Abstract
Background and purpose:
The effects of bergamot essential oil (BEO; Citrus bergamia, Risso) on excitotoxic neuronal damage was investigated in vitro.
Experimental approach:
The study was performed in human SH-SY5Y neuroblastoma cells exposed to N-methyl-D-aspartate (NMDA). Cell viability was measured by dye exclusion. Reactive oxygen species (ROS) and caspase-3 activity were measured fluorimetrically. Calpain I activity and the activation (phosphorylation) of Akt and glycogen synthase kinase-3β (GSK-3β) were assayed by Western blotting.
Key results:
NMDA induced concentration-dependent, receptor-mediated, death of SH-SY5Y cells, ranging from 11 to 25% (0.25–5 mM). Cell death induced by 1 mM NMDA (21%) was preceded by a significant accumulation of intracellular ROS and by a rapid activation of the calcium-activated protease calpain I. In addition, NMDA caused a rapid deactivation of Akt kinase and this preceded the detrimental activation of the downstream kinase, GSK-3β. BEO (0.0005–0.01%) concentration dependently reduced death of SH-SY5Y cells caused by 1 mM NMDA. In addition to preventing ROS accumulation and activation of calpain, BEO (0.01%) counteracted the deactivation of Akt and the consequent activation of GSK-3β, induced by NMDA. Results obtained by using specific fractions of BEO, suggested that monoterpene hydrocarbons were responsible for neuroprotection afforded by BEO against NMDA-induced cell death.
Conclusions and Implications:
Our data demonstrate that BEO reduces neuronal damage caused in vitro by excitotoxic stimuli and that this neuroprotection was associated with prevention of injury-induced engagement of critical death pathways.
Keywords: bergamot essential oil, neuroprotection, NMDA receptors, excitotoxicity, cell death, SH-SY5Y neuroblastoma cells, Akt, GSK-3β, calpain I
Introduction
Excessive activation of N-methyl-D-aspartate (NMDA) subtype of glutamate receptors, and hence abnormal Ca2+ influx through the receptor-associated cation channel, largely contribute to glutamate-mediated neuronal death (Lynch and Guttmann, 2002). The increase in intracellular calcium triggers robust activation of calcium-dependent enzymes, including nitric oxide (NO) synthase (NOS), and mitochondrial calcium overload and dysfunction leading to generation of NO, reactive oxygen species (ROS) and peroxynitrite which initiate neuronal cell demise (Dawson et al., 1991; Corasaniti et al., 1992; Lafon-Cazal et al., 1993; Dugan et al., 1995; Stout et al., 1998; Moncada and Bolanos, 2006). In addition to NOS, Ca2+ entry through the NMDA receptor-gated cation channel activates the neutral cysteine protease calpain I (Croall and DeMartino, 1991) and activation of calpain I has been implicated in excitotoxic neuronal death both in vitro and in vivo (Siman et al., 1989; Roberts-Lewis et al., 1994; Corasaniti et al., 1996; Lankiewicz et al., 2000; Bano et al., 2005).
