stimulation on days 2, 4 and 6 post-injury, compared with animals receiving corresponding perineural treatment with vehicle solution (n=13). *P<0.05 paw withdrawal response force for vehicle group on a post-injury day versus WIN 55,212–2 group: ANOVA, Tukey test. (b) Continuous perineural delivery of WIN 55,212–2 (1.4 μg μl−1 h−1) reduces the mean percentage response to cooling stimulation compared with vehicle treatment. *P<0.05 paw withdrawal responses for vehicle group on a post-injury day versus WIN 55,212–2 group; Kruskal–Wallis ANOVA, Student–Newman–Keuls.
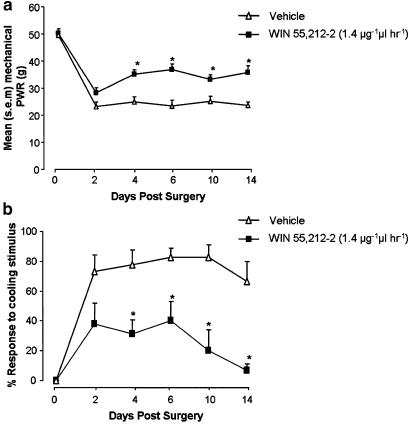
Figure 7.
Extended behavioural testing of PNL-injured animals receiving perineural WIN 55,212–2 or vehicle treatments: Mean paw withdrawal responses (PWR) after mechanical and cooling stimulation applied ipsilateral to injury at days 2, 4, 6, 10 and 14 after PNL, in rats receiving perineural WIN 55,212–2 treatment (1.4 μg μl−1 h−1) or vehicle solution by means of a mini-osmotic pump designed for 7-day delivery. (a) Mean paw withdrawal response force after punctate mechanical stimulation in vehicle and WIN 55,212–2-treated rats. *P<0.05 mean paw withdrawal response force in WIN 55,212–2-treated rats (n=6) versus vehicle-treated rats (n=6); ANOVA, Tukey test. (b) Mean percentage paw withdrawal responses after cooling stimulation in vehicle and WIN 55,212–2-treated rats. *P<0.05 mean percentage paw withdrawal response in WIN 55,212–2-treated rats versus vehicle-treated rats; ANOVA, Tukey test.
The histological section in Figure 1b shows the site of a PNL injury to rat sciatic nerve tissue. Injection of thionin dye into an implanted perineural catheter was used to indicate the likely delivery site of solutions pumped via a perineural catheter. Thionin staining was observed in nerve tissue surrounding the injury site.
Perineural WIN 55,212–2 delivery
Continuous perineural delivery of WIN 55,212–2 (1.4 μg μl−1) to the site of a PNL injury reduced hypersensitivity to mechanical and cooling stimuli that develops after nerve injury. The mean mechanical force required for a reflex paw withdrawal response of the injured hind limb was increased in animals receiving WIN 55,212–2 treatment, compared with control animals receiving vehicle solution (Figure 2a). This difference was significant on each testing day: 2, 4 and 6 days after the establishment of the nerve injury, and represents a reduction of PNL-induced mechanical hypersensitivity. WIN 55,212–2 treatment had the same effect on PNL-induced behavioural hypersensitivity measured in response to cooling stimulation applied to the injured hind paw: the mean post-injury response to cooling stimulation was reduced when compared with vehicle treatment (Figure 2b). WIN 55,212–2 applied to the contralateral side to injury did not alter paw withdrawal thresholds to either stimulus modality, compared with vehicle treatment (P>0.05 ANOVA).
To control for the possible effects of implanting a perineural catheter on hind paw sensory thresholds, both the catheter and a mini-osmotic pump were implanted into rats without an accompanying ligation injury to the sciatic nerve. In these sham-operated animals, there were no differences between paw withdrawal responses ipsilateral to the perineural catheter, as compared with those on the contralateral side (for either mechanical or cooling types of stimulation). The mean paw withdrawal responses (±s.e.m.) to mechanical stimulation ipsilateral to the perineural catheter – measured at baseline and on days 2, 4 and 6 post-surgery – ranged between 58.8±2.6 and 44.6±2.7 g. These values did not vary significantly from those recorded on the contralateral side (53.7±2.6–46.3±4.8 g; P>0.05 ANOVA). This result was also observed for responses to cooling stimulation as the mean paw withdrawal response rates were <20% for either hind paw. There were no significant differences between responses measured ipsilateral to the perineural catheter and those measured on the contralateral side (P=0.958 ANOVA).
