Abstract
Background and purpose:
The nitrogen-containing bisphosphonates are drugs used successfully in the treatment of osteoporosis. They act inhibiting farnesyl diphosphate synthase. This mechanism may also produce anti-inflammatory effects. The therapeutic activity of alendronate was tested in vivo using a model of inflammatory bowel disease.
Experimental approach:
The trinitrobenzenesulfonic acid model of colitis in the rat was used. Rats were treated orally with alendronate and its efficacy compared with that of oral sulphasalazine or vehicle, starting 2 h after colitis induction. The status of the animals was assessed 5 days later.
Key results:
Alendronate treatment (25 or 75 mg kg-1 day-1) resulted in a decrease in the colonic damage score and loss of body weight (at 25 mg kg-1 day-1 only). This was associated to a dramatic reduction in the mRNA levels of interleukin 1β (IL-1β), monocyte chemoattractant protein 1 (MCP-1) and interleukin 1 receptor antagonist (IL-1ra). The magnitude of the beneficial effect was comparable to that of sulphasalazine (at a 6-20 fold higher dose). Thus sulphasalazine post-treatment reduced the mRNA levels of IL-1β/IL-1ra and MCP-1 to the same extent as alendronate and additionally lowered colonic alkaline phosphatase activity, but failed to affect body weight loss or colonic damage score. Alendronate failed to exert beneficial effects when administered intraperitoneally.
Conclusions and Implications:
Oral but not intraperitoneal alendronate significantly protected the colon in experimental rat colitis. Inflammatory bowel disease patients might benefit from exposure to oral alendronate.
Keywords: alendronate, trinitrobenzenesulfonic acid, experimental colitis, bisphosphonates, inflammatory bowel disease, interleukin 1
Introduction
The phrase inflammatory bowel disease (IBD) refers to two different but closely related conditions, ulcerative colitis and Crohn's disease. Both are chronic relapsing diseases of the intestine that cause a significant deterioration of the quality of life of patients and which have a substantial (and increasing) prevalence (Sands, 2000). Despite an intense investigative effort, the aetiology of IBD remains unknown, but recent studies strongly suggest that IBD represents an uncontrolled and exacerbated response to luminal antigens that are innocuous for the normal population. There are significant differences in the pathology of both IBD variants. Thus Crohn's disease appears to be a well-defined Th1-driven condition characterized by the increased production of interferon γ, IL-12/IL-23, IL-18, IL-21 and osteopontin, whereas ulcerative colitis exhibits a Th2 profile, with augmented IL-5, IL-4 and IL-13 (Monteleone et al., 2006). Furthermore, Crohn's disease has been linked to gene variants such as those affecting CARD15/NOD2, SLC22A4 and SLC22A5, although the mechanisms involved are not well established (Siminovitch, 2006). However, despite these differences in both conditions there is a marked increase in the biosynthesis of nonspecific inflammatory markers, including eicosanoids, adhesion molecules, metalloproteinases, oxidants/free radicals, chemokines, etc (Monteleone et al., 2006). Hence it is not surprising that nearly all drug treatments for IBD downregulate intestinal inflammation in a non-specific fashion. These drugs, including corticoids, aminosalicylates or azathioprine, among others, can often manage IBD successfully, but have a plethora of serious adverse effects which limit their application. Therefore, the search for new treatments with a low profile of adverse effects is much warranted (Sands, 2000; Van Assche et al., 2005).
One of the possible strategies that can be applied to limit the inflammatory response is to target protein prenylation, which is essential for the bioactivity of a number of small GTPases such as Rho and Rho-like enzymes, which in turn are involved in the activation of the p38 and JNK MAP kinases and NF-κB, among other pro-inflammatory pathways. One important drug family, the statins, exert anti-inflammatory effects that are accounted for, at least in part, by this mechanism (Abeles and Pillinger, 2006). These include intestinal anti-inflammatory activity in a preclinical model of IBD, acting by a mechanism independent of cholesterol lowering (Sasaki et al., 2003).
The inhibitory effect on protein prenylation is characteristic of another drug class, which is otherwise unrelated, namely the bisphosphonates, a group of drugs used primarily in the treatment and prevention of osteoporosis. The oldest members of the bisphophonate family, such as clodronate and etidronate, inhibit bone resorption through induction of osteoclast apoptosis. However, the members of the so called second generation of bisphosphonates (nitrogen-containing) appear to act by an entirely different mechanism, that is interference with signal transduction mechanisms by inhibition of farnesyl diphosphate synthase in the cholesterol biosynthetic pathway (Coxon et al., 2006). This results in inhibition of osteoclastic bone resorption and even stimulation of bone matrix new synthesis. The clinical efficacy of bisphosphonates in this context, including risedronate, alendronate and pamidronate, among others, is well established by clinical trials (Mathoo et al., 2004). Of note, bisphosphonates are often associated with corticoids in the treatment of IBD in an attempt to reduce the impact of the latter on the bone. However, the therapeutic potential of bisphosphonates in intestinal inflammation has not been explored to date. Therefore, we set out to verify whether one such drug, alendronate, has beneficial effects in the trinitrobenzenesulfonic acid (TNBS) model of rat colitis, using sulphasalazine a standard treatment for IBD, for comparison.
Methods
Animals
Female Wistar rats (150–200 g) obtained from the Laboratory Animal Service of the University of Granada were housed in makrolon cages, maintained in a 12 h light-dark cycle, fed standard rodent chow (Panlab A04, Panlab, Barcelona, Spain) and water ad libitum throughout the experiment. This study was carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health and was approved by the Animal Welfare Committee of the University of Granada.
Induction of colitis and treatment protocol
After a 7-day acclimatization period, rats were weighed and randomly distributed in the different experimental groups of seven rats each. Colitis was induced by the administration of an enema containing 0.25 ml of a solution of TNBS (10 mg) in EtOH (50% v v−1). Animals were killed after 5 days. Three different experiments were performed. In the first one the animals that received the TNBS enema were treated daily starting 2 h after colitis induction with either alendronate (25 mg kg−1 day−1, alendronate group), sulphasalazine (500 mg kg−1 day−1, sulphasalazine group) or vehicle (1% methylcellulose, TNBS group) in parallel experiments until the day they were killed. A control group, which received saline instead of TNBS, was included for comparison.