Survival signaling pathways engaged by activation of Akt kinase (also known as protein kinase B (PKB), mediate endogenous prosurvival responses to neuronal stress induced by an excitotoxic, that is ischemic, insult (see Fukunaga and Kawano, 2003). The serine/threonine kinase Akt functions as a major downstream target of phosphatidylinositol 3 kinase (PI3K) and, after being phosphorylated, it promotes cell survival by phosphorylating and inhibiting several proteins including Bad, caspase-9 and glycogen synthase kinase-3β (GSK-3β) (see Brazil and Hemmings, 2001). Several in vivo studies have indicated that activation of the PI3K/Akt pathway plays an important role in rescuing neurones of the ischemic penumbra from delayed cell death (see Fukunaga and Kawano, 2003). In animal models of experimental brain ischemia, enhanced Akt phosphorylation has been detected in the ischemic penumbra but not in the ischemic core (Noshita et al., 2001). Double staining experiments demonstrate that phospho-Akt (p-Akt)-positive neurones do not show DNA fragmentation (Noshita et al., 2001), suggesting that phosphorylation-mediated activation of Akt kinase contributes to neuroprotection. However, enhancement of p-Akt expression is transient and it is followed by a dramatic decrease of p-Akt within 24 h or more after ischemia, depending on the experimental model of brain ischemia (focal or global, transient or permanent) (Zhao et al., 2005). Under these experimental conditions, pretreatment with neurotrophic factors able to activate the PI3K/Akt pathway, confers neuroprotection by maintaining the phosphorylation of Akt and thus its activity (see Fukunaga and Kawano, 2003; Maiese et al., 2004; Amantea et al., 2005). However, it should be stressed that a restriction to clinical application of neurotrophic factors is represented by their peptidic moiety which limits their penetration into the brain (Wu, 2005). As a consequence, the availability of compounds endowed with neuroprotective properties and able to cross the blood–brain barrier would be extremely valuable in the development of neuroprotective therapies.
Recently, interest in natural products regarded as potential source of neuroprotective agents has been renewed. In the frame of a research project aimed at investigating the neuropharmacological profile of the essential oil of bergamot (Citrus bergamia, Risso), a citrus growing almost exclusively in the south of Italy, in a restricted area of the Calabrian coast, we have assessed the neuroprotective properties of the oil.
According to the Farmacopea Ufficiale Italiana (1991), the bergamot essential oil (BEO), is obtained by cold pressing of the epicarp and, partly, of the mesocarp of the fresh fruit of bergamot. The citrus is cultivated for its essential oil, a product in great demand by perfumery and cosmetic industries but also employed by pharmaceutical, food and confectionery industries. BEO comprises a volatile fraction (93–96% of total) containing monoterpene and sesquiterpene hydrocarbons (such as limonene, α- and β-pinene, β-myrcene, γ-terpinene, terpinolene, sabinene and β-bisabolene) and oxygenated derivatives (such as linalool, neral, geranial, linalyl acetate, neryl acetate and geranyl acetate) and a nonvolatile fraction (4–7% of total) containing waxes, polymethoxylated flavones, coumarins and psoralens such as bergapten (5-methoxypsoralen) and bergamottine (5-geranyloxypsoralen) (Mondello et al., 1993; Dugo et al., 2000). The most abundant compounds found in the volatile fraction are the monoterpene hydrocarbons limonene, γ-terpinene, and β-pinene, the monoterpene alcohol, linalool, and the monoterpene ester, linalyl acetate, which altogether constitute more than 90% of the whole oil (Mondello et al., 1995; Verzera et al., 1996, 2003). Oxygenated compounds, namely linalool and linalyl acetate, mark the flavor notes of BEO, whereas the hydrocarbon fraction does not have a fundamental role in determining the olfactory character of BEO. The nonvolatile residue is a natural odor fixative which influences the olfactory properties of the oil; however, it contains about 0.2% bergapten which is responsible for the phototoxicity of BEO (Zaynoun et al., 1977; Ashwood-Smith et al., 1980); therefore, a bergapten-free extract of the essence (BEO-BF) together with a natural essence deprived of the hydrocarbon fraction and of bergapten (BEO-HF/BF) are prepared by extractive industries for perfumery and cosmetic uses.
In the present study, we examined whether BEO could reduce excitotoxic neuronal damage caused by exposure of human SH-SY5Y neuroblastoma cells to NMDA. The finding that BEO protects from NMDA-induced cell death prompted us to investigate the intracellular pathways through which BEO affords neuroprotection together with the identification of the fraction of the oil responsible for neuroprotection. Collectively, our data demonstrate that BEO reduces excitotoxic damage in vitro and that neuroprotection is associated with prevention of ROS accumulation, inhibition of calpain I activation and prevention of injury-induced decrease of p-Akt and phospho-GSK-3β (p-GSK-3β) levels.