Dose-related effects of perineural WIN 55,212–2
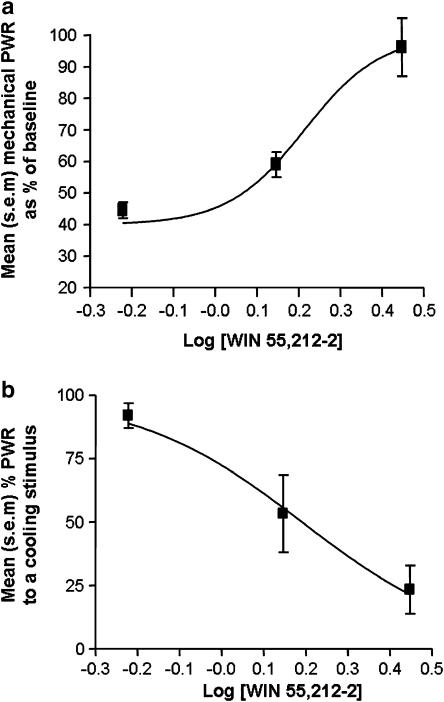
WIN 55,212–2 was continuously delivered to the site of a PNL injury at three separate concentrations: 0.6, 1.4 and 2.8 (μg μl−1). At the lowest delivery rate of 0.6 μg μl−1 h−1, WIN 55,212–2 treatment failed to have a significant effect on reflex hypersensitivity, when compared with animals receiving parallel treatment with the vehicle solution (P>0.05 mean paw withdrawal responses to mechanical or cooling stimulation in WIN 55,212–2-treated rats versus vehicle-treated rats at each time point, ANOVA, Tukey or Student–Newman–Keuls tests, n=5). WIN 55,212–2 delivery at rates of 1.4 and 2.8 μg μl−1 h−1 were both effective at reducing reflex hypersensitivity to mechanical and cooling stimulation at 2, 4 and 6 days after PNL injury (P<0.05 mean paw withdrawal response for WIN 55,212–2 treated versus vehicle treated at each time point, ANOVA, n=6). The effect of perineural WIN 55,212–2 treatment on mechanical paw withdrawal responses at these concentrations was dose related with an estimated EC50 value of 1.55 μg μl−1 h−1 (95% CI, [1.11–2.16 μg μl−1 h−1]) (Figure 3a). The WIN 55,212–2-mediated reduction in response to cooling stimulation after injury was also dose related, producing a comparable estimated EC50 value of 1.52 μg μl−1 h−1 (95% CI[1.07–2.18 μg μl−1 h−1]) (Figure 3b).
Figure 3.
Log dose–response curves for mean paw withdrawal responses (PWR) on the ipsilateral side to injury in animals receiving continuous perineural delivery of WIN 55,212–2 at a delivery rate of 0.6–2.8 μg μl−1 h−1 at day 4 after PNL injury (n=5–6 per dose group). (a) Log dose–response curve plotting mean paw withdrawal responses to punctate mechanical stimulation (expressed as a percentage of baseline responses). (b) Log dose–response curve plotting mean percentage paw withdrawal responses to cooling stimulation.
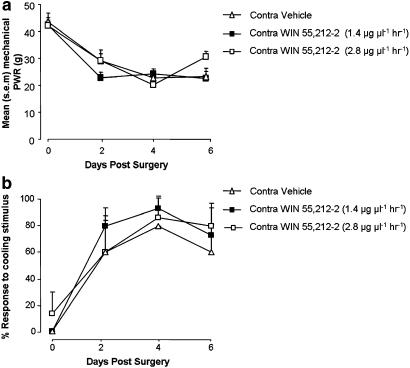
Contralateral delivery of WIN 55,212–2 at effective ipsilateral perineural doses
The perineural catheter delivery system was designed to achieve localized peripheral delivery of WIN 55,212–2 to the site of a nerve injury at doses that are reported in the literature as being ineffective at reducing neuropathic pain behaviour when delivered by a systemic route (Bridges et al., 2001; Fox et al., 2001). To control for the possible systemic activity of WIN 55,212–2 delivered perineurally via mini-osmotic pumps, the same two peripherally effective doses of WIN 55,212–2 (1.4 and 2.8 μg μl−1 h−1) were delivered to the contralateral side to nerve injury. Sensory testing demonstrated that there were no significant increases in the mean mechanical force of stimulation required to elicit a withdrawal response from the injured hind paw of animals treated contralaterally with either dose of WIN 55,212–2, when compared with the vehicle-treated control group (Figure 4a). Similarly, there were also no significant reductions in responses to cooling stimulation in WIN 55,212–2-treated rats, versus those treated contralaterally with the vehicle solution (Figure 4b). Withdrawal responses of the contralateral ‘treated' paws in these animals were also measured. Statistical comparisons between contralateral responses to WIN 55,212–2- and vehicle-treated groups reported no significant changes in behavioural sensitivity to either cooling or mechanical stimulation (P>0.05 Kruskal–Wallis ANOVA, P>0.05 ANOVA Tukey test, respectively).
Figure 4.
Contralateral delivery of WIN 55,212–2. Paw withdrawal responses (PWR) to mechanical and cooling stimuli at 2, 4 and 6 days after PNL injury measured from the injured hind limb during perineural mini-osmotic pump delivery of WIN 55,212–2 (1.4 and 2.8 μg μl−1 h−1) or vehicle solution to the uninjured side (n=3 per group). (a) Mean paw withdrawal response force for mechanical stimulation in each treatment group. P>0.05 contralateral WIN 55,212–2 (1.4 μg μl−1h−1)-treated rats versus contralateral vehicle-treated rats; Kruskal-Wallis ANOVA, Dunn's test. Contralateral WIN 55,212–2 (2.8 μg μl−1 h−1)-treated rats versus contralateral vehicle-treated rats, ANOVA, Tukey test. (b) Mean percentage paw withdrawal response to cooling stimulation in each treatment group. P>0.05 contralateral WIN 55,212–2 (1.4 μg μl−1 h−1)-treated rats versus contralateral vehicle-treated rats; ANOVA, Student–Newman–Keuls. P>0.05 contralateral WIN 55,212–2 (2.8 μg μl−1 h−1)-treated rats versus contralateral vehicle-treated rats; ANOVA, Student–Newman–Keuls.