The second experiment was similar but a suspension of Fosamax tablets (Merck Sharp and Dohme) was used as alendronate treatment. This approach was followed to increase the dose to 75 mg kg−1 day−1, which resulted in enhanced anti-inflammatory activity. In a third experiment, alendronate was administered by the i.p. route (10 mg kg−1 day−1) and compared with the control groups.
Assessment of colonic damage
Animal body weight and food consumption were recorded daily. Animals were scored for diarrhoeal status (0–3), killed by cervical dislocation and the colon was removed and placed on an ice-cold plate, cleaned of fat and mesentery and blotted on filter paper. Each specimen was weighed and its length measured under a constant load (2 g). The intestinal segments were subsequently divided longitudinally in 3–4 pieces and immediately frozen in liquid nitrogen for biochemical determinations. Macroscopically visible damage was scored on a 0–24 scale by an observer unaware of treatment according to the criterion shown in Table 1.
Table 1.
Scoring criteria applied to the visible lesions in the rat colona
| Adhesions | 0 No adhesions |
| 1 Difficult dissection | |
| 2 Visible adhesions | |
| 3 ‘Wrapped' intestine | |
| Obstruction | 0 No obstruction |
| 1 Need for gentle manual cleaning | |
| 2 Fecal impaction | |
| Thickening | 0 Similar to uninflamed intestine |
| 1 Thicker than normal (∼1–2 mm) | |
| 2 Much thicker than normal (>2 mm) | |
| Hyperemia | 0 Similar to uninflamed intestine |
| 1 Mild and generalized or intense but localized hyperemia | |
| 2 Intense and localized hyperemia | |
| 3 Frank hemorrage | |
| Shortening | 0 Normal colonic length (>15 cm) |
| 1 Colonic length <15 cm | |
| 2 Colonic length <14 cm | |
| 3 Colonic length <13.5 cm | |
| Necrosis | 0 No signs of necrosis |
| 1 Small areas of necrosis | |
| 2 Patchy necrosis (cobblestone appearance) | |
| 3 Focal necrosis, ∅<0.8 cm | |
| 4 Focal necrosis, ∅>0.8 cm | |
| 5 Extended necrotic lesion | |
| Other signs | +2 proximal dilation |
| +2 deformity | |
| +1 fragility (tendency to break) | |
| +1 scarring |
All scores were assigned by one investigator unaware of the treatment.
Alkaline phosphatase activity was measured spectrophotometrically using disodium p-nitrophenylphosphate as substrate (Sanchez de Medina et al., 2004) and the results expressed as mU mg protein−1. The sensitivity to the inhibitor levamisole (1 mM), which is modulated by inflammation, was also determined and expressed as % inhibition (Sanchez de Medina et al., 2004). Myeloperoxidase activity was determined as an index of neutrophil accumulation. The enzyme activity was measured spectrophotometrically, according to the technique described by Krawisz et al. (1984) with minor modifications. The results are expressed as myeloperoxidase units (μmol min−1) per gram of wet tissue.
Western blot
Colonic levels of nitric oxide synthase (iNOS) and cyclo-oxygenase (COX-2) were determined by immunoblotting. Colonic samples were homogenized in cold lysis buffer containing 1% Igepal CA-630, 20 mM HEPES-Na pH 7.5, 10 mM ethylene glycol bis (β-amino ethylether)-N,N,N′,N′,-tetraaceticacid (EGTA), 40 mM β-glycerophosphate, 25 mM MgCl2, 2 mM sodium orthovanadate and freshly added protease inhibitors (phenyl-methylsulphonyl fluoride, aprotinin, leupeptin, 1,10-phenanthroline). The protein content was measured by the bicinchoninic acid assay (Vilaseca et al., 1990b), using bovine serum albumin (BSA) as standard. Samples were boiled for 4 min in Laemmli buffer, then 75 μg were separated by 7% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The nitrocellulose membranes were blocked for at least 1 h at room temperature in Tris-Buffered saline-0.1% Tween-20 (TBS-T) containing 5% (w v−1) nonfat dry milk and then incubated with TBS-T containing BSA 5% and the primary antibody at 4°C overnight. The dilutions of antibodies used were: 1:3000 for iNOS (Transduction Laboratories, BD Biosciences, Madrid, Spain) and COX-2 (Cayman Chemical Company). A primary antibody against α-actin was used as loading control. After three washes of 5 min with TBS-T, peroxidase-conjugated anti-mouse IgG was used as secondary antibody. Then, enhanced chemiluminiscence (Perkin Elmer, Life Sciences, Boston, MA, USA) detection was performed.
Immunohistochemical analysis
The samples for the histological study were taken from the edge of the necrotic area, coded, fixed in formaldehyde and processed for routine analysis using haematoxylin/eosin staining. The sections were scored for microscopic damage (including tissue oedema) by an investigator (AN) unaware of the sample identity as described previously (Stucchi et al., 2000). Immunohistochemistry was performed with COX-2 and iNOS polyclonal antibodies (Oxford Biomedical Research and Santa Cruz Biotechnology, Heidelberg, Germany respectively) at a 1:400 and 1:100 dilution for 30 min at room temperature. The developing system was Dako ChemMateTM Universal Kits (LSBA), following manufacturer's instructions. Immunoreactivity was visualized with 3–30 diaminobenzidine tetrachloride (Sigma) and hydrogen peroxide (0.01%) and slides were counterstained for 2 min with Gill's haematoxylin. Dilutions and washing were made in TBS (Castells et al., 2006). Negative control slides were made by substituting the primary antibody with TBS or with normal pig serum.