Methods
Cell culture, treatments and cytotoxicity study
Adherent SH-SY5Y human neuroblastoma cells, obtained from ICLC-IST (Genoa, Italy), were cultured in RPMI 1640 (containing 0.407 mM MgSO4-7H2O) supplemented with heat-inactivated fetal bovine serum (10% v/v), 1 mM sodium pyruvate and 2 mM glutamine at 37°C in a 5% CO2 atmosphere. The cells, cultured in 75 cm2 flasks, were seeded in 35 mm six-well plates. Twenty-four hours after plating, the growth medium was replaced with fresh normal medium (control cultures) or with medium supplemented with NMDA (0.25–5 mM) for 24 h. Addition of 1 or 5 mM NMDA brought the pH of the culture medium from 7.60 to 7.55 and 7.08, respectively. For antagonism studies, MK801, CGP40116 and 7-nitroindazole were applied to SH-SY5Y cells 10 min before the addition of NMDA (1 mM) and they were present during the 24 h exposure time. In experiments involving BEO and its fractions, BEO-BF and BEO-BF/HF, these were added to cell cultures 60 min before NMDA application. Preliminary experiments were carried out in to characterize the effects of BEO on cell viability; SH-SY5Y cultures were exposed for 24 h to graded dilutions of BEO before evaluating cell viability. Cell viability was assessed by cell exclusion of trypan blue (0.4% w/v) and cell death was reported as the percentage of stained (non viable) vs total cells counted (Corasaniti et al., 2001). Data were expressed as mean±s.e.m. percentage cell death.
Fluorimetric caspase-3 activity assay
NMDA-treated SH-SY5Y cells were harvested at the indicated times in ice-cold phosphate-buffered saline (PBS) and lysed in ice-cold lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM MgCl2, 5 mM EDTA, 0.1% 3[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), 5 mM dithiothreitol, 10 μg ml−1 pepstatin A, 10 μg ml−1 leupeptin and 10 μg ml−1 aprotinin). Cell suspensions were briefly sonicated, centrifuged at 12 000 g for 10 min at 4°C and protein concentration in supernatants was determined by the DC protein assay (Bio-Rad Laboratories, Milan, Italy). The fluorimetric assay for caspase-3 activity was performed as follows. Cell supernatants were diluted in assay buffer (100 mM HEPES, pH 7.4, 5 mM EDTA, 0.1% CHAPS, 5 mM dithiothreitol and 10% glycerol) to a final concentration of 0.6 μg protein per μl and incubated in triplicate in a 96-well clear-bottom plate with the fluorogenic substrate acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC; 50 μM; Bachem, Bubendorf, Switzerland). Production of fluorescent-free AMC, released by caspase-3 activity, was monitored over 60 min at 37°C using a microplate fluorometer (Victor2 multilabel counter, Perkin-Elmer Life Sciences; excitation, 355 nm; emission, 460 nm). The specific contribution of caspase-3 activity in each cell extract was determined by preincubating parallel sample aliquots with the caspase-3 preferring inhibitor acetyl-Asp-Glu-Val-Asp-aldehyde (Ac-DEVD-CHO; 50 μM; Bachem) for 10 min at 37°C before the addition of the caspase substrate; the difference between the substrate cleavage activity in the absence and presence of Ac-DEVD-CHO was regarded as specific caspase-3 activity. The increase in fluorescence was linear for 40 min after addition of the fluorogenic substrate. Data were analyzed by linear regression within the linear range of the enzymatic reaction and the results expressed as relative fluorescence units (RFU) per min per mg of protein.