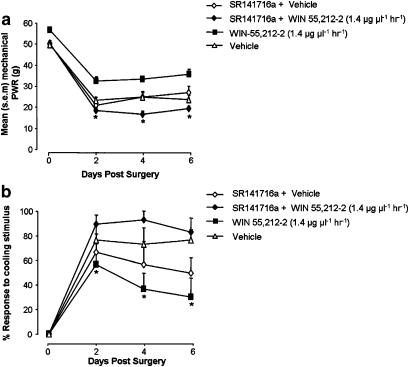
Co-delivery of WIN 55,212–2 and the CB1 antagonist SR141716a
A separate set of experiments were carried out to investigate whether the effect of WIN 55,212–2 delivery to the site of PNL injury involved signalling at CB1 receptors. Owing to animal licence restrictions (which prohibited the use of an additional systemic drug delivery route in animals with perineural catheters), WIN 55,212–2 and the receptor antagonist SR141716a were loaded into the same pump and co-delivered perineurally. Parallel groups of animals received either WIN 55,212–2+SR141716a, WIN 55,212–2 alone, vehicle, or SR141716a+vehicle solution. A 6.25 μg μl−1 h−1 dose of SR141716a was chosen as this was 4.5 × higher than the standard effective dose of WIN 55,212–2 1.4 μg μl−1 h−1 and equated to a dose of ∼1 mg kg−1 – reported previously as an effective systemic dose of SR141716a in behavioural experiments (Bridges et al., 2001; Pascual et al., 2005). When co-applied to the site of the PNL injury with WIN 55,212–2, the CB1 antagonist attenuated the reduction in hypersensitivity produced by WIN 55,212–2 (1.4 μg μl−1 h−1) alone. The mean withdrawal response threshold to mechanical stimulation was significantly reduced in SR141716a+WIN 55,212–2-treated rats, compared with those treated with WIN 55,212–2 only (Figure 5a). The co-application of SR141716a with WIN 55,212–2 also significantly increased the response rate to cooling stimulation, compared with WIN 55,212–2 treatment alone (Figure 5b).
Figure 5.
Co-application of CB1 receptor antagonist SR141716a and WIN 55,212–2 to the nerve injury site. Ipsilateral mean paw withdrawal responses (PWR) to mechanical (a) and cooling stimulation (b) in three groups of PNL-injured animals receiving either perineural WIN 55,212–2 (1.4 μg μl−1 h−1), WIN 55,212–2 (1.4 μg μl−1 h−1) and SR141716a (6.25 μg μl−1 h−1) (SR141716a+WIN 55,212–2), or SR141716a (6.25 μg μl−1 h−1) and vehicle solution (SR141716a+vehicle) (n=6 per group). (a) Mean paw withdrawal response force to mechanical stimulation at days 2, 4 and 6 after PNL injury. *P<0.05 WIN 55,212–2-treated rats versus SR141716a+WIN 55,212–2-treated rats: Kruskals–Wallis ANOVA, Dunn's test. (b) Mean percentage response to cooling stimulation at days 2, 4 and 6 after PNL injury. *P<0.05 WIN 55,212–2-treated rats versus SR141716a+WIN 55,212–2-treated rats; Kruskals–Wallis ANOVA, Student–Newman–Keuls.
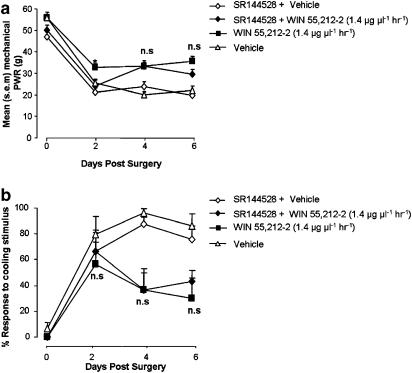
Co-delivery of WIN 55,212–2 and the CB2 antagonist SR144528
WIN 55,212–2 (1.4 μg μl−1 h−1) was co-delivered perineurally with the CB2 receptor antagonist SR144528 (6.25 μg μl−1 h−1) in animals receiving a PNL injury. Parallel groups received WIN 55,212–2 treatment alone or SR144528+vehicle treatment. Co-application of SR144528 did not alter the effect of WIN 55,212–2 treatment on paw withdrawal responses to cooling stimuli. Responses to cooling stimulation in SR144528+WIN 55,212–2-treated rats were not significantly different from rats receiving WIN 55,212–2 treatment alone (Figure 6b). This was also the case for mechanical responses, except at the earliest testing time point, which was day 2 after PNL injury (Figure 6a). On this testing day, the mean mechanical withdrawal threshold for the SR144528+WIN 55,212–2 treatment group was significantly lower than for the WIN 55,212–2 group, but not the SR144528+vehicle treatment group (P<0.05 ANOVA, Tukey test).
Figure 6.
Co-application of CB2 receptor antagonist SR144528 and WIN 55,212–2 to the nerve injury site. Ipsilateral mean paw withdrawal responses (PWR) to mechanical (a) and cooling stimulation (b) in three groups of PNL-injured animals receiving either perineural WIN 55,212–2 (1.4 μg μl−1 h−1), WIN 55,212–2(1.4 μg μl−1 h−1) and SR144528 (6.25 μg μl−1 h−1) (SR144528+WIN 55,212–2) or SR144528 (6.25 μg μL−1 h−1)+vehicle solution (SR144528+vehicle) (n=6 per group). (a) Mean paw withdrawal response force to mechanical stimulation at days 2, 4 and 6 after PNL injury. *P<0.05 WIN 55,212–2-treated rats versus SR144528+WIN 55,212–2-treated rats; ANOVA, Tukey. At post-surgery day 2 only. (b) Mean percentage response to cooling stimulation at days 2, 4 and 6 after PNL injury. NS, P>0.05 WIN 55,212–2-treated rats versus SR144528+WIN 55,212–2-treated rats; Kruskal–Wallis ANOVA, Student–Newman–Keuls.