Analysis of gene expression by reverse transcriptase-polymerase chain reaction
Colonic fragments were frozen immediately post mortem in liquid nitrogen. Total RNA was isolated by single-step, guanidium thiocyanate-phenol-chloroform extraction with the TRIzol Reagent (Invitrogen, Paisley, UK) according to the manufacturer's instructions. RNA (5 μg sample−1) was reverse-transcribed into complementary DNA (cDNA) using reverse transcriptase (First-Strand cDNA Synthesis Kit, Amersham Biosciences, Barcelona, Spain) and following the instructions as indicated. The expression of the 18 S ribosomal unit was routinely examined as a loading standard. The following primer sequences (5′ → 3′) were used: for Muc-2, forward: 5′-GCTCAATCTCAGAAGGCGACAC-3′ and reverse: 5′-CCAGATAACAATGATGCCAGAGC-3′; for Muc-3, forward: 5′-CACAAAGGCAAGAGTCCAGA-3′ and 5′-ACTGTCCTTGGTGCTGAATG-3′; for trefoil factor 3 (TFF-3), forward: 5′-ATGGAGACCAGAGCCTTCTG-3′ and reverse 5′-ACAGCCTTGTGCTGACTGTA-3′; for interleukin-1β (IL-1β), forward: 5′-AATGACCTGTTCTTTGAGGCTGAC-3′ and reverse 5′-CGAGATGCTGCTGTGAGATTTGAAG-3′; for interleukin 1 receptor antagonist (IL-1ra), forward: 5′-GAGTCAGCTGGCCACCTG-3′ and reverse 5′-CAGACTTGACACAAGACAGGCAC-3′; for transforming growth factor-β (TGF-β), forward: 5′-GCTAATGGTGGACCGCAACAAC-3′ and reverse 5′-CACTGCTTCCCGAATGTCTGAC-3′; for macrophage chemoattractant protein 1 (MCP1), forward 5′-CACTATGCAGGTCTCTGTCACG-3′ and reverse 5′-CTGGTCACTTCTACAGAAGTGC-3′; and for 18S, forward: 5′-CCATTGGAGGGCAAGTCTGGTG-3′ and reverse 5′-CGCCGGTCCAAGAATTTCACC-3′. The polymerase chain reaction was performed in a 25 μl volume containing 1 μl of RT product (cDNA) and 23 μl PCR master mix: 10 × buffer, 1U Taq DNA polymerase (Amersham Biosciences), 5 mM of each dNTPs (Roche, Mannheim, Germany) and 2 pM concentration of each primer. For each primer pair, control experiments were performed to determine the range of cycles, in which a given amount of cDNA would be amplified in a linear fashion. The cycle numbers and hybridization temperatures for each PCR reaction were as follows: MUC2, 27 cycles and 59°C, MUC3, 25 cycles and 56°C, TFF-3, 27 cycles and 59°C, IL-1β, 30 cycles and 57°C, IL-1ra, 34 cycles and 59°C, TGF-β, 33 cycles and 57°C, MCP1, 40 cycles and 56°C and 18 S, 15 cycles and 60°C. Semiquantitative analyses of photographs of ethidium bromide-stained DNA gels (2% agarose) were performed with Scion Image (Scion Corporation, Frederick, MD, USA). The data were normalized to transcript levels for the constitutively expressed 18 S gene.
Statistical analysis
All results are expressed as mean±s.e.m. Differences among means were tested for statistical significance using one way analysis of variance and a posteriori least significance tests. Statistical significance was set at P<0.05. All analyses were carried out with SigmaStat 2.0 (Jandel Corporation, San Rafael, CA, USA).
Materials
Except where indicated, all chemicals and primers were obtained from Sigma (Madrid, Spain).
Results
TNBS colitis
Administration of 10 mg of TNBS intrarectally induced a severe colonic inflammatory reaction characterized by mucosal necrosis (Figure 1), submucosal fibrosis and oedema, bowel wall thickening and shortening of colonic length (Table 2), as described previously (Morris et al., 1989; Sanchez de Medina et al., 1996). These features were associated with marked anorexia and loss of body weight (Table 2), which were strongly correlated.
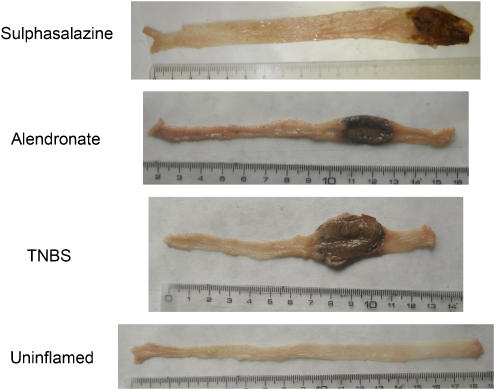
Figure 1.
Macroscopic appearance of rat TNBS colitis in the different experimental groups. Photographs are representative of the macroscopic features of the large intestine in each group. Uninflamed: noncolitic group; TNBS: colitic animals treated with vehicle; alendronate: colitic animals treated with alendronate (25 mg kg−1 day−1); sulphasalazine: colitic animals treated with sulphasalazine (500 mg kg−1 day−1). Darkened areas correspond to epithelial necrosis.
Table 2.
Effect of alendronate on macroscopic parameters and AP activity in rat TNBS colitis
| Colonic weight/length ratio (mg/cm) | Extension of necrosis (cm) | Damage score | Body weight gain after 5 days | AP activity (mU/mg protein) | |
|---|---|---|---|---|---|
| Uninflamed | 53.8±1.8 | 0 | 0 | +2.3±1.6 | 71.1±4.8 |
| TNBS | 201.0±11.9+ | 3.2±0.5+ | 11.0±0.5+ | −10.8±2.3+ | 169.6±36.7+ |
| Alendronate | 180.0±22.9+ | 3.1±0.6+ | 7.4±1.1+* | −5.3±3.9+* | 171.1±43.0+ |
| Sulphasalazine | 143.7±41.5+ | 2.2±0.9+ | 8.1±6.5+ | −12.8±2.0+ | 110.24±35.8+* |
Abbreviations: AP, alkaline phosphatase; TNBS, trinitrobenzenesulfonic acid
The doses of alendronate and sulphasalazine were 25 and 500 mg kg−1 day−1, respectively. Values are means±s.e.m., n=7.
Different from Control group, P<0.05
different from TNBS group, P<0.05.
Body weight gain is expressed as percentage change from the start of the experiment.
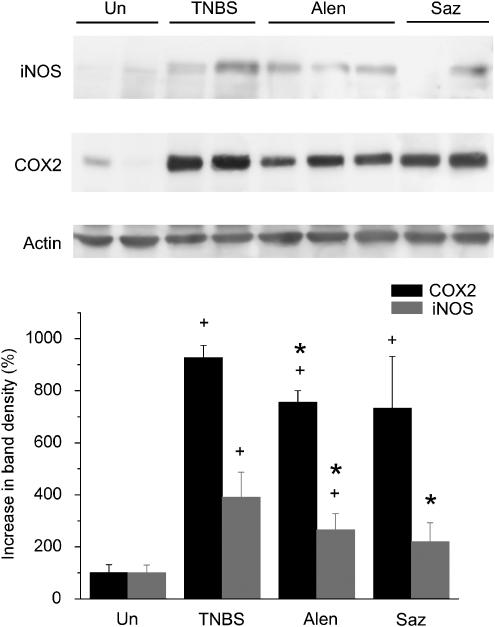
Colon inflammation was also measured biochemically. The results reveal that trinitrobenzenesulfonic acid (TNBS) colitis was associated with a significant increase in myeloperoxidase activity, a marker of neutrophil infiltration (not shown), as well as of alkaline phosphatase (AP) activity, recently proposed as an inflammatory marker (Table 2) (Sanchez de Medina et al., 2004). In addition, the expression of COX-2 and iNOS, as assessed by Western blot, was significantly upregulated compared to the uninflamed group (Figure 2).
