Abstract
Background and purpose:
Studies in vitro suggest that the standardised extract of Ginkgo biloba, EGb-761 has anti-inflammatory properties and modulatory effects on key pain-related molecules. This study investigated the analgesic and anti-inflammatory effects of EGb-761 on carrageenan-induced inflammatory and hindpaw incisional pain.
Experimental approach:
Adult male Wistar rats (n=6-10/group; 250-420 g) were injected intradermally with carrageenan into the left hindpaw or anaesthetised with isoflurane (2%) and a longitudinal 1 cm incision was made through the skin, fascia and plantaris muscle of the hindpaw. EGb-761 (3, 10, 30, 100 or 300 mg kg−1), diclofenac (5 mg kg−1) or drug-vehicle was administered 3 h post-carrageenan/post-surgery. Hindpaw withdrawal latency (in seconds) to thermal stimulation, response threshold (in grams) to mechanical stimulation and paw volume were measured.
Key results:
Carrageenan induced significant mechanical allodynia, thermal hyperalgesia and paw oedema at 6 h post-carrageenan, while paw incision surgery induced significant mechanical allodynia and thermal hyperalgesia at 6 and 24 h post-surgery. Administration of EGb-761 dose-dependently inhibited thermal hyperalgesia and was equally effective as diclofenac (5 mg kg−1) in both the carrageenan and hindpaw incision model. EGb-761 had no effect on carrageenan- or incision-induced mechanical allodynia or paw oedema. Diclofenac significantly reduced mechanical allodynia in both models and carrageenan-induced paw oedema.
Conclusions and Implications:
EGb-761 dose-dependently alleviates acute inflammatory and surgically induced thermal hyperalgesia and is comparable to diclofenac, a commonly prescribed non-steroidal anti-inflammatory drug. This indicates that EGb-761 has analgesic potential in acute inflammatory pain.
Keywords: inflammation, analgesia, mechanical allodynia, thermal hyperalgesia, Ginkgo biloba, post-surgical pain
Introduction
Medicinal herbs are commonly used to treat a variety of illnesses with little or no understanding of their mechanism of action. Pharmacological assessment of these herbs is often made difficult as typically extracts are not standardized or may contain multiple constituents that induce several effects and interact in a synergistic, antagonistic or additive way (Maclennan et al., 2002). A patented extract derived from the leaves of the Ginkgo biloba tree, EGb-761, is commonly used in dietary supplements for ailments, such as peripheral arterial disease, Alzheimer's disease, short-term memory loss, depressed mood and anxiety (Kleijnen and Knipschild, 1992; Curtis-Prior et al., 1999; Watanabe et al., 2001). In Germany, EGb-761 has been approved to treat cerebral insufficiency in the elderly (marketed as Tebidon by Schwabe Pharmaceuticals, Germany).
Recent evidence suggests that this extract may have neuromodulatory effects in the central nervous system. Mice given food supplemented with EGb-761 for 4 weeks showed transcriptional alterations in transthyretin, AMPA-2 receptor channels and neuronal tyrosine/theonine phosphatase 1 mRNA in hippocampus and cortex, all of which are thought to have neuroprotective roles (Watanabe et al., 2001). Ginkgo biloba has also been reported to have anti-inflammatory properties mediated through modulation of key pain-related signalling molecules, such as cyclooxygenase-2 (COX-2), tumour necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS) and nitric oxide (NO). For instance, studies in vitro using lipopolysaccharide (LPS) stimulated RAW264.7 macrophage cells have demonstrated that pretreatment with EGb-761 dose-dependently inhibits TNF-α, NO release and iNOS protein expression (Wadsworth and Koop, 2001a, 2001b). A dose-dependent decrease in NO and TNF-α concentration has also been demonstrated in serum samples in response to LPS challenge in rat (Wadsworth and Koop, 2001a, 2001b). In addition, treatment with a Ginkgo biloba extract, similar in composition to EGb-761, was reported to reduce carrageenan-induced hindpaw oedema and capsaicin induced hindpaw licking in rat (Abdel-Salam et al., 2004), indicating that such extracts may have anti-inflammatory and analgesic properties.
The aim of this study was to investigate the therapeutic effects of EGb-761 on pain and inflammation. Specifically, this study set out to investigate the effects of oral administration of EGb-761 on hypersensitivity and inflammation in a model of acute inflammatory pain and in the hindpaw incision model of post-surgical pain in rat. Results show that systemic administration of EGb-761 dose-dependently inhibits carrageenan- and hindpaw incision-induced thermal hyperalgesia but not mechanical allodynia or carrageenan-induced paw oedema.
Methods
Animals
The study was approved by the Institute's Ethics and Welfare committee and all procedures were performed according to the UK Animal Scientific Procedures Act (1986). Animals were treated in accordance with the Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals as issued by the International Association for the study of Pain. Adult male Wistar rats (250–420 g) were used in these studies. Animals were bred in house and kept from birth in pairs and subjected to a light/dark cycle of 12 h with water and food available ad libitum.