Extension of behavioural testing beyond the perineural WIN 55,212–2 delivery period
To test whether the effect of WIN 55,212–2 treatment on behavioural hypersensitivity was extended beyond the 7-day delivery duration of the mini-osmotic pump, behavioural testing was carried out on days 10 and 14 after injury and pump implantation. Upon post-mortem removal of the pumps at 14 days, the pump lumen was completely transparent and the catheter was no longer patent, indicating that solution delivery to the perineural area had been terminated. Behavioural testing on days 10 and 14 after PNL injury indicated that behavioural hypersensitivity in WIN 55,212–2-treated animals was still below that of the vehicle-treated control group. When tested at 10 and 14 days after injury, the mean force of mechanical stimulation eliciting paw withdrawal was still significantly higher in the WIN 55,212–2-treated group, compared with the vehicle group (Figure 7a). Likewise, on days 10 and 14, responses to cooling stimulation in WIN 55,212–2-treated rats were still significantly reduced, when compared with vehicle-treated controls (Figure 7b).
Discussion and conclusions
Neuropathic pain is often difficult to manage clinically because treatment with conventional analgesics can lead to unsatisfactory levels of pain relief in patients (Hempenstall et al., 2005; Rice and Hill, 2006). As a result, the development of novel treatments for neuropathic pain conditions is an important area of therapeutic need. Some conditions associated with neuropathic pain present an unexploited window for preventative therapy (e.g. herpes zoster) and prophylactic therapies are particularly sparse. This study describes a new method of peripheral cannabinoid delivery that is achieved by applying WIN 55,212–2 locally to the site of a nerve injury using a mini-osmotic pump connected to a perineural supply catheter. We hypothesized that this method of direct cannabinoid delivery to the injury site would prevent the development of nerve injury-associated hypersensitivity in rats. We found that WIN 55,212–2 (1.4–2.8 μg μl−1 h−1), delivered perineurally, partially reversed the reduction in withdrawal responses of the injured limb to mechanical and cooling stimulation applied to the plantar surface of the paw.
The highest perineural dose of WIN (2.8 μg μl−1 h−1) used in this study, equates to a dose of 0.012 mg kg−1 h−1and a total daily dose of 0.28 mg kg−1 day−1, both are below the range reported to be systemically effective for analgesia when given as a bolus injection in neuropathic rats (0.4–2.5 mg kg−1) (Bridges et al., 2001; Fox et al., 2001). To examine a possible systemic action of perineurally delivered cannabinoids, the pump catheters were directed to the contralateral side to nerve injury. Behavioural testing revealed no significant effect of contralateral WIN 55,212–2 treatment on reflex behaviour (tested in the injured paw) when compared with vehicle-treated controls. This indicates the absence of a systemic analgesic effect with either dose of perineural WIN 55,212–2 treatment and implicates the activation of peripheral CB receptors in mediating the antihypersensitivity effects of WIN 55,212–2.
The activation of peripheral cannabinoid receptors have been similarly implicated in the reduction of neuropathic pain behaviour after peripheral delivery of cannabinoids directly to the site of sensory testing in the hind paw (Fox et al., 2001; Elmes et al., 2004; Guindon and Beaulieu, 2006). In fact, bolus intraplantar injections of WIN 55,212–2 reduced mechanical hypersensitivity in the same PNL injury model as used in this study (Fox et al., 2001). The effect size (a ∼2-fold reduction in responses to mechanical stimulation in the injured paw, compared with vehicle treatment, 1 h after intraplantar application) is comparable with the magnitude of the effect on mechanical responses reported for perineural WIN 55,212–2 delivery. Antinociceptive effects on reflex behaviour to sensory stimuli have also been reported following delivery of cannabinoids to the hind paw (Elmes et al., 2004; Nackley et al., 2004) and these are likely to be attributed to the inhibitory effects of cannabinoids on the excitatory functions of cutaneous nociceptors (Richardson et al., 1998b; Ellington et al., 2002; Dovrak et al., 2003; Rukwied et al., 2003). The same cutaneous action may contribute to increasing withdrawal thresholds in neuropathic rats. However, this study provides evidence that cannabinoid receptors, found at a separate location near the nerve injury site, are also likely to play a role in the effects of peripheral cannabinoid treatment on nerve injury-induced stimulus hypersensitivity.
The successful delivery of biologically active WIN 55,212–2 (1.5 μg h−1) to nervous tissues using mini-osmotic pumps has been demonstrated in a previous study using Wistar rats (Galve-Roperh et al., 2000). Likewise, perineural catheters have also been used to successfully deliver substances to injured sciatic nerves: BDNF was applied directly to axotomized rat tibial nerves via a catheter delivery system and mini-osmotic pump, to produce significant axon regeneration (Boyd and Gordon, 2002). In agreement with our data, the direct delivery of a non-steroidal anti-inflammatory drug (NSAID) analgesic compound to the site of a chronic constriction injury to the sciatic nerve, also reduced behavioural hypersensitivity in rats (Takahashi et al., 2004). In our study, thionin dye injected via an implanted catheter stained sciatic nerve tissue surrounding the site of the nerve injury, providing histological evidence that solutions delivered by this route had access to damaged nerve tissue.
The concentration of WIN 55,212–2 used in our study (1.4 μg μl−1 or 2.7 mM) is also consistent with the effective 2.9 mM concentration of WIN 55,212–2 shown to produce antitumour effects in cerebral tissue after delivery by mini-osmotic pump (Galve-Roperh et al., 2000). The pump concentration was set higher than the concentration used to activate cannabinoid receptors on individual primary afferent neurones (Khasabova et al., 2002) because there is likely to be a considerable dilution effect following perineural delivery, acting to reduce the effective concentration of WIN 55,212–2 reaching nerve tissue. The concentration of WIN 55,212–2 used is still lower than the bolus doses of 30 μg (57 mM) injected into the hind paw skin, which is reported as being effective at reducing mechanical hypersensitivity in PNL-injured rats and those receiving a streptozocin-induced diabetic neuropathy (Fox et al., 2001; Ulugol et al., 2004).
The hypothesis that peripheral cannabinoid receptors can be activated at the site of injury (independently of those in the CNS) to alleviate pain responses, is supported by a growing body of evidence (Pertwee, 2001; Rice, 2005; Lever and Rice, 2006). After an inflammatory injury to the hind paw, for example, both the effects of oedema and thermal hypersensitivity can be attenuated by an intraplantar injection of the endocannabinoid anandamide at a systemically inactive dose (Richardson et al., 1998a, 1998b). Local application of WIN 55,212–2 also reduced pain behaviour following a heat- or formalin-induced injury to the hind paw via the activation of peripheral CB1 receptors (Calignano et al., 1998; Johanek and Simone, 2004).
Consistent with these data, the analgesic effects of WIN 55,212–2 in this study were also reversed by co-application of the CB1 receptor antagonist SR141716a. Likewise, SR141716a injected locally in the hind paw also reversed the inhibitory effects of WIN 55,212–2 on mechanical hypersensitivity in the same PNL nerve injury model (Fox et al., 2001). Furthermore, the antihypersensitivity effects of injecting anandamide into the hind paw, in an animal model of neuropathic pain, were locally reversed using a CB1 antagonist (Guindon and Beaulieu, 2006). Together, these studies support the involvement of peripheral CB1 receptors in mediating the effects of peripheral WIN 55,212–2 on behavioural hypersensitivity in neuropathic pain models.
In our study, the attenuation of mechanical hypersensitivity was initially reversed by CB2 receptor antagonists delivered perineurally, an effect that was not extended beyond the day 2 testing time point. The same antagonist effect at day 2 was not observed for responses to cooling stimuli, although such an effect may have been masked by the unusually low response of the group receiving the CB2 receptor antagonist and vehicle (against which the effect of the antagonist combined with WIN 55,212–2 was measured). In support of the involvement of CB2 receptors in the modulation of early nociceptive hypersensitivity responses, CB2 receptor antagonists have also been reported to reverse only the early analgesic effects of peripheral WIN 55,212–2 treatment measured after heat injury of the rat hind paw (Johanek and Simone, 2004). A peripheral CB2-mediated analgesic action affecting mechanical hypersensitivity after a nerve injury, has similarly been demonstrated by the intraplantar injection of a CB2-selective agonist (Ibrahim et al., 2003; Elmes et al., 2004). In some studies, peripheral CB2 receptor signalling has also been implicated in mediating the local anti-inflammatory effects of WIN 55,212–2 (Oka et al., 2006) and its reduction of inflammatory hyperalgesia (Nackley et al., 2003). As inflammation is suggested to play a role in the initial stages of the development of neuropathy (Lindenlaub and Sommer, 2000), it is possible that a CB2-mediated anti-inflammatory mechanism contributes to the reduction of pain responses by WIN 55,212–2 in the early stages after nerve injury.
Given the actions of cannabinoids on peripheral tissues, several mechanisms could putatively operate to produce the analgesic effects of WIN 55,212–2 treatment after nerve injury. Neuroinflammatory and neurodegenerative process are known to play an important role in the development and maintenance of nerve injury asssociated pain and contribute to its severity (Lindenlaub and Sommer, 2000). In a study by Costa et al. (2004), WIN 55,212–2 was systemically delivered immediately after a nerve injury and treatment was sustained during the period associated with neuropathic pain development. In addition to a reduction in pain behaviour, the investigators also measured a concomitant decrease in the level of pro-inflammatory mediators associated with neuropathic pain. After nerve injury, pro-inflammatory cytokines, including tumour neurosis factor α (TNFα), are thought to be produced by Schwann cells and macrophages invading damaged nerve tissue. Specific anti-TNFα treatment has been demonstrated to reduce hypersensitivity after nerve injury, implying a role for the inflammatory process in the aetiology of neuropathic pain (Sommer et al., 1998). In the time frame of WIN 55,212–2 treatment, the anti-inflammatory effects of this cannabinoid may act to reduce inflammation that occurs after an initial mechanical insult to nerve tissue, and thus, may reduce the contribution of this process to the sensitization of primary afferent responses that, in turn, produce lower paw withdrawal thresholds.
An early impact on inflammatory pro-algesic processes is likely to be CB2 mediated because this receptor is predominantly expressed on immune cells (Walter and Stella, 2004) and its activation has been demonstrated to reduce levels of TNFα, as well as other inflammatory cytokines (Puffenbarger et al., 2000; Molina-Holgado et al., 2003). The presence of WIN 55,212–2 during the initial stages of an injury may act to reduce the level of secondary nerve damage due to inflammatory processes. In addition, this may be an explanation for the apparent non-time-dependent nature of the effects of perineural WIN 55,212–2 on hypersensitivity, as these effects were extended after delivery of the cannabinoid had ceased. An examination of the effect of varying the timing of WIN-55,212–2 delivery, in relation to creation of peripheral nerve lesion, was not performed in this study. However, previous reports have demonstrated the efficacy of peripherally administered WIN 55,212–2 to evoke antihypersensitivity effects when initiated either 1 day or 12–15 days after the establishment of a nerve injury (Fox et al., 2001; Costa et al., 2004).