Figure 2.
Colonic protein expression of COX-2 (70 kilodaltons) in the different experimental groups. Uninflamed: noncolitic group; TNBS: colitic animals treated with vehicle; alendronate: colitic animals treated with alendronate (25 mg kg−1 day−1); sulphasalazine: colitic animals treated with sulphasalazine (500 mg kg−1 day−1). Expression was assessed by Western blot and densitometry was performed with the Scion Image software. A representative gel is shown. +P<0.05 vs uninflamed group; *P<0.05 vs TNBS group.
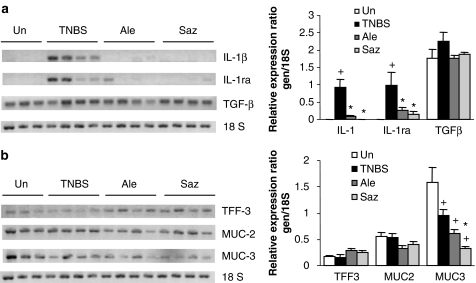
Further examination of the inflammatory status revealed that the mRNA levels of IL-1β and IL-1ra were upregulated by the TNBS challenge (Figure 3). IL-1β is a potent proinflammatory cytokine, whereas IL-1 receptor antagonist (IL-1ra) is a naturally occurring antagonist; both are expressed in the mucosa of patients with IBD (Hannum et al., 1990; Stokkers et al., 1998). The TGF-β transcript levels were higher in the TNBS than in the uninflamed group, but the difference did not reach statistical significance. On the other hand, MUC2 levels did not change significantly after colitic induction, whereas MUC3 levels were decreased. Finally, the colonic expression of TFF3, a peptide produced by goblet cells with important cytoprotective functions in the gut, was also measured, but no significant changes were detected (Figure 3).
Figure 3.
Effect of alendronate on IL-1β, IL-1ra and TGF-β (a), MUC2, MUC3 and TFF-3 (b) mRNA levels in colon specimens. Representative gels (of at least triplicate experiments) and densitometric analysis are shown. Uninflamed: noncolitic group; TNBS: colitic animals treated with vehicle; alendronate: colitic animals treated with alendronate (25 mg kg−1 day−1); sulphasalazine: colitic animals treated with sulphasalazine (500 mg kg−1 day−1). Expression was assessed by semiquantitative RT-PCR and densitometry was performed with the Scion Image software. Bars represent mean±s.e.m.; +P<0.05 vs uninflamed group, *P<0.05 vs TNBS group.
Effect of sulphasalazine on TNBS colitis
Sulphasalazine was included as a standard established drug in the treatment of IBD for comparison purposes. This drug has been previously shown to be efficacious in TNBS colitis (Daddaoua et al., 2005; Woodruff et al., 2005). Our data show that sulphasalazine had a beneficial impact administered as a postreatment in TNBS colitis. Sulphasalazine treated rats exhibited a significant decrease in the colonic AP activity compared to non-treated rats (Table 2), while IL-1β mRNA levels were normalized (Figure 3). On the other hand, there was no significant change in colonic weight to length ratio, damage score, body weight, myeloperoxidase, COX2, MUC2 or TGF-β levels (Table 2, Figures 2 and 3, and data not shown). The effect on iNOS was significant but of low magnitude. IL-1ra levels were strongly reduced (but less than those of IL-1β), and MUC3 was downregulated compared with non-treated animals (Figure 3). TFF-3 expression was slightly higher in sulphasalazine-treated rats, but without reaching the statistical threshold for significance.
Effect of alendronate on TNBS colitis
Oral treatment of rats with alendronate (25 mg kg−1 day−1) reduced body weight loss when compared with untreated colitic rats (Table 2). Postmortem evaluation of the colon showed a significant improvement, which resulted in a reduction of tissue damage score by approximately 33% in comparison with non-treated colitic animals (Table 2). The photographs in Figure 1 show the features of TNBS inflammation, including wall thickness and colon length shortening. Amelioration of these parameters was observed after alendronate treatment, which resulted in decreased colonic damage score (Table 2).
The intestinal protective effect exerted by alendronate was associated with a significant, though slight, inhibition of COX-2 and iNOS protein expression in the colon (Figure 2) when compared to the TNBS group. In addition, alendronate was associated with a dramatic decrease in colonic IL-1β and IL-1ra expression (Figure 3). The extent of the inhibitory effect was comparable to that of sulphasalazine treated rats. Colonic TFF3 mRNA was higher in the alendronate group than in the TNBS group but this difference did not reach statistical difference. On the other hand, no significant differences were detected in the expression levels of TGF-β, MUC2 or MUC3 compared to the TNBS group (Figure 3).
Effect of higher dose of alendronate (Fosamax) on TNBS colitis
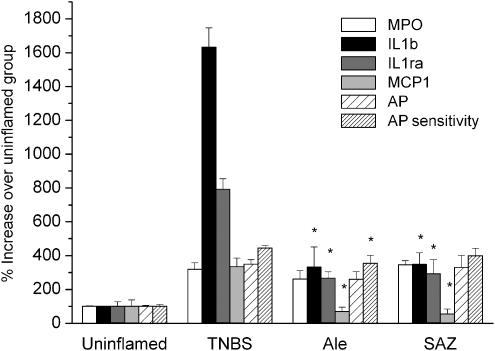
The aim of this second set of experiments was twofold. First, to obtain new samples for further determinations, including immunohistochemical analysis. Second, to assess the effect of a higher dose of alendronate. Because of the short supply of alendronate available from chemical vendors we decided to use a commercial form, Fosamax, administered as a suspension of the powdered tablets in methylcellulose. This treatment resulted in an increased therapeutic effect on extension of necrosis and damage score (Table 3), while the impact on IL-1β was similar (Figure 4). There was also a striking reduction in monocyte chemoattractant protein 1 (MCP-1). Again, the effect of alendronate was similar or even higher than that of sulphasalazine, which failed to ameliorate colonic weight to length ratio or AP sensitivity, parameters that were significantly reduced with Fosamax. COX2 and iNOS protein levels were slightly reduced by both sulphasalazine and Fosamax, as in the first set of experiments (not shown). It should be noted that although the Fosamax excipients could not be added to the reference groups, they are unlikely to be involved in the pharmacological effect, because alendronate was active by itself in the first experiment.