Carrageenan model
Adult male Wistar rats (n=6–10 per group) received a 50 μl intradermal injection of carrageenan (3%) into the left hindpaw and responses to thermal and mechanical hindpaw stimulation and paw oedema were measured before (time 0) and 2, 4, 6 and 24 h post-carrageenan. EGb-761 (3, 10, 30, 100 and 300 mg kg−1), diclofenac (5 mg kg−1) or drug-vehicle (30% ethanol: 70% water) was administered orally via a gavage needle at 3 h after carrageenan. Distilled water (5 ml) was administered orally to a separate group of animals (n=3) to control for potential antinociceptive effects of vehicle treatment.
Hindpaw incision model
Adult male Wistar rats (n=6 per group) were anaesthetized with isoflurane (2%) and the incision site was sterilized using a 10% povidone–iodine solution before surgery. The plantar aspect of the left hindpaw was placed through a hole in a sterile paper drape and a longitudinal 1 cm incision was made through the skin, fascia and plantaris muscle of the hindpaw, as described by Brennan et al. (1996). The wound was closed using Nexabond surgical glue (Genusxpress, UK). The whole procedure took 15 min. Control animals were subjected to anaesthesia only (AO) for 15 min (n=7). Responses to thermal and mechanical stimulation of the hindpaws were taken before (time 0) and 2, 4, 6, 24, 48, 72 and 192 h (8 days) after surgery. EGb-761 (3, 10, 30, 100 and 300 mg kg−1), diclofenac (5 mg kg−1) or drug-vehicle (30% ethanol: 70% water) was administered orally via a gavage needle 3 h post-surgery. Distilled water (5 ml) was administered orally to a separate group of animals (n=3) to control for potential antinociceptive effects of vehicle treatment.
Hindpaw withdrawal responses to thermal and mechanical stimulation
One week before treatment animals underwent acclimatization to the testing environment by placing them in the behavioural testing boxes for 30–60 min twice daily. During this period, animals were habituated to the testing devices. Baseline response thresholds to thermal or mechanical stimulation of both hindpaws were taken on two occasions the day before treatment and then at time 0 (immediately before carrageenan injection/paw incision). A minimum of three readings were taken on each paw at each time point.
Animals were placed in an elevated transparent perspex box and thermal hyperalgesia measured using the Ugo Basile Plantar Test (Hargreaves test), which directs an infrared beam onto the plantar surface of the hindpaw, until a withdrawal reflex is evoked. As the rat withdraws its paw, the sudden drop in reflected energy stops the timer. Withdrawal latency is recorded (to the nearest 0.1 s). A cutoff of 25 s was applied in the absence of a response.
Two types of allodynia have been characterized after the type of stimulus that leads to their induction, dynamic allodynia elicited by light stroking, or static allodynia elicited by steadily applying gentle pressure (Ochoa and Yarnitsky, 1993), such as that applied by von Frey probes. Static allodynia was assessed in the present study using a dynamic plantar aesthesiometer (Ugo Basile, Italy). Animals were placed on an elevated wire mesh bottom cage and responses to punctate mechanical stimulation assessed using the aesthesiometer. The unit raises a straight metal filament (0.5 mm diameter) until it touches the plantar surface of the hindpaw and begins to exert an upwards force until the paw is withdrawn or the preset cutoff is reached (50 g). The force required to elicit a withdrawal responses is measured in grams.
Paw oedema
Paw oedema following carrageenan injection was measured using a plethsysmometer (Ugo Basile, Italy), which measures water displacement when the paw is submerged into a water cell and enables calculation of paw volume (cm3). The volume of both paws was measured and data represented as the percentage difference in volume between the ipsilateral and contralateral paws.
Statistical analysis
Data are represented as the means±standard error of the mean (s.e.m.). Threshold response data were analysed using two-way mixed factorial analysis of variance (ANOVA) (SPSS, v12, USA), where treatment is the between-subject factor and time post-carrageenan/surgery is the within-subjects (repeated measures) factor. Mean differences were calculated using simple effects analyses, applying Bonferroni adjustment factor for multiple comparisons. The maximum effect (Emax: represented as a percent according to the formula: (maximal decrease in withdrawal threshold after treatment−baseline withdrawal threshold)/baseline withdrawal threshold × 100), and time to maximum effect (Tmax) were also calculated for each animal as the maximum change in withdrawal threshold (after carrageenan or surgery) from baseline responses and the time of measurement of the Emax, respectively. These data were analysed using one-way ANOVA with post hoc Tukey's test (SPSS, v12).
