Abstract
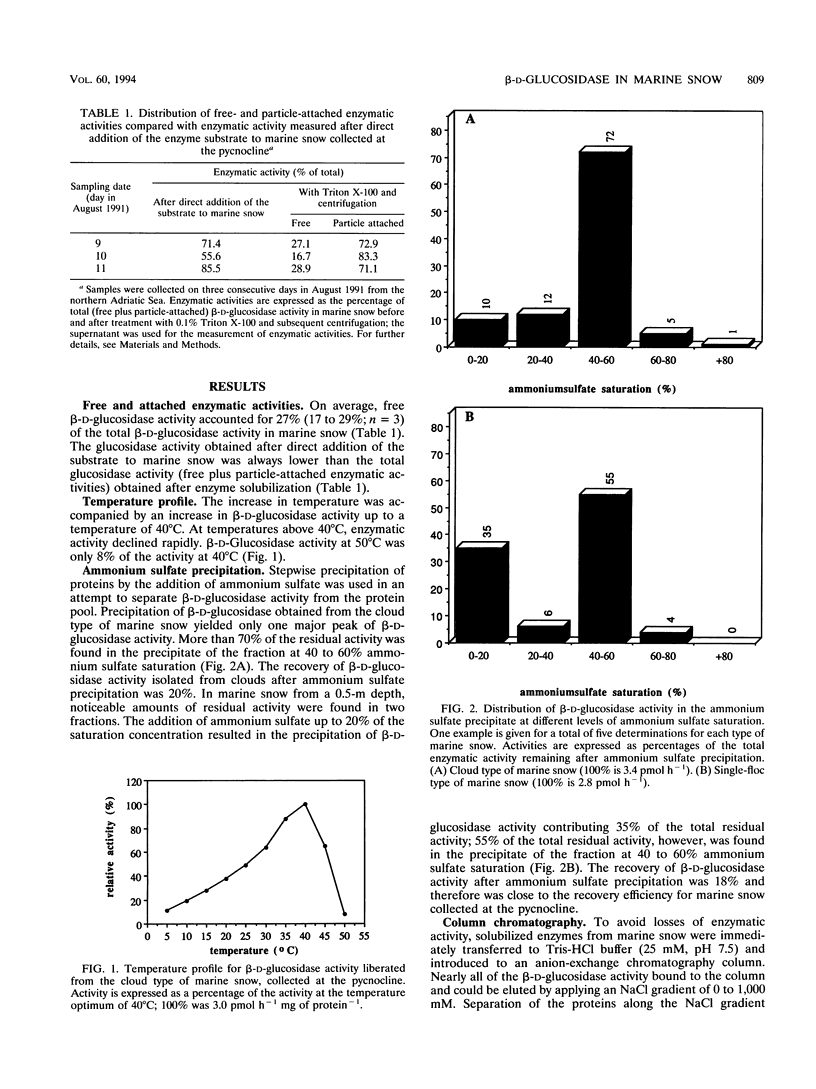
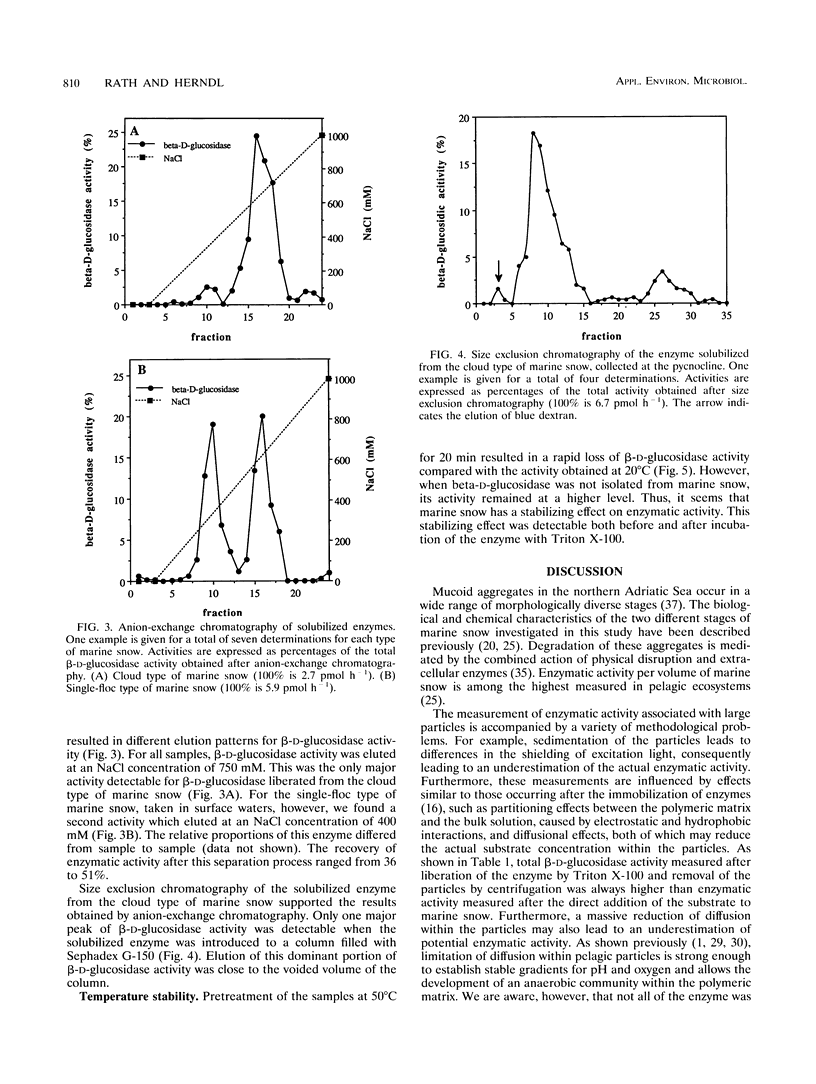
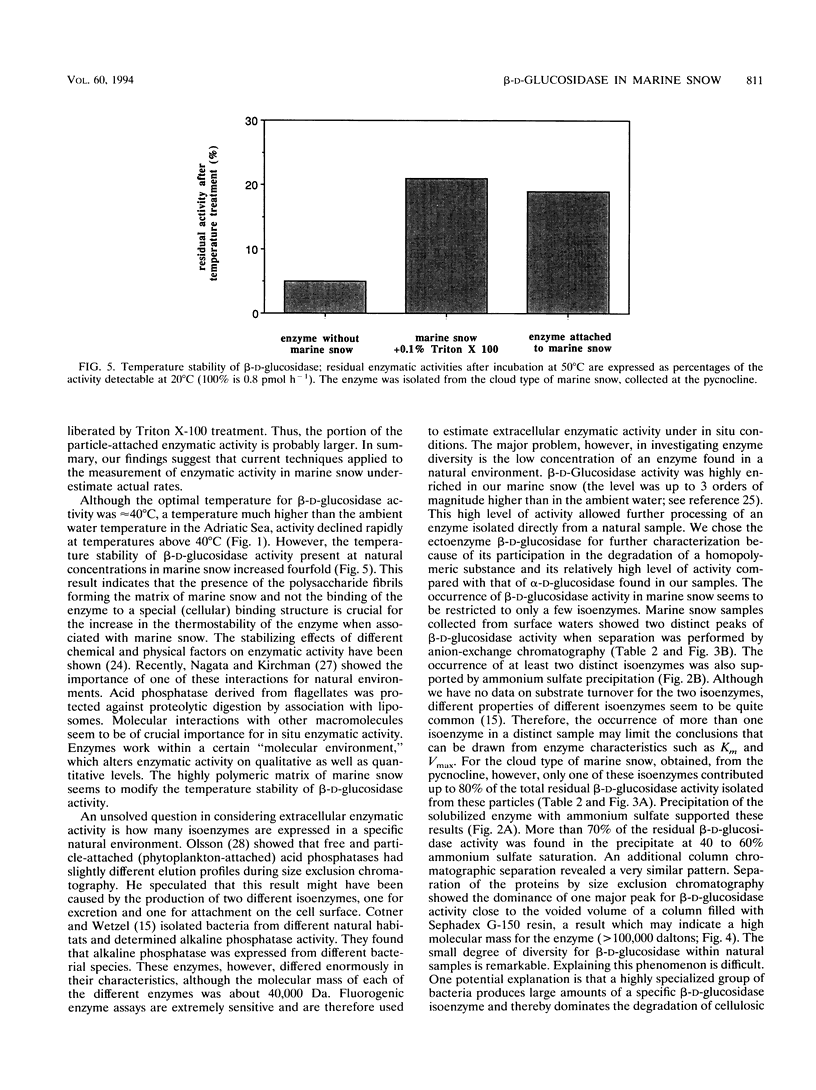
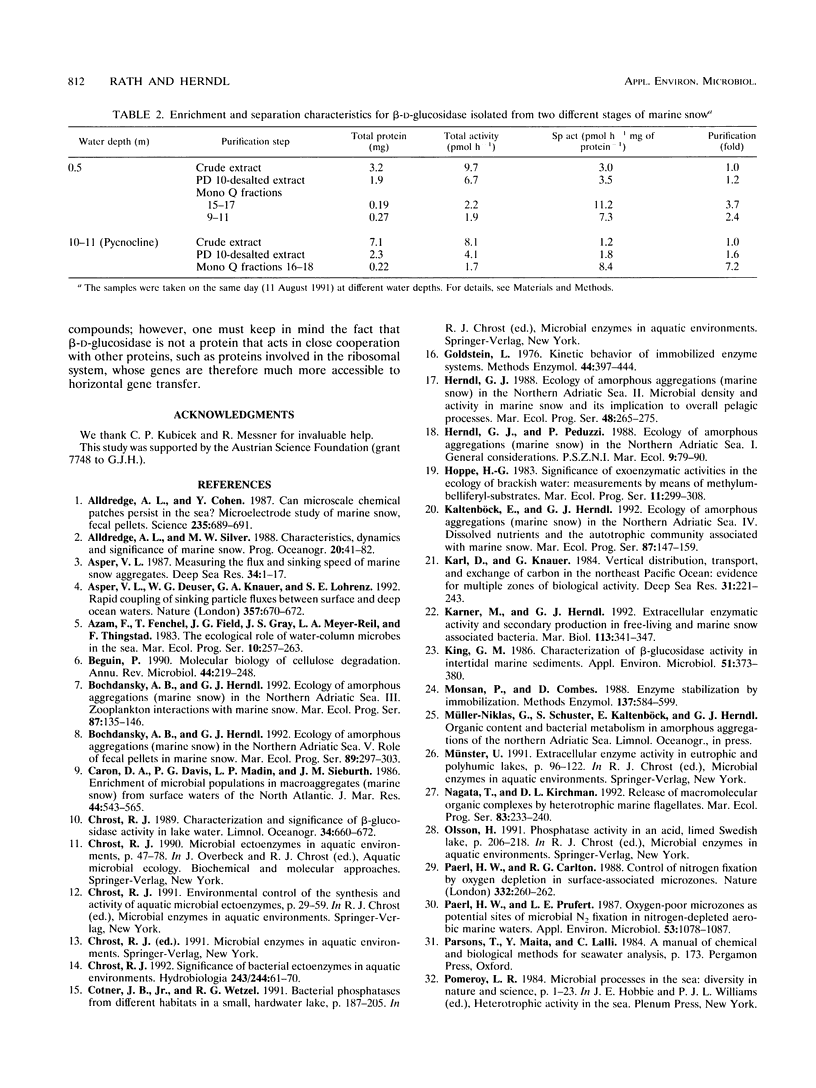
Large amorphous aggregates commonly described as marine snow were sampled in the water column of the northern Adriatic Sea in August 1991. β-d-Glucosidase activity associated with these aggregates was characterized. Enzymatic activity was measured with the fluorogenic substrate analog 4-methylumbelliferyl-β-d-glucoside and found to be mainly associated with particles; on average, only 24% of the whole β-d-glucosidase activity remained in the supernatant after centrifugation. Although the temperature optimum for β-d-glucosidase activity was ≈ 40°C, incubation of the previously liberated particle-attached enzyme at 50°C for 20 min caused a >90% reduction of enzymatic activity relative to the activity at 40°C. The level of inactivation of β-d-glucosidase was much lower, however, when whole marine snow was incubated, indicating qualitative modifications of β-d-glucosidase in marine snow. Separation of β-d-glucosidase by different approaches indicated that the diversity of isoenzymes is restricted. In samples taken from the pycnocline, only one major isoenzyme was present in noticeable amounts. This isoenzyme contributed up to 70% of the whole β-d-glucosidase activity detectable by two different chromatographic separations (anion-exchange chromatography and size exclusion chromatography). Although the same isoenzyme was dominant in marine snow taken from surface waters (0.5-m depth), we found a second peak of activity which eluted at lower NaCl concentrations from the anion-exchange column. Generally, the diversity of isoenzymes exhibiting β-d-glucosidase activity seems to be surprisingly small in amorphous pelagic aggregates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alldredge A. L., Cohen Y. Can microscale chemical patches persist in the sea? Microelectrode study of marine snow, fecal pellets. Science. 1987 Feb 6;235(4789):689–691. doi: 10.1126/science.235.4789.689. [DOI] [PubMed] [Google Scholar]
- Béguin P. Molecular biology of cellulose degradation. Annu Rev Microbiol. 1990;44:219–248. doi: 10.1146/annurev.mi.44.100190.001251. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Kinetic behavior of immobilized enzyme systems. Methods Enzymol. 1976;44:397–443. doi: 10.1016/s0076-6879(76)44031-4. [DOI] [PubMed] [Google Scholar]
- King G. M. Characterization of beta-Glucosidase Activity in Intertidal Marine Sediments. Appl Environ Microbiol. 1986 Feb;51(2):373–380. doi: 10.1128/aem.51.2.373-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsan P., Combes D. Enzyme stabilization by immobilization. Methods Enzymol. 1988;137:584–598. doi: 10.1016/0076-6879(88)37055-2. [DOI] [PubMed] [Google Scholar]
- Paerl H. W., Prufert L. E. Oxygen-poor microzones as potential sites of microbial n(2) fixation in nitrogen-depleted aerobic marine waters. Appl Environ Microbiol. 1987 May;53(5):1078–1087. doi: 10.1128/aem.53.5.1078-1087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somville M. Measurement and study of substrate specificity of exoglucosidase activity in eutrophic water. Appl Environ Microbiol. 1984 Dec;48(6):1181–1185. doi: 10.1128/aem.48.6.1181-1185.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Fryda S. J., Robinson I. M. Cellulolytic bacteria from pig large intestine. Appl Environ Microbiol. 1984 Jan;47(1):219–221. doi: 10.1128/aem.47.1.219-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