To account for the efficacy of relatively low doses of perineural WIN 55,212–2 used in this study, it is possible to hypothesize that the antihypersensitivity actions of this exogenous cannabinoid are boosted locally at the nerve injury site by injury-associated synthesis of endocannabinoids, as has been shown in the case of spinal demyelination (Baker et al., 2001). The effects of perineural WIN 55,212–2 are also likely to be amplified by increases in cannabinoid receptors, as there is evidence for the ipsilateral upregulation of CB1 and CB2 receptors in response to nerve injury (Lim et al., 2003; Wotherspoon et al., 2005; Beltramo et al., 2006). Specifically, the accumulation of CB2 receptor immunoreactivity in sciatic nerve tissue, proximal to a unilateral ligation injury, is evidence for local, injury-associated receptor increases in nerve tissue (Wotherspoon et al., 2005). In addition, populations of cannabinoid receptors may be frequently replenished during the development of the nerve injury, during to the continual invasion of circulating cannabinoid receptor-expressing immune cells (this may also help to prevent the development of tolerance effects that are sometimes attributed to continuous drug delivery systems). Localized, injury-associated increases in endocannabinoid production and cannabinoid receptor expression could be used to explain the absence of a contralateral effect of peripheral WIN 55,212–2 delivery on behavioural hypersensitivity.
Evidence suggests that the activation of cannabinoid receptor signalling by WIN 55,212–2 in neurones can be neuroprotective by reducing the extent of excitotoxic cell damage after a tissue injury (Bereiter et al., 2002; Zhuang et al., 2005). In the case of nerve injury inducing axonal damage to peripheral sensory neurones, the result of excitotoxic damage can be manifested as an increase in activity levels in the cell bodies of these cells (Song et al., 2003; Devor, 2006). Signalling of CB1 receptors is coupled to inhibitory G-protein effector systems, which act as a brake to cell excitability. In vitro experiments demonstrated that WIN 55,212–2 was capable of inhibiting calcium responses via the activation of CB1 receptors found on large-sized DRG neurones that subtend myelinated axons and stain positively with neurofilament antibodies (Khasabova et al., 2002). This kind of CB1-mediated neuroprotective mechanism has been proposed to explain the reduction in the extent of neurodegeneration and loss of myelinated (neurofilament positive) neurones seen after autoimmune destruction of nerve tissue in mice (Baker et al., 2003; Pryce et al., 2003). The loss of myelinated fibres is also correlated to the extent of nociceptive hypersensitivity measured after a partial sciatic nerve injury (Lindenlaub and Sommer, 2000). Considering these data, it is possible that perineural delivery of WIN 55,212–2 may have instigated a neuroprotective effect on myelinated DRG neurones expressing CB1 receptors, to reduce the extent of pro-algesic nerve fibre demyelination.
In this study, the reduction in behavioural hypersensitivity achieved by applying WIN 55,212–2 to the site of a nerve injury – away from the site of sensory testing at systemically inactive doses – is the first indication that cannabinoids may act directly at a nerve injury site to prevent aspects of neuropathic pain behaviour. With this perineural delivery mechanism, it is possible to separate the peripheral analgesic effects of WIN 55,212–2 from the antinociceptive actions of cannabinoids at the terminals of sensory neurons in the skin transducing the testing stimuli. This was a possible confounding factor in experiments where cannabinoids were applied to the site of sensory testing in the hind paw.
Our study demonstrates the ability of WIN 55,212–2 to reduce hypersensitivity, via the activation of peripheral cannabinoid receptors. This finding is of potential therapeutic importance because it may represent a way of delivering the analgesic benefit of cannabinoid compounds without their centrally mediated psychoactive side effects. In addition, this approach may be useful where there is the possibility of preventative cannabinoid therapy in conditions associated with focal neuropathic pain, where regional administration is feasible, and where such a prophylactic window of opportunity exists – for example, during acute herpes zoster to prevent the development of post-herpetic neuralgia.
Acknowledgments
This work was supported by the Wellcome Trust (London Pain Consortium).
Abbreviations
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- DMSO
dimethylsulphoxide
- DRG
dorsal root ganglion
- PNL
partial nerve ligation
- PWR
paw withdrawal response
- RSA
rat serum albumin
Conflict of interest
The authors state no conflict of interest.
References
- Ahluwalia J, Urban L, Bevan S, Nagy I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurones by activating both the cannabinoid 1 receptor and the vannilloid receptor 1 in vitro. Eur J Neurosci. 2003;17:2611–2618. doi: 10.1046/j.1460-9568.2003.02703.x. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, et al. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J. 2001;15:300–302. doi: 10.1096/fj.00-0399fje. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Giovannoni G, Thompson AJ. The therapeutic potential of cannabinoids. Lancet Neurol. 2003;2:291–298. doi: 10.1016/s1474-4422(03)00381-8. [DOI] [PubMed] [Google Scholar]
- Bell MR, D'Ambra TE, Kumar V, Eissenstat MA, Herrmann JL, Wetzel JR, et al. Anti-nociceptive (aminoalkyl)indoles. J Med Chem. 1991;34:1099–1110. doi: 10.1021/jm00107a034. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Bernardini R, Bertorelli M, Campanella E, Nicolussi S, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Bereiter DF, Hirata H. Topical cannabinoid agonist, WIN55,212–2, reduces cornea-evoked trigeminal brainstem activity in the rat. Pain. 2002;99:547–556. doi: 10.1016/S0304-3959(02)00271-3. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur J Neurosci. 2002;15:613–626. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- Bridges D, Ahmad KS, Rice ASC. The synthetic cannabinoid WIN55,212–2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br J Pharmacol. 2001;133:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D, Rice ASC, Egertova M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience. 2003;119:803–812. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Lekan HA, Kim SH, Chung JM. Behavioral manifestations of an experimental model for peripheral neuropathy produced by spinal nerve ligation in the primate. Pain. 1994;56:155–166. doi: 10.1016/0304-3959(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Costa B, Colleoni M, Conti S, Trovato AE, Bianchi M, Sotgiu ML, et al. Repeated treatment with the synthetic cannabinoid WIN 55,212–2 reduces both hyperalgesia and production of pronociceptive mediators in a rat model of neuropathic pain. Br J Pharmacol. 2004;141:4–8. doi: 10.1038/sj.bjp.0705587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovrak M, Watkinson A, McGlone F, Rukwied R. Histamine induced responses are attenuated by a cannabinoid receptor in human skin. Inflamm Res. 2003;52:238–245. doi: 10.1007/s00011-003-1162-z. [DOI] [PubMed] [Google Scholar]
- Devor M. Sodium Channels and mechanisms of neuropathic pain. J Pain. 2006;7:S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Elmes SJR, Jhaveri MD, Smart D, Kendall DA, Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. Eur J Pharmacol. 2004;20:2311–2320. doi: 10.1111/j.1460-9568.2004.03690.x. [DOI] [PubMed] [Google Scholar]
- Ellington HC, Cotter MA, Cameron NE, Ross RA. The effect of cannabinoids on capsaicin-evoked calcitonin gene-related peptide (CGRP) release from the isolated paw skin of diabetic and non-diabetic rats. Neuropharmacology. 2002;42:966–975. doi: 10.1016/s0028-3908(02)00040-0. [DOI] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, et al. The role of central and peripheral cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Sanchez C, Cortes LM, Del Pulgar TG, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000;6:31–39. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- Guindon J, Beaulieu P. Antihyperalgesic effects of local injections of anandamide, ibuprofen, rofecoxiib and their combinations in a model of neuropathic pain. Neuropharmacology. 2006;50:814–823. doi: 10.1016/j.neuropharm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Hasnie FS, Breuer J, Parker S, Wallace VCJ, Blackbeard J, Lever I, et al. Further characterisation of a rat model of varicella zoster virus (VZV)-associated pain: relationship between mechanical hypersensitivity and anxiety-related behaviour; and the influence of analgesic drugs. Neuroscience. 2006;144:1495–1508. doi: 10.1016/j.neuroscience.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempenstall K, Nurmikko TJ, Johnson RW, A'Hern R, Rice ASC. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2:628–644. doi: 10.1371/journal.pmed.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen HU, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. Br Med J. 2005;330:11. doi: 10.1136/bmj.38267.664086.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg U, Eliav E, Bennett GJ, Kopin IJ. The analgesic effects of R(+)-WIN55,212–2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci Lett. 1997;221:157–160. doi: 10.1016/s0304-3940(96)13308-5. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodortova A, et al. CB-2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102:3039–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar SI, Hasnie FS, Sellaturay S, Rice ASC. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 agonist palmitoylethanolamide investigated in models of visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Johanek LM, Simone DA. Activation of peripheral cannabinoid receptors attenuates cutaneous hyperalgesia produced by a heat injury. Pain. 2004;109:432–442. doi: 10.1016/j.pain.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Khasabova IA, Simone DA, Seybold VS. Cannabinoids attenuate depolarisation-dependent Ca2+ influx in intermediate-size primary afferent neurones of adult rats. Neuroscience. 2002;115:613–625. doi: 10.1016/s0306-4522(02)00449-9. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Rice ASC. Handbook of Experimental Pharmacology. Springer-Verlag: Berlin, Heidelberg; 2006. Cannabinoids; pp. 259–300. [Google Scholar]
- Lim G, Sung B, Ji R-R, Mao J. Upregulation of spinal cannabinoid-1-receptors following nerve injury, enhances the effects of Win 55,212–2 on neuropathic pain behaviours in rats. Pain. 2003;105:275–283. doi: 10.1016/s0304-3959(03)00242-2. [DOI] [PubMed] [Google Scholar]