Drugs
Carrageenan (λ-carrageenan type IV; Sigma, UK) was dissolved in saline to make a 3% solution 24 h before the start of each study and stored at 4°C. The standardized extract of Ginkgo biloba (EGb-761) was a gift from Dr Schwabe Pharmaceuticals, Germany. It was dissolved in (70% water: 30% ethanol) to give doses of 3, 10, 30, 100 and 300 mg kg−1 in a dose volume of 5 ml. EGb-761 was solubilized by heating and vortexing and stored at 4°C for 24 h before administration. The extract EGb-761 contains 24% flavone glycosides (quercetin; kaempferol; isorhamnetin) and 6% terpenoids (2.8–3.4% ginkgolides A, B and C; 2.6–3.2% bilobalide). Diclofenac (Sigma, UK) was dissolved in (70% water: 30% ethanol) to give a dose of 5 mg kg−1 in a dose volume of 5 ml.
Results
Carrageenan-induced hypersensitivity
Animals injected with carrageenan and treated with drug-vehicle displayed significant thermal hyperalgesia, mechanical allodynia and paw oedema in the injected paw. No change in thermal or mechanical responses or paw volume was observed in the contralateral non-injected paw at any point during the recording period. There was no significant difference in the magnitude or duration of thermal hyperalgesia, mechanical allodynia or paw oedema between drug-vehicle and water treatment groups (data not shown).
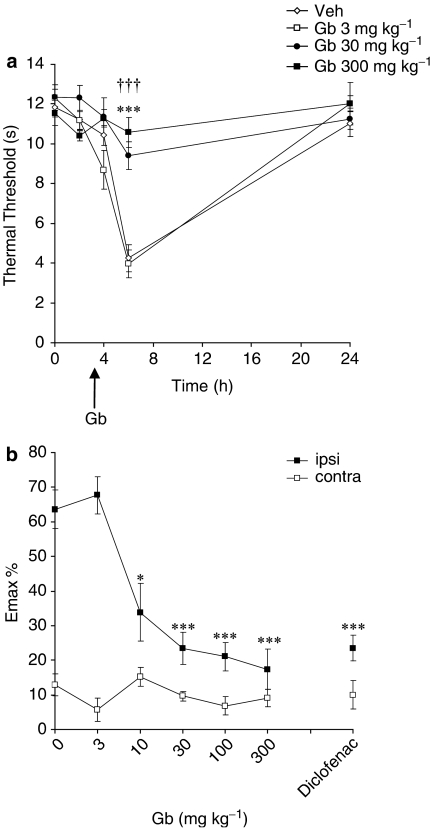
A maximum decrease in thermal response latency of 64±6% was observed in the carrageenan-injected paw at 6 h in animals treated with drug-vehicle (P<0.001 vs pre-carrageenan response latencies). Oral administration of EGb-761 significantly and dose dependently (10–300 mg kg−1) attenuated thermal hyperalgesia (Figure 1). Emax data revealed that 30, 100 and 300 mg kg−1 EGb-761 inhibited thermal hyperalgesia (all P<0.001 vs vehicle; Figure 1b) with similar efficacy to diclofenac (P<0.001 vs vehicle; Figure 1b). The lowest effective dose of EGb-761, 10 mg kg−1, also significantly attenuated thermal hyperalgesia at 6 h (P<0.05 vs vehicle). EGb-761 and diclofenac had no effect on thermal response latencies in the contralateral paw (Figure 1b).
Figure 1.
The effect of EGb-761 (Gb), or vehicle administered 3 h post-carrageenan on thermal hyperalgesia. (a) The mean thermal withdrawal latency (in seconds) measured in the ipsilateral hindpaw in adult male rats (n=7–10 per group). Responses were measured before, 2, 4, 6 and 24 h post-carrageenan. Arrow represents time of Gb administration. The results show that 30 mg kg−1 (†††) and 300 mg kg−1 (***) significantly attenuated hyperalgesia: P<0.001 vs vehicle. (b) The effect of all doses of EGb-761 tested (3, 10, 30, 100 and 300 mg kg−1), diclofenac (5 mg kg−1) or vehicle on thermal hyperalgesia in both hindpaws. Maximum change in response latency in the ipsilateral (ipsi) and contralateral (contra) paw post-carrageenan is represented as mean % change (Emax %) from the pre-carrageenan/baseline response thresholds. Significant attenuation of hyperalgesia: *P<0.05; ***P<0.001 vs vehicle.