Table 3.
Effect of Fosamax (containing alendronate) on macroscopic parameters in rat TNBS colitis
| Colonic weight/length ratio (mg cm−1) | Diarrhea score | Extension of necrosis (cm) | Damage score | Body weight gain after 5 days | |
|---|---|---|---|---|---|
| Uninflamed | 65.4±2.2 | 0 | 0 | 0 | 4.9±0.5 |
| TNBS | 155.0±14.2+ | 2.0±0.4+ | 3.5±0.4+ | 9.4±0.7+ | −7.2±1.5+ |
| Alendronate | 125.4±15.6+* | 1.4±0.3+ | 2.1±0.7+* | 5.5±1.2+* | −12.0±1.9+* |
| Sulphasalazine | 141.5±5.7+ | 1.7±0.2+ | 3.5±0.5+ | 8.8±0.4+ | −11.6±0.8+* |
Abbreviations: TNBS, trinitrobenzenesulfonic acid.
The doses of Fosamax (containing alendronate) and sulphasalazine were 75 and 500 mg kg−1 day−1, respectively. Values are means±s.e.m., n=7.
Different from Control group, P<0.05.
Different from TNBS group, P<0.05.
Body weight gain is expressed as percentage change from the start of the experiment.
Figure 4.
Effect of alendronate (Fosamax) on TNBS colitis. Control values were: 2.6±0.1 U g−1 tissue (myeloperoxidase; MPO), 83.4±4.5 mU mg−1 protein (AP), 16.5±1.9% (AP sensitivity). TNBS: vehicle-treated colitic rats; SAZ: colitic rats treated with sulphasalazine (500 mg kg−1); Ale: colitic rats treated with alendronate (75 mg kg−1). *Different from TNBS group, P<0.05. All means in the TNBS group are significantly different from the control (not shown).
The histological analysis (Figure 5) showed that the majority of the TNBS control animals presented more than 50% of epithelial surface ulceration at the edge of the affected region, generally associated with marked transmural infiltration by inflammatory cells. The infiltrate was composed of neutrophils in the lamina propria, whereas the submucosa and muscle layers presented also eosinophils, lymphocytes and plasma cells. Submucosal and specially muscularis oedema contributed slightly to bowel wall thickening (scored as 1.6±0.3). The histological score was 16.3±3.9. In the sulphasalazine group there was only focal ulceration or even complete absence thereof, except in two rats, which were comparable to the TNBS group. However, the histological score was not decreased significantly (10.4±3.1). This may be related to the high value assigned to mucosal hyperplasia and goblet cell depletion, which usually precede mucosal healing and thus might be interpreted as signs of tissue recovery. This uneven effect of therapy was also observed in the alendronate group, in which three of six rats exhibited focal or no ulceration and low scores, while the other three rats were similar to those of the TNBS group. As a result the histological score, although lower than the TNBS group (13.7±3.3), was not altered significantly. Both sulphasalazine and alendronate reduced muscularis oedema significantly (0.7±0.3 and 0.7±0.2 respectively, P<0.05 vs. TNBS).
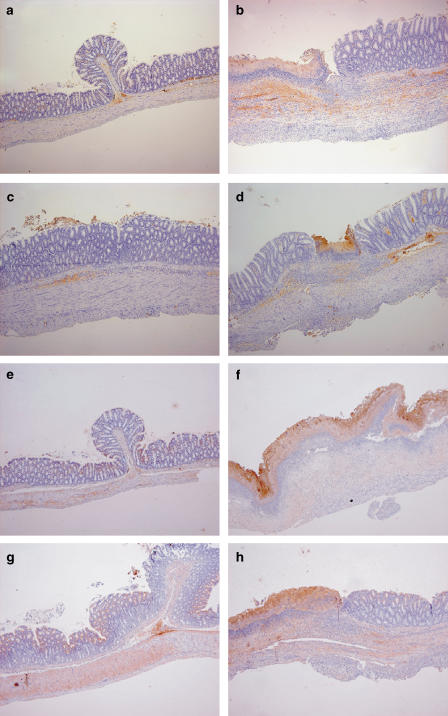
Figure 5.
Immunohistochemical analysis of the effect of alendronate (Fosamax) on rat TNBS colitis. A piece of the distal colon was fixed in formaldehyde and stained with antibodies specific for either COX2 (a–d) or iNOS (e–h). The images are representative of the groups of control rats (a, e) and TNBS colitic rats treated with vehicle (b, f), sulphasalazine 500 mg kg−1 (c, g) or alendronate 75 mg kg−1 (d, h). Original magnification: × 5.
Immunohistochemical analysis revealed that COX2 immunoreactivity was restricted to lymphoplasmacytic cells in all cases, although markedly enhanced by TNBS colitis. Treatment with either alendronate or sulphasalazine resulted in lower COX2 levels. On the other hand, iNOS, which appeared localized mainly to the epithelium and secondarily to the muscle layer, was also increased in TNBS colitic animals, but largely unaffected by both drug treatments.
Effects of intraperitoneally administered alendronate
The aim of this set of experiments was to test the influence of the route of administration in the effect of alendronate. The dose selected was relatively high, considering the low oral bioavailability of this drug (Lin et al., 1994; Porras et al., 1999). Interestingly, this approach blunted the therapeutic impact of alendronate and actually resulted in a worse clinical outcome, as reflected by the increased body weight loss and extension of necrosis (Table 4). The other parameters examined were not changed.
Table 4.
Effect of intraperitoneally administered alendronate on macroscopic and biochemical parameters in rat TNBS colitis
| Weight to length ratio (mg cm−1) | Extension of necrosis (cm) | Damage score | Body weight gain after 5 days | MPO (U g−1) | AP (mU mg protein−1) | |
|---|---|---|---|---|---|---|
| Uninflamed | 67.5±2.6 | 0 | 0 | 3.0±0.6 | 2.6±0.2 | 53.0±2.7 |
| TNBS | 158.8±27.8+ | 3.6±0.7+ | 7.0±1.6+ | −4.3±3.3+ | 40.2±6.7+ | 104.6±11.1+ |
| Alendronate | 124.7±6.6+ | 4.1±0.3+* | 7.0±0.6+ | −16.3±1.4+* | 46.8±6.4+ | 98.6±7.4+ |
Abbreviations: TNBS, trinitrobenzenesulfonic acid.
The dose of alendronate was 10 mg kg−1 day−1. Values are means±s.e.m., n=7.
Different from control group, P<0.05.
Different from TNBS group, P<0.05.
Body weight gain is expressed as percentage change from the start of the experiment.
Discussion
IBD, comprising Crohn's disease and ulcerative colitis, poses a clear challenge to the biomedical community in terms of both basic and applied research. Thus despite the intense investigative effort dedicated to IBD in the past few years, a causative factor is yet to be identified. Thus the pharmacological strategies are necessarily empirical and are common to a number of inflammatory disorders. Although these treatments are generally effective, they have significant adverse effects and refractoriness is not infrequent.
On the other hand, IBD is a predisposing factor to osteoporosis, and high rates of reduced bone mineral density are reported in patients with IBD (Bernstein and Leslie, 2005). The risk is increased in the elderly and in underweight patients, as well as in those treated with corticoids, although it should be noted that the overall increase in fractures is relatively low. The approach to fracture prevention in IBD patients should be applied to the patients showing the highest risk and comprises removal of systemic corticoids, supplementation with vitamin D and calcium, weight-bearing exercise and treatment with bisphosphonates (Bernstein and Leslie, 2005). The second-generation (nitrogen containing) bisphosphonates, including alendronate and risedronate, are widely used for the prevention and treatment of osteoporosis and related disorders (Licata, 2005). Alendronate (Haderslev et al., 2000), ibandronate (von Tirpitz et al., 2003) and pamidronate (Bartram et al., 2003) have been employed successfully to increase bone mineral density in IBD.
Although incompletely characterized, the mechanism of action of nitrogen containing bisphosphonates is related to direct inhibition of farnesyl diphosphate synthase in the cholesterol biosynthetic pathway. This results in diminished protein geranylgeranylation, which is essential for the basic cellular processes required for osteoclastic bone resorption (Reszka and Rodan, 2003; Green, 2004; Rogers, 2004; Licata, 2005). In addition, nitrogen containing bisphosphonates may induce osteoclast apoptosis, but this action is not necessary for their inhibition of bone resorption. This mechanism is similar to that of the hypocholesterolemic agents (statins), which inhibit 3-hydroxy-3-methylglutaryl coenzyme A reductase but ultimately also reduce protein prenylation (Stancu and Sima, 2001). In fact, this property of statins accounts for the wide spectrum of pharmacological actions ascribed to this drug group, which includes both anti-inflammatory (Shovman et al., 2002) and anti-osteoporotic effects (Yaturu, 2003), although clinical evidence of the latter is still controversial (Koida et al., 2004; Gonyeau, 2005). Pravastatin has been specifically shown to be effective in rat experimental colitis induced by dextran sulphate sodium (Sasaki et al., 2003). Therefore, bisphosphonates may have beneficial effects in the gut. We selected alendronate for testing in one of the most widely used models of IBD, namely TNBS colitis (Neurath et al., 2000).
Our data demonstrate that alendronate is effective in reducing the severity of the colonic inflammatory reaction, when administered after induction of colitis, as assessed by reduced body weight loss, colonic damage score, IL-1β, MCP-1, COX2 and iNOS expression. IL-1β is one of the predominant cytokines in rat TNBS colitis and it is expressed at higher levels than tumor necrosis factor or interferon-γ, whereas MCP-1 is an important monocyte chemoattractant and activator. Both are increased in IBD (Banks et al., 2003; Ludwiczek et al., 2004). The effects on COX2 and iNOS are comparatively minor and are probably of lesser importance. The biological activity of IL-1β is in part regulated by the endogenous inhibitor IL-1ra, which specifically inhibits IL-1 activities by binding to IL-1 receptors, but does not display agonist activity (Hawthorne et al., 1992). In the intestinal mucosa, epithelial cells and lamina propria mononuclear cells are the major sources of IL-1ra. An imbalance between the production of IL-1β and IL-1ra has been described in freshly isolated intestinal mucosal cells and in colonic mucosal biopsies obtained from inflamed intestinal tissue of IBD patients (Vilaseca et al., 1990a). Although upregulation of IL-1ra seems to be an appropriate response to control IL-1-mediated inflammation, this response is not sufficient. A large excess of IL-1ra is required to block the binding of IL-1 to the IL-1 type I receptor (Rask-Madsen et al., 1992).
It should be noted that these effects were attained with a presumably rather low effective dose, because oral bioavailability of alendronate, as with other bisphosphonates, is around 1% only (Lin et al., 1994; Porras et al., 1999). Thus the amount of alendronate that reaches systemic circulation may be as low as 0.25–0.75 mg kg−1. Previous pharmacokinetic studies indicate that alendronate is preferentially distributed to the bone or cleared by the kidney (Lin et al., 1994; Porras et al., 1999). It should be noted nonetheless that the dose is relatively high compared with the standard 10 mg used in humans. In an attempt to increase bioavailability, we performed an additional experiment in which alendronate was administered as a post-treatment by the i.p. route with a reduced dose (10 mg kg−1 day−1). This approach did not result in significant anti-inflammatory activity, but instead produced a marked loss of body weight, suggesting toxicity. This clearly suggests that the therapeutic effect of oral alendronate may be owing to a direct effect on the mucosal tissue by the drug as it reaches the colon. This may explain the lack of anti-inflammatory effect observed previously with subcutaneously administered pamidronate in TNBS colits although still exerting significant effects on the bone (Lin et al., 2000). Thus it appears that low intestinal absorption favors the anti-inflammatory effect of alendronate in the distal region of the intestine, although it is plausible that a higher local uptake would result in an increased therapeutic benefit.
The protective effect of alendronate was comparable to that of sulphasalazine, a standard drug used in IBD treatment (Sands, 2000). Alendronate was better in terms of body weight gain, colonic damage score and COX2 expression, whereas sulphasalazine was the only one that reduced colonic alkaline phosphatase activity, a marker of intestinal inflammation related to both leukocyte infiltration and epithelial phenotypic changes (Sanchez de Medina et al., 2004). Interestingly, both drug treatments tended to increase TFF3 and decrease MUC2 despite the lack of changes brought about by inflammation. The increase in TFF3 might represent a mechanism of anti-inflammatory action, as this peptide has mucosal protective and regenerative properties, which ultimately result in intestinal antiinflammatory effects (Vandenbroucke et al., 2004; Poulsen et al., 2005). The relatively modest effect of sulphasalazine must be interpreted in terms of the dosing protocol applied, that is post-treatment rather than pre-treatment. It is well known that drug treatments, including sulphasalazine, are generally less effective when given after colitis has been induced than when administered as a preventive measure (Daddaoua et al., 2005). This in turn highlights the positive results obtained with alendronate. As sulphasalazine is considered to act by mechanisms unrelated to farnesyl diphosphate synthase inhibition (Nikolaus et al., 2000), it is conceivable that it may have synergistic effects with alendronate.
In conclusion, we have demonstrated that alendronate is effective in the TNBS model of rat colitis, a widely employed preclinical model of IBD, with an activity comparable to that of sulphasalazine. Our results suggest that IBD patients under risk of osteoporotic fractures could additionally benefit from bisphosphonate therapy. Additional experiments are warranted to establish the mechanistic basis of alendronate effect. On the other hand, pharmacokinetic manipulation might provide more pronounced therapeutic effects of alendronate by increasing access of the colonic mucosa to the drug.
Acknowledgments
We thank the technical assistance of Dr Mercedes González. This study was supported by grants of the Instituto de Investigación Carlos III (PI051651 and PI051625). Olga Martínez Augustin was funded by the Ramón y Cajal program and the I3 program of the Ministry of Education and Science. Isabel Ballester and Rocío López-Posadas are the recipients of fellowships by the Fundación Ramón Areces and the Spanish Ministry of Education and Science, respectively.
Abbreviations
- COX2
cyclooxygenase 2
- IL-1β
interleukin 1β
- IL-Ira
interleukin 1 receptor antagonist
- IBD
inflammatory bowel disease
- iNOS
inducible nitric oxide synthase
- TNBS
trinitrobenzenesulfonic acid
- TGF-β
transforming growth factor-β
- TFF3
trefoil factor 3
Conflict of interest
The authors state no conflict of interest.
References
- Abeles AM, Pillinger MH. Statins as antiinflammatory and immunomodulatory agents: a future in rheumatologic therapy. Arthritis Rheum. 2006;54:393–407. doi: 10.1002/art.21521. [DOI] [PubMed] [Google Scholar]
- Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn's disease. J Pathol. 2003;199:28–35. doi: 10.1002/path.1245. [DOI] [PubMed] [Google Scholar]
- Bartram SA, Peaston RT, Rawlings DJ, Francis RM, Thompson NP. A randomized controlled trial of calcium with vitamin D, alone or in combination with intravenous pamidronate, for the treatment of low bone mineral density associated with Crohn's disease. Aliment Pharmacol Ther. 2003;18:1121–1127. doi: 10.1111/j.1365-2036.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- Bernstein CN, Leslie WD. Therapy insight: Osteoporosis in inflammatory bowel disease – advances and retreats. Nat Clin Pract Gastroenterol Hepatol. 2005;2:232–239. doi: 10.1038/ncpgasthep0169. [DOI] [PubMed] [Google Scholar]
- Castells A, Paya A, Alenda C, Rodriguez-Moranta F, Agrelo R, Andreu M, et al. Cyclooxygenase 2 expression in colorectal cancer with DNA mismatch repair deficiency. Clin Cancer Res. 2006;12:1686–1692. doi: 10.1158/1078-0432.CCR-05-1581. [DOI] [PubMed] [Google Scholar]
- Coxon FP, Thompson K, Rogers MJ. Recent advances in understanding the mechanism of action of bisphosphonates. Curr Opin Pharmacol. 2006;6:307–312. doi: 10.1016/j.coph.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Daddaoua A, Puerta V, Zarzuelo A, Suarez MD, Sanchez de Medina F, Martinez-Augustin O. Bovine glycomacropeptide is anti-inflammatory in rats with hapten-induced colitis. J Nutr. 2005;135:1164–1170. doi: 10.1093/jn/135.5.1164. [DOI] [PubMed] [Google Scholar]
- Gonyeau MJ. Statins and osteoporosis: a clinical review. Pharmacotherapy. 2005;25:228–243. doi: 10.1592/phco.25.2.228.56954. [DOI] [PubMed] [Google Scholar]
- Green JR. Bisphosphonates: preclinical review. The Oncologist. 2004;9 Suppl 4:3–13. doi: 10.1634/theoncologist.9-90004-3. [DOI] [PubMed] [Google Scholar]
- Haderslev KV, Tjellesen L, Sorensen HA, Staun M. Alendronate increases lumbar spine bone mineral density in patients with Crohn's disease. Gastroenterology. 2000;119:639–646. doi: 10.1053/gast.2000.16518. [DOI] [PubMed] [Google Scholar]
- Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, et al. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Hawthorne AB, Daneshmend TK, Hawkey CJ, Belluzzi A, Everitt SJ, Holmes GK, et al. Treatment of ulcerative colitis with fish oil supplementation: a prospective 12 month randomised controlled trial. Gut. 1992;33:922–928. doi: 10.1136/gut.33.7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koida M, Fukuyama R, Nakamuta H. Osteoporosis requires bone-specific statins. Curr Pharm Des. 2004;10:2605–2613. doi: 10.2174/1381612043383827. [DOI] [PubMed] [Google Scholar]
- Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- Licata AA. Discovery, clinical development, and therapeutic uses of bisphosphonates. Ann Pharmacother. 2005;39:668–677. doi: 10.1345/aph.1E357. [DOI] [PubMed] [Google Scholar]
- Lin CL, Moniz C, Chow JW. Treatment with fluoride or bisphosphonates prevents bone loss associated with colitis in the rat. Calcif Tissue Int. 2000;67:373–377. doi: 10.1007/s002230001162. [DOI] [PubMed] [Google Scholar]
- Lin JH, Chen IW, Deluna FA. On the absorption of alendronate in rats. J Pharm Sci. 1994;83:1741–1746. doi: 10.1002/jps.2600831218. [DOI] [PubMed] [Google Scholar]
- Ludwiczek O, Vannier E, Borggraefe I, Kaser A, Siegmund B, Dinarello CA, et al. Imbalance between interleukin-1 agonists and antagonists: relationship to severity of inflammatory bowel disease. Clin Exp Immunol. 2004;138:323–329. doi: 10.1111/j.1365-2249.2004.02599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathoo JM, Cranney A, Papaioannou A, Adachi JD. Rational use of oral bisphosphonates for the treatment of osteoporosis. Curr Osteoporos Rep. 2004;2:17–23. doi: 10.1007/s11914-004-0010-6. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Fina D, Caruso R, Pallone F. New mediators of immunity and inflammation in inflammatory bowel disease. Curr Opin Gastroenterol. 2006;22:361–364. doi: 10.1097/01.mog.0000231808.10773.8e. [DOI] [PubMed] [Google Scholar]
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Neurath M, Fuss I, Strober W. TNBS-colitis. Int Rev Immunol. 2000;19:51–62. doi: 10.3109/08830180009048389. [DOI] [PubMed] [Google Scholar]
- Nikolaus S, Folscn U, Schreiber S. Immunopharmacology of 5-aminosalicylic acid and of glucocorticoids in the therapy of inflammatory bowel disease. Hepatogastroenterology. 2000;47:71–82. [PubMed] [Google Scholar]
- Porras AG, Holland SD, Gertz BJ. Pharmacokinetics of alendronate. Clin Pharmacokinet. 1999;36:315–328. doi: 10.2165/00003088-199936050-00002. [DOI] [PubMed] [Google Scholar]
- Poulsen SS, Kissow H, Hare K, Hartmann B, Thim L. Luminal and parenteral TFF2 and TFF3 dimer and monomer in two models of experimental colitis in the rat. Regul Pept. 2005;126:163–171. doi: 10.1016/j.regpep.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen J, Bukhave K, Laursen LS, Lauritsen K. 5-Lipoxygenase inhibitors for the treatment of inflammatory bowel disease. Agents Actions. 1992;36 Suppl 1:C37–C46. [PubMed] [Google Scholar]
- Reszka AA, Rodan GA. Mechanism of action of bisphosphonates. Curr Osteoporos Rep. 2003;1:45–52. doi: 10.1007/s11914-003-0008-5. [DOI] [PubMed] [Google Scholar]
- Rogers MJ. From molds and macrophages to mevalonate: a decade of progress in understanding the molecular mode of action of bisphosphonates. Calcif Tissue Int. 2004;75:451–461. doi: 10.1007/s00223-004-0024-1. [DOI] [PubMed] [Google Scholar]
- Sanchez de Medina F, Galvez J, Romero JA, Zarzuelo A. Effect of quercitrin on acute and chronic experimental colitis in the rat. J Pharmacol Exp Ther. 1996;278:771–779. [PubMed] [Google Scholar]
- Sanchez de Medina F, Martinez-Augustin O, Gonzalez R, Ballester I, Nieto A, Galvez J, et al. Induction of alkaline phosphatase in the inflamed intestine: a novel pharmacological target for inflammatory bowel disease. Biochem Pharmacol. 2004;68:2317–2326. doi: 10.1016/j.bcp.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Sands BE. Therapy of inflammatory bowel disease. Gastroenterology. 2000;118:S68–S82. doi: 10.1016/s0016-5085(00)70007-2. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Bharwani S, Jordan P, Joh T, Manas K, Warren A, et al. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor pravastatin reduces disease activity and inflammation in dextran-sulfate induced colitis. J Pharmacol Exp Ther. 2003;305:78–85. doi: 10.1124/jpet.102.044099. [DOI] [PubMed] [Google Scholar]
- Shovman O, Levy Y, Gilburd B, Shoenfeld Y. Antiinflammatory and immunomodulatory properties of statins. Immunol Res. 2002;25:271–285. doi: 10.1385/IR:25:3:271. [DOI] [PubMed] [Google Scholar]
- Siminovitch KA. Advances in the molecular dissection of inflammatory bowel disease. Semin Immunol. 2006;18:244–253. doi: 10.1016/j.smim.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5:378–387. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkers PC, Van Aken BE, Basoski N, Reitma PH, Tytgat GN, Van Deventer SJ. Five genetic markers in the interleukin 1 family in relation to inflammatory bowel disease. Gut. 1998;43:33–39. doi: 10.1136/gut.43.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucchi AF, Shofer S, Leeman S, Materne O, Beer E, McClung J, et al. NK-1 antagonist reduces colonic inflammation and oxidative stress in dextran sulfate-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1298–G1306. doi: 10.1152/ajpgi.2000.279.6.G1298. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Vermeire S, Rutgeerts P. Medical treatment of inflammatory bowel diseases. Curr Opin Gastroenterol. 2005;21:443–447. [PubMed] [Google Scholar]
- Vandenbroucke K, Hans W, Van Huysse J, Neirynck S, Demetter P, Remaut E, et al. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology. 2004;127:502–513. doi: 10.1053/j.gastro.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Vilaseca J, Salas A, Guarner F, Rodriguez R, Malagelada JR. Participation of thromboxane and other eicosanoid synthesis in the course of experimental inflammatory colitis. Gastroenterology. 1990a;98:269–277. doi: 10.1016/0016-5085(90)90814-h. [DOI] [PubMed] [Google Scholar]
- Vilaseca J, Salas A, Guarner F, Rodriguez R, Martinez M, Malagelada JR. Dietary fish oil reduces progression of chronic inflammatory lesions in a rat model of granulomatous colitis. Gut. 1990b;31:539–544. doi: 10.1136/gut.31.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Tirpitz C, Klaus J, Steinkamp M, Hofbauer LC, Kratzer W, Mason R, et al. Therapy of osteoporosis in patients with Crohn's disease: a randomized study comparing sodium fluoride and ibandronate. Aliment Pharmacol Ther. 2003;17:807–816. doi: 10.1046/j.1365-2036.2003.01448.x. [DOI] [PubMed] [Google Scholar]
- Woodruff TM, Arumugam TV, Shiels IA, Newman ML, Ross PA, Reid RC, et al. A potent and selective inhibitor of group IIa secretory phospholipase A2 protects rats from TNBS-induced colitis. Int Immunopharmacol. 2005;5:883–892. doi: 10.1016/j.intimp.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Yaturu S. Skeletal effects of statins. Endocr Pract. 2003;9:315–320. doi: 10.4158/EP.9.4.315. [DOI] [PubMed] [Google Scholar]