- Lindenlaub T, Sommer C. Partial sciatic nerve transection as a model of neuropathic pain: a qualitative neuropathological study. Pain. 2000;98:97–106. doi: 10.1016/S0304-3959(00)00354-7. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Lu J, Keniston L, Mayer DJ. Two distinctive anti-nociceptive systems in rats with pathological pain. Neurosci Lett. 2000;280:13–16. doi: 10.1016/s0304-3940(99)00998-2. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, Pinteaux E, Moore JD, Molina-Holgado E, Guaza C, Gibson RM, et al. Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J Neurosci. 2003;23:6470–6474. doi: 10.1523/JNEUROSCI.23-16-06470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nackley AG, Suplita II, Hohmann AG. Aperipheral cannabinoid mechanism suppresses spinal fos protein expression and pain behaviour in a rat model of inflammation. Neuroscience. 2003;117:650–670. doi: 10.1016/s0306-4522(02)00870-9. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 2004;92:3562–3574. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- Oka S, Wakui J, Gokoh M, Kishimoto S, Sugiura T. Suppression by WIN55212–2, a cannabinoid receptor agonist, of inflammatory reactions in mouse ear: Interference with the actions of an endogenous ligand, 2-arachidonoylglycerol. Eur J Pharmacol. 2006;538:154–162. doi: 10.1016/j.ejphar.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Pascual D, Goicoechea C, Suardiaz M, Martin MI. A cannabinoid agonist WIN55,212–2 reduces neuropathic nociception induced by paclitaxel in rats. Pain. 2005;118:23–34. doi: 10.1016/j.pain.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoids and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Pozzi O, Misiano P, Clark GD, Visentin L. Antagonism between the anti-inflammatory activity of the cannabinoid WIN 55212–2 and SR 141716A. Pharmacology. 2003;69:158–163. doi: 10.1159/000072669. [DOI] [PubMed] [Google Scholar]
- Pryce G, Ahmed Z, Hankey DJR, Jackson SJ, Croxford JL, Pocock JM, et al. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain. 2003;126:2191–2202. doi: 10.1093/brain/awg224. [DOI] [PubMed] [Google Scholar]
- Puffenbarger R, Boothe AC, Cabral GA. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29:58–69. [PubMed] [Google Scholar]
- Rice ASC.Cannabinoids Melzack and Wall: Textbook of Pain 2005Elsevier: London; In: Koltzenburg M, McMahon SB (eds) [Google Scholar]
- Rice ASC, Hill RG. New treatments for neuropathic pain. Ann Rev Med. 2006;57:535–551. doi: 10.1146/annurev.med.57.121304.131324. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Aanonsen L, Hargreaves KM. Antihyperalgesic effects of spinal cannabinoids. Eur J Pharmacol. 1998a;345:145–153. doi: 10.1016/s0014-2999(97)01621-x. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998b;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Ross RA, Coutts AA, McFarlane SM, Anavi-Goffer S, Irving AJ, Pertwee RG, et al. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Rukwied R, Watkinson A, McGlone F, Dvorak M. Cannabinoid agonists attenuate capsaicin-induced responses in human skin. Pain. 2003;102:283–288. doi: 10.1016/S0304-3959(02)00401-3. [DOI] [PubMed] [Google Scholar]
- Sagar DR, Kelly S, Millns PJ, O'Shaughnessey CT, Kendall D, Chapman V. Inhibitory effects on CB1 and CB2 receptor agonists on responses of DRG neurones and dorsal horn neurones in neuropathic rats. Eur J Neurosci. 2005;22:371–379. doi: 10.1111/j.1460-9568.2005.04206.x. [DOI] [PubMed] [Google Scholar]
- Savinaninen JR, Kokkola T, Salo OMH, Poso A, Jarvinen T, Laitinen JT. Identification of WIN55212–3 as a competitive neutral antagonist of the human cannabinoid CB2 receptor. Br J Pharmacol. 2005;145:636–645. doi: 10.1038/sj.bjp.0706230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, Wright CE, Angus JA. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124–131. doi: 10.1016/j.pain.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Yoram S. A novel behavioural model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Sokal DM, Elmes SJR, Kendall DA, Chapman V. Intraplantar injection of anandamide inhibits mechanically-evoked responses of spinal neurones via activation of CB2 receptors in anaesthetised rats. Neuropharmacology. 2003;45:404–411. doi: 10.1016/s0028-3908(03)00195-3. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schmidt C, George A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol. 1998;151:138–142. doi: 10.1006/exnr.1998.6797. [DOI] [PubMed] [Google Scholar]
- Song X-J, Zhang J-M, Hu S-J, LaMotte RH. Somata of nerve-injured sensory neurones exhibit enhances responses to inflammtory mediators. Pain. 2003;104:701–709. doi: 10.1016/S0304-3959(03)00167-2. [DOI] [PubMed] [Google Scholar]
- Ständer S, Schmelz M, Metze D, Luger TM, Rukweid R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibres and adnexal structures in human skin. J Dermatol Sci. 2005;38:177–188. doi: 10.1016/j.jdermsci.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kawaguchi M, Shimada K, Konishi N, Furuya H, Nakashima T. Peri-sciatic administration of indomethacin early after nerve injury can attenuate the development of tactile allodynia in a rat model of L5 single spinal nerve injury. Neurosci Lett. 2004;356:37–40. doi: 10.1016/j.neulet.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Tognetto M, Amadesi S, Harrison S, Creminon C, Trevisani M, Carreras M, et al. Anandamide excites central terminals of dorsal root ganglion neurons via vanilloid receptor-1 activation. J Neurosci. 2001;21:1104–1109. doi: 10.1523/JNEUROSCI.21-04-01104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulugol A, Karadag HC, Ipci Y, Tarner M, Dokmeci I. The effect of WIN 55,212–2, a cannabinoid agonist, on tactile allodynia in diabetic rats. Neurosci Lett. 2004;371:167–170. doi: 10.1016/j.neulet.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Wallace VCJ, McMahon SB, Rice ASC. 11th World Congress on Pain Abstracts 2005. IASP Press: USA; 2005. The Characterisation of Rodent Models of HIV – gp120 and Antiretroviral – Associated Painful Peripheral Neuropathy; pp. 67–P44. [Google Scholar]
- Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Zhuang S-Y, Bridges D, Grigorenko E, McCloud S, Boon A, Hampson RE, et al. Cannabinoids produce neuroprotection by reducing intracellular calcium release from ryanodine-sensitive stores. Neuropharmacology. 2005;48:1086–1096. doi: 10.1016/j.neuropharm.2005.01.005. [DOI] [PubMed] [Google Scholar]