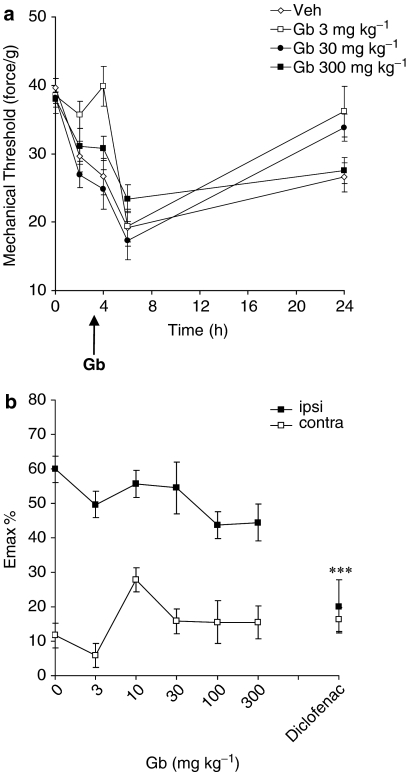
A maximum decrease in mechanical response threshold of 60±4% was observed in the ipsilateral paw at 6 h in animals treated with drug-vehicle (P<0.001 vs pre-carrageenan response thresholds; Figure 2). Oral administration of EGb-761 had no effect on carrageenan-induced mechanical allodynia (Figure 2). In contrast, diclofenac (5 mg kg−1) significantly inhibited mechanical allodynia (P<0.001 vs vehicle; Figure 2b). EGb-761 and diclofenac had no effect on mechanical response thresholds in the contralateral paw (Figure 2b).
Figure 2.
The effect of EGb-761 (Gb) or vehicle administered 3 h post-carrageenan on mechanical allodynia. (a) The mean mechanical threshold (in force/g) measured in the ipsilateral hindpaw in adult male rats (n=7–10). Responses were measured before, 2, 4, 6 and 24 h post-carrageenan. Arrow represents time of Gb administration. (b) The effect of all doses of EGb-761 (3, 10, 30 and 300 mg kg−1), diclofenac (5 mg kg−1) or vehicle on mechanical allodynia in both hindpaws. Maximum change in mechanical response threshold in the ipsilateral (ipsi) and contralateral (contra) paw post-carrageenan is represented as mean % change (Emax %) from the pre-carrageenan/baseline response thresholds. EGb-761 had no effect on carrageenan-induced allodynia. Significant attenuation of allodynia by diclofencac; ***P<0.001 vs vehicle.
Carrageenan-induced paw oedema
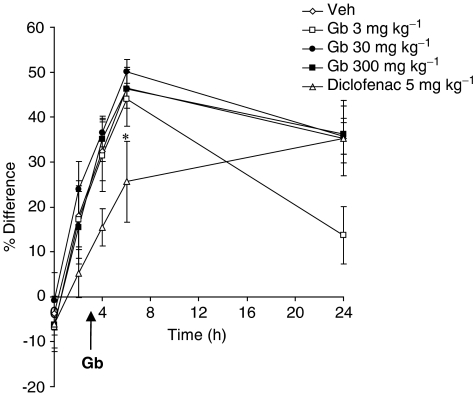
Animals injected with carrageenan and treated with vehicle displayed significant oedema in the ipsilateral paw 6 h post-carrageenan (P<0.05 vs pre-carrageenan values; Figure 3). Treatment with EGb-761 had no effect on carrageenan-induced paw oedema. In contrast, diclofenac reduced significantly paw oedema induced by carrageenan (P<0.05 vs vehicle; Figure 3).
Figure 3.
The effect of EGb-761 (Gb), diclofenac or vehicle administered 3 h post-carrageenan on paw oedema. Paw volume (cm3) was measured in the ipsilateral and contralateral hindpaws. Paw oedema is represented as the % difference between the ipsilateral and contralateral paw volume. Responses were measured before, 2, 4, 6 and 24 h post-carrageenan. EGb-761 had no effect on carrageenan-induced paw oedema. Significant reduction in paw oedema by diclofenac: *P<0.05.
Hindpaw incision-induced hypersensitivity
No change in mechanical or thermal responses was detected in animals subjected to AO at any time point during the observation period (Figures 4 and 5). Hindpaw incision induced thermal hyperalgesia (P<0.001 vs AO treated animals) and mechanical allodynia (P<0.001 vs AO treated animals) in the incised paw. No significant change in thermal or mechanical response thresholds was observed in the contralateral paw at any point during the recording period. There was no significant difference in hindpaw incision-induced thermal hyperalgesia or mechanical allodynia between drug-vehicle and water treatment groups (data not shown).
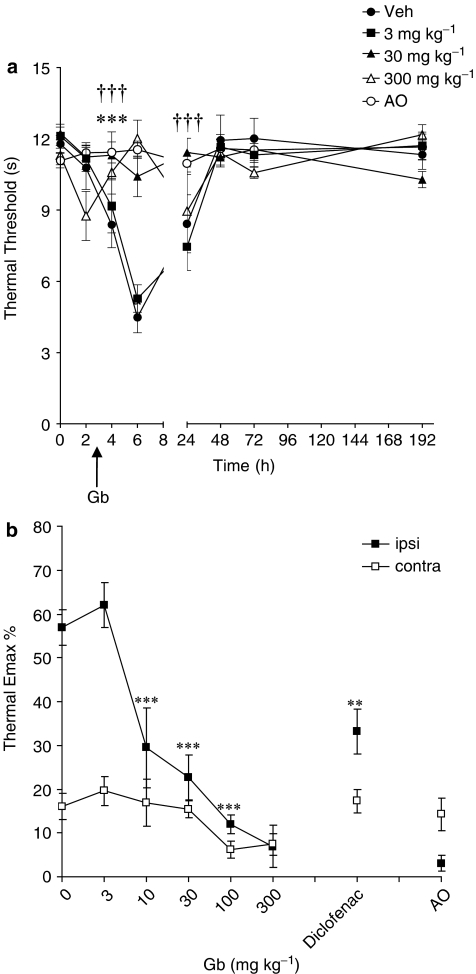
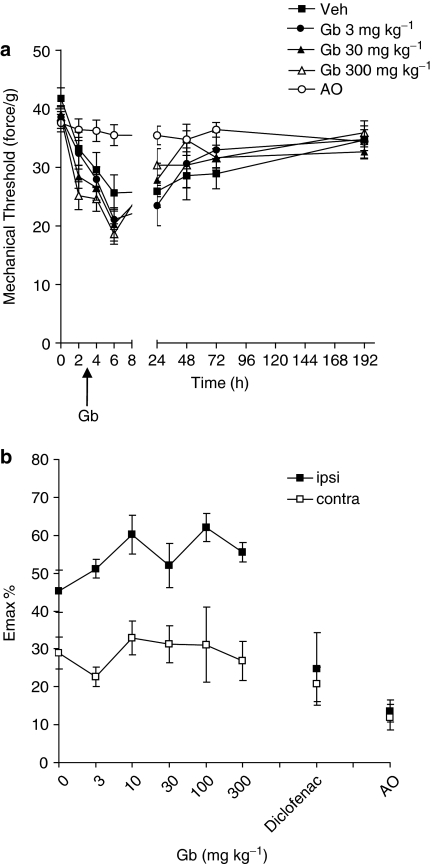
Figure 4.
The effect of EGb-761 (Gb) or vehicle administered 3 h post-surgery on thermal hyperalgesia. (a) The mean thermal withdrawal latency (in seconds) measured in the incised paw in adult male rats (n=6–7 per group). Responses were measured before, 2, 4, 6, 24, 48, 72 and 192 h post-surgery. 30 mg kg−1 (†††) and 300 mg kg−1 (***) significantly attenuated hyperalgesia: P<0.001 vs vehicle. (b) The effect of all doses of EGb-761 (3, 10, 30, 100 and 300 mg kg−1), diclofenac (5 mg kg−1) or vehicle on thermal hyperalgesia. Maximum change in thermal latency in the ipsilateral (ipsi) and contralateral (contra) paw post-surgery is represented as mean % change (Emax %) from the pre-surgery/baseline response thresholds. Significant attenuation of hyperalgesia: **P<0.01; ***P<0.001 vs vehicle. AO, anaesthesia only.
Figure 5.
The effect of EGb-761 (Gb) or vehicle administered 3 h post-surgery on mechanical allodynia. (a) The mean mechanical threshold (in force/g) measured in the ipsilateral hindpaw in adult male rats (n=7–10). Responses were measured before, 2, 4, 6, 24, 48, 72 and 192 h post-surgery. Arrow represents time of Gb administration. (b) The effect of all doses of EGb-761 (3, 10, 30, 100 and 300 mg kg−1), diclofenac (5 mg kg−1) or vehicle administered 3 h post-surgery on mechanical allodynia in both hindpaws. Maximum change in mechanical response threshold in the ipsilateral (ipsi) and contralateral (contra) paw post-surgery is represented as mean % change (Emax %) from the pre-surgery/baseline response thresholds. EGb-761 had no effect on paw incision-induced allodynia. AO, anaesthesia only.
Thermal hyperalgesia was maximal 6 h after surgery in drug-vehicle-treated animals (P<0.001 vs pre-surgery responses) and persisted until 24 h (P<0.05 vs pre-surgery responses). Oral administration of EGb-761 significantly and dose-dependently (10–300 mg kg−1) attenuated thermal hyperalgesia (Figure 4a). Emax data revealed that 10, 30, 100 and 300 mg kg−1 EGb-761-inhibited thermal hyperalgesia (all P<0.001 vs vehicle). Further analysis revealed that high-dose EGb-761 (100 and 300 mg kg−1) inhibited thermal hyperalgesia with greater effect than diclofenac (P<0.05 vs 100 and 300 mg kg−1 EGb-761; Figure 4b). The antihyperalgesic effects of 30 and 100 mg kg−1 EGb-761 and diclofenac were still evident at 24 h post-surgery (all P<0.05). EGb-761 and diclofenac had no effect on thermal response latencies in the contralateral paw (Figure 4b).
Mechanical allodynia was also maximal at 6 h in drug-vehicle-treated animals (P<0.05 vs pre-surgery responses) and persisted until 24 h (P<0.05 vs pre-surgery responses). EGb-761 had no effect on paw incision-induced mechanical allodynia (Figure 5). In contrast, diclofenac treatment significantly inhibited paw-incision induced mechanical allodynia at 4, 6 and 72 h (P<0.05 vs vehicle; data not shown). EGb-761 and diclofenac had no effect on mechanical response threshold in the contralateral paw (Figure 5b).
Discussion and conclusion
The present study demonstrates that systemic administration of the standardized Ginkgo biloba extract, EGb-761, dose-dependently inhibits thermal hyperalgesia induced by carrageenan or hindpaw incision, but not the mechanical allodynia or carrageenan-induced paw oedema. Of interest, EGb-761 was as effective as or better than, diclofenac (5 mg kg−1) in inhibiting thermal hyperalgesia, but not mechanical allodynia. This is the first report of EGb-761 reversing thermal hyperalgesia in models of acute inflammatory and post-incisional pain in rat.
There is little evidence in the literature of Gingko biloba having analgesic properties. A study by Abdel-Salam et al. (2004) reported that a Ginkgo biloba extract (50, 100 mg kg−1) reduced the time spent licking the hindpaw after capsaicin injection. This group also reported that this extract delays reaction time on the hot plate in normal animals. Studies carried out in this laboratory have failed to show any analgesic effect of EGb-761 on thermal or mechanical nociception in normal animals (unpublished observations). In the present study, EGb-761 did reverse thermal hyperalgesia but not mechanical allodynia in both pain models. Hyperalgesia and allodynia to tactile stimulation are classic symptoms for individuals with inflammatory and post-surgical pain (LaBuda et al., 2006). Tissue injury-induced hyperalgesia and allodynia are directly associated with an increase in sensitivity of peripheral nociceptor afferent terminals at the site of damage and with an activity-dependent increase in central neuronal excitability, resulting in peripheral and central sensitization (Woolf and Chong, 1993). As EGb-761 was administered systemically, the site of action cannot be determined. EGb-761 had no effect on paw oedema, indicating that it may act centrally to produce antihyperalgesic effects, although further studies are required to support this. The mechanism of action of Ginkgo biloba extracts have not been fully determined but there is considerable evidence to show that they block induction of iNOS and release of NO in in vitro and in vivo models (Wadsworth and Koop, 2001a, 2001b; Ilieva et al., 2004). NO is known to play a significant role in nociceptive processing in the spinal cord (Meller and Gebhart 1993). For instance, Meller et al. (1994) demonstrated that intrathecal administration of the NO inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) reversed carrageenan-induced thermal hyperalgesia but not mechanical allodynia. In models of neuropathic pain, intrathecal administration of L-NAME was also reported to block thermal hyperalgesia (Salter et al., 1996), whereas administration of a NO donor, NOC-18, was found to accelerate the development of thermal hyperalgesia (Inoue et al., 1998). Interestingly, Linden and Seybold (1999) reported that thermal but not mechanical hyperalgesia is mediated by activation of the neurokinin 3 receptor in spinal cord, and that this activity is blocked by pretreatment with L-NAME, suggesting a role for NO. It is hypothesized, therefore, that the blockade of thermal hyperalgesia by EGb-761 observed in the present study may be due, in part, to blockade of NO in the spinal cord. Further studies are underway to investigate this hypothesis.
Of interest in the present study was the relatively long duration of action of EGb-761 in the hindpaw incision model. For instance, thermal hyperalgesia was still blocked at 24 h post-surgery, that is, 21 h after administration of EGb-761. An extract of Ginkgo biloba is reported to have a half-life of approximately 4.5 h (Moreau et al., 1988) but the precise half-life is complicated by the presence of multiple active constituents in the whole extract (Maclennan et al., 2002). For example, the half-lives of the terpenoids ginkgolide A, B and C are 1.7, 2 and 2.2 h, respectively (Biber and Koch, 1999), whereas bilobalide has a half-life of 3 h (Kleijnen and Knipschild, 1992). Thus, the antihyperalgesia observed at 24 h is unlikely to be due directly to the constituents of EGb-761 but rather to their effects on downstream targets, like NO and COX-2, that were still present at 24 h post-surgery.
In the present study, EGb-761 had no effect on paw oedema regardless of dose or time of administration. This is in contrast to a study by Abdel-Salam et al. (2004) who reported that oral administration of a Ginkgo biloba extract similar to EGb-761 (50, 100 mg kg−1), given 30 min after carrageenan, significantly reduced paw oedema. Variations in extract composition could account for the contrasting results. Studies investigating the anti-inflammatory properties of Ginkgo biloba have reported that treatment with Ginkgo biloba extract (30–120 mg kg−1; per os) reduces inflammation and acute colonic damage induced by acetic acid (Mustafa et al., 2006). Other studies investigating anti-inflammatory properties of flavanoids, quercetin and kaempferol 3-O-sophoroside, have also demonstrated reduced carrageenan-induced hindpaw oedema in mice (Rotelli et al. 2003; Toker et al., 2004). An anti-inflammatory role for Ginkgetin, a constituent of Ginkgo biloba has also been reported. Administration of Ginkgetin reduced joint inflammation by 86% in a rat model of arthritis (Kim et al., 1999) and inhibited croton oil induced ear oedema in mice (Kwak et al., 2002). The lack of effect of EGb-761 on paw oedema in the present study would suggest that EGb-761 does not have significant anti-inflammatory properties in the carrageenan model. However, the possibility of a more subtle regulatory effect on local inflammatory mediators, such as iNOS, COX-2 and TNF-α in the paw, is possible, as previous studies have shown that Gingko biloba extracts inhibit induction of these mediators (Wadsworth and Koop, 2001a, 2001b; Ilieva et al., 2004).
Extracts of Ginkgo biloba contain two main groups of active constituents: ginkgo flavone glycosides (flavanoids) and terpene lactones. Recent in vivo evidence suggests that flavanoids may be effective analgesics. For example, oral administration of the flavanoid, quercetin, was reported to reverse thermal hyperalgesia in a mouse model of diabetic neuropathic pain (Anjaneyulu and Chopra, 2003) and inhibit p-benzoquinone-induced writhing in mice (Toker et al., 2004). Similarly, the flavanoid, kaempferol 3-O-sophoroside, was also reported to have significant analgesic activity in the acetic acid- and p-benzoquinone-induced writhing test in mice (Palanichamy and Nagarajan, 1990; Toker et al., 2004). This evidence suggests that the flavanoid fraction of EGb-761 (which accounts for 24% of the standardized extract) may contribute to the anti-hyperalgesic properties of EGb-761 observed in the current study.
In summary, the present study demonstrates that oral administration of EGb-761 dose-dependently inhibits thermal hyperalgesia induced by intradermal injection of carrageenan into the hindpaw or hindpaw incision in rat to a similar or greater extent than diclofenac. EGb-761 had no effect on mechanical allodynia or paw oedema induced by carrageenan. These data demonstrated that EGb-761 has significant antihyperalgesic activity comparable with commonly used non-steroidal anti-inflammatory drugs in the treatment of acute inflammatory pain.
Acknowledgments
We thank Mr Brian Leiper and Mrs Veronica Graham for their technical assistance, and Dr Shwabe Pharmaceuticals for the gift of EGb-761.
Abbreviations
- AO
anaesthesia only
- COX-2
cyclooxygenase-2
- Emax
maximum effect
- iNOS
inducible nitric oxide synthase
- L-NAME
Nω-nitro-L-arginine methyl ester
- LPS
lipopolysaccharide
- s.e.m.
standard error of the mean
- Tmax
time to maximum effect
- TNF-α
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Abdel-Salam ME, Baiumy AR, El-Batran S, Arbid SM. Evaluation of the anti-inflammatory, anti-nociceptive and gastric effects of Ginkgo Biloba in the rat. Pharmacol Res. 2004;49:133–142. doi: 10.1016/j.phrs.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Anjaneyulu M, Chopra K. Quercetin, a biflavonoid, attenuates thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1001–1005. doi: 10.1016/S0278-5846(03)00160-X. [DOI] [PubMed] [Google Scholar]
- Biber A, Koch E. Bioavailability of ginkgolides and bilobalide from extracts of ginkgo biloba using GC/MS. Planta Med. 1999;65:192–193. doi: 10.1055/s-2006-960467. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- Curtis-Prior P, Vere D, Fray P. Therapeutic value of Ginkgo biloba in reducing symptoms of decline in mental function. J Pharm Pharmacol. 1999;1:535–541. doi: 10.1211/0022357991772817. [DOI] [PubMed] [Google Scholar]
- Ilieva I, Ohgami K, Shiratori K, Koyama Y, Yoshida K, Kase S, et al. The effects of Ginkgo Biloba extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp Eye Res. 2004;79:181–197. doi: 10.1016/j.exer.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Inoue T, Mashimo T, Shibata M, Shibuta S, Yoshiya I. Rapid development of nitric oxide-induced hyperalgesia depends on an alternate to the cGMP-mediated pathway in the rat neuropathic pain model. Brain Res. 1998;792:263–270. doi: 10.1016/s0006-8993(98)00147-4. [DOI] [PubMed] [Google Scholar]
- Kim HK, Son KH, Chang HW, Kang SS, Kim HP. Inhibition of rat adjuvant-induced Arthritis by Ginkgetin, a Biflavone from Ginkgo biloba leaves. Planta Med. 1999;65:465–467. doi: 10.1055/s-2006-960815. [DOI] [PubMed] [Google Scholar]
- Kleijnen J, Knipschild P. Ginkgo biloba. Lancet. 1992;340:1136–1139. doi: 10.1016/0140-6736(92)93158-j. [DOI] [PubMed] [Google Scholar]
- Kwak WJ, Han CK, Son KH, Chang HW, Kang SS, Park BK, et al. Effects of Ginkgetin from Ginkgo Biloba leaves on cyclooxygenases and in vivo skin inflammation. Planta Med. 2002;68:316–321. doi: 10.1055/s-2002-26742. [DOI] [PubMed] [Google Scholar]
- LaBuda JC, Koblish M, Tuthill P, Dolle RE, Little PJ. Antinociceptive activity of the selective iNOS inhibitor AR-C102222 in rodent models of inflammatory, neuropathic and post-operative pain. Eur J Pain. 2006;10:505–512. doi: 10.1016/j.ejpain.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Linden DR, Seybold VS. Spinal neurokinin3 receptors mediate thermal but not mechanical hyperalgesiavia nitric oxide. Pain. 1999;80:309–317. doi: 10.1016/s0304-3959(98)00222-x. [DOI] [PubMed] [Google Scholar]
- Maclennan KM, Darlington CL, Smith PF. The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog Neurobiol. 2002;67:235–257. doi: 10.1016/s0301-0082(02)00015-1. [DOI] [PubMed] [Google Scholar]
- Meller ST, Cummings CP, Traub GF, Gebhart GF. The role of nitric oxide in the development and maintenance of the Hyperalgesia produced by intraplantar injection of carrageenan in the rat. Neuroscience. 1994;60:367–374. doi: 10.1016/0306-4522(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52:127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- Moreau JP, Eck CR, McCabe J, Skinner S.Absorption, distribution, and excretion of tagged Ginkgo biloba leaf extract in the rat Rökan (Ginkgo biloba) 1988Springer: New York; 37–45.In: Fünfgeld EW (ed)Recent Results in Pharmacology and Clinic [Google Scholar]
- Mustafa A, El-Medany A, Hagar HH, El-Medany G. Ginkgo biloba attenuates mucosal damage in a rat model of ulcerative colitis. Pharm Res. 2006;53:324–330. doi: 10.1016/j.phrs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Ochoa JL, Yarnitsky D. Mechanical hyperalgesias in neuropathic pain patients: dynamic and static subtypes. Ann Neurol. 1993;33:465–472. doi: 10.1002/ana.410330509. [DOI] [PubMed] [Google Scholar]
- Palanichamy S, Nagarajan S. Analgesic activity of Cassia alata leaf extract and kaempferol 3-O-sophoroside. J Ethnopharmacol. 1990;29:3–78. doi: 10.1016/0378-8741(90)90099-f. [DOI] [PubMed] [Google Scholar]
- Rotelli AE, Guardia T, Juarez AO, de la Rocha N, Pelzer LE. Comparative study of flavenoids in experimental models of inflammation. Pharm Res. 2003;48:601–606. doi: 10.1016/s1043-6618(03)00225-1. [DOI] [PubMed] [Google Scholar]
- Salter M, Strijbos PJLM, Neale S, Duffy C, Follenfant RL, Garthwaite J. The nitric oxide-cyclic GMP pathway is required for nociceptive signalling at specific loci within the somatosensory pathway. Neurosci. 1996;73:649–655. doi: 10.1016/0306-4522(96)00060-7. [DOI] [PubMed] [Google Scholar]
- Toker G, Kupeli E, Memisoglu M, Yesliada E. Flavenoids with antinociceptive and anti-inflammatory activities from the leaves of Tilia argentea (silver linden) J Ethnopharmacol. 2004;95:393–397. doi: 10.1016/j.jep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wadsworth TL, Koop DR. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced signalling pathways involved in the release of nitric oxide. Chem Biol Interact. 2001a;137:43–58. doi: 10.1016/s0009-2797(01)00208-3. [DOI] [PubMed] [Google Scholar]
- Wadsworth TL, Koop DR. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced signalling pathways involved in the release of tumour necrosis factor-α. Biochem Pharmacol. 2001b;62:963–974. doi: 10.1016/s0006-2952(01)00734-1. [DOI] [PubMed] [Google Scholar]
- Watanabe CMH, Wolffram S, Ader P, Rimbach G, Packer L, Maguire JJ, et al. The in vivo neuromodulatory effects of the herbal medicine Ginkgo biloba. Proc Natl Acad Sci USA. 2001;98:6577–6580. doi: 10.1073/pnas.111126298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Chong MS. Preemptive analgesia treating postoperative pain by preventing the establishment of central sensitisation. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]