Determination of ROS production
Accumulation of intracellular ROS, a term that includes both free radicals, such as peroxyl, alkoxyl and hydroxyl radicals, and nonradicals, such as peroxynitrite and hydrogen peroxide that are oxidizing agent and/or are easily converted into radicals (see Halliwell and Whiteman, 2004), was estimated by fluorescence assay as described previously (Russo et al., 2005), using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA; Molecular Probes, Eugene, OR, USA) as a probe (Mattson et al., 1995). H2DCF-DA readily diffuses into the cells where intracellular esterases cleave the acetate group of H2DCF-DA from the molecule to yield H2DCF, which is trapped within the cells. Intracellular ROS (see Halliwell and Whiteman, 2004) oxidize H2DCF to form the highly fluorescent compound DCF. SH-SY5Y cells were subcultured from confluent 75 cm2 flasks and seeded in 96-well plates. Twenty-four hours after plating, the growth medium was replaced with fresh medium containing H2DCF-DA (20 μM final concentration) and 2.5 mM probenecid; after 30 min at 37°C, the cells were washed twice to remove the extracellular H2DCF-DA and incubated in a pre-warmed buffer (phosphate-buffered saline (PBS) supplemented with 1.2 mM CaCl2 and 10 mM glucose, pH 7.4) in the absence or presence of NMDA. In experiments involving BEO, this was applied to SH-SY5Y cells before the loading with H2DCF-DA at the same preincubation time used for the cytotoxicity study. Fluorescence was monitored every 5 min for 60 min following the addition of NMDA by using a microplate fluorometer (Victor2 multilabel counter, Perkin-Elmer Life Sciences; excitation, 495 nm; emission, 530 nm). Data were analyzed according to the following formula: (Tx−T0/T0) × 100, where Tx is the DCF fluorescence measured at the indicated time and T0 is the DCF fluorescence measured at the beginning of the analysis, and reported as the mean±s.e.m. of eight wells per experimental group.
Preparation of cell lysates
Cell monolayers in 100-mm plates were washed with ice-cold PBS and lysed with lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% Triton, 1 nM okadaic acid, a cocktail of protease inhibitors (code P8340, Sigma, Milan, Italy) and a cocktail of phosphatase inhibitors (code 524625, Calbiochem, La Jolla, CA, USA). Following 5 min incubation on ice, the lysates were collected in microcentrifuge tubes, briefly sonicated and centrifuged at 20 800 g for 15 min at 4°C. Protein concentration in the supernatants of cell lysates was determined by the DC protein assay (Bio-Rad Laboratories, Milan, Italy).
Western-blot analysis
Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 8% for Akt and GSK-3β and 6% for α-spectrin) and electrotransferred to nitrocellulose membranes (Optitran BA-S 83, Schleicher & Schuell Bioscence, Dassel, Germany). Primary antibodies were incubated overnight at 4°C followed by a horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Immunoreactivity was visualized by enhanced chemiluminescent detection (Amersham Biosciences, GE Healthcare, Milan, Italy) and exposure to X-ray films (Hyperfilm ECL, Amersham Biosciences). Autoradiographic films were scanned and densitometric analysis was carried out using Quantiscan software (Biosoft, Cambridge, UK). The following primary antibodies were used: a rabbit polyclonal antibody for Akt at 1:2000 dilution (Cell Signaling Technology, Beverly, MA, USA), a rabbit polyclonal antibody for p-Akt (Ser473) at 1:2000 dilution (Cell Signaling Technology), a rabbit polyclonal antibody against GSK-3β at 1:2000 dilution (Cell Signaling Technology), a rabbit polyclonal antibody for p-GSK-3β (Ser9) at 1:1000 dilution (Cell Signaling Technology), a mouse anti-spectrin (nonerythroid) monoclonal antibody at 1:4000 dilution (MAB1622; Chemicon International Inc., Temecula, CA, USA), a mouse monoclonal anti-actin antibody at 1:2000 dilution (clone AC-40; Sigma), a mouse monoclonal anti-α-tubulin antibody at 1:40 000 dilution (clone B-5-1-2; Sigma). Horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (Pierce Biotechnology, Rockford, IL, USA) were used as secondary antibodies.
Statistical analysis
Data are expressed as the mean±s.e.m. of the indicated number of independent experiments and evaluated statistically for difference by one-way analysis of variance (ANOVA) followed by Tukey–Kramer or by Dunnett tests for multiple comparisons. A value of P<0.05 was considered to be significant.
Drugs
The essential oil of bergamot (BEO) and its fractions were kindly provided by the company ‘Simone Gatto' (San Pier Niceto, Messina, Italy) together with the certificate of analysis carried out by the ‘Stazione Sperimentale per le Industrie delle Essenze e dei Derivati dagli Agrumi' (SSEA, Reggio Calabria, Italy). The fractions employed were bergapten-free extract of the essence (BEO-BF) and the natural essence deprived of hydrocarbon fraction and of bergapten (BEO-BF/HF). According to the percentages reported in the literature (Mondello et al., 1995; Verzera et al., 1996, 2003), among other substances present in lower percentages, BEO contained 37.98% D-limonene, 30.02% linalyl acetate, 9.83% linalool, 7.17% γ-terpinene and 6.15% β-pinene. In BEO-BF/HF, the percentages of the above-mentioned compounds were 0.38% limonene, 70.26% linalyl acetate, 18.95% linalool, 0.62% γ-terpinene and 0.03% β-pinene. BEO and its fractions were diluted 1:10 in a 1:9 water/ethanol solution and then further diluted in culture medium to obtain final concentrations of 0.0005, 0.005 and 0.01%; identical volumes of ethanol were added to culture medium to investigate potential effects on NMDA-induced cell death.
MK801 (dizocilpine) and 7-nitroindazole were from Sigma; D-(E)-2-aminomethyl-5-phosphono-3-pentenoic acid (CGP40116) was kindly provided by Ciba-Geigy (Basel, Switzerland). 2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) and 6-bromoindirubin-3′-oxime (GSK-3 Inhibitor IX) were from Calbiochem and they were supplied as a 10 mM solution in dimethyl sulfoxide (DMSO) and further diluted in culture medium; the maximal concentrations of DMSO in culture medium were 0.2% for LY294002 and 0.01% for GSK-3 Inhibitor IX, which did not affect the percentage cell death caused by NMDA.
Results
Characterization of NMDA-induced cytotoxicity in SH-SY5Y cultures
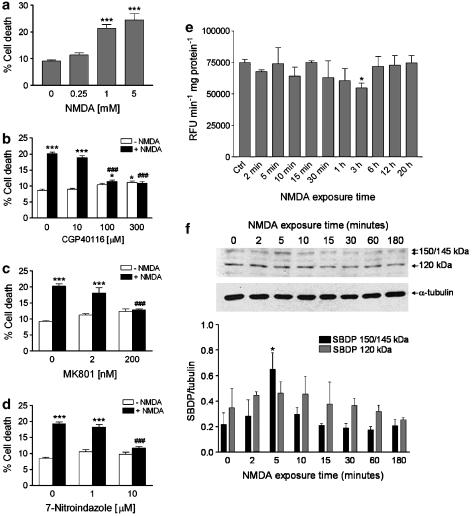
Human SH-SY5Y neuroblastoma cells express NMDA subtype of glutamate receptors (Sun and Murali, 1998) and exposure to the excitotoxin induces concentration-dependent cell death (Figure 1a). Death caused by NMDA in SH-SY5Y cultures occurs via stimulation of NMDA subtypes of glutamate receptors because CGP40116, a selective competitive NMDA receptor antagonist (Sauer et al., 1993), and the use-dependent channel blocker, MK801, prevented in a concentration-dependent manner cell death elicited by 1 mM NMDA (Figure 1b and c).
Figure 1.
Characterization of NMDA-induced cytotoxicity in SH-SY5Y cultures. (a) Treatment of SH-SY5Y cells with 1.0 mM NMDA for 24 h induces significant cytotoxic effects as assessed by trypan blue staining; no greater cytotoxicity was observed by incubating the cells with a higher concentration (5.0 mM) of the excitotoxin. A lower concentration (0.25 mM) of NMDA did not significantly affect cell viability. Cell death induced by NMDA (1 mM) was prevented by selective, (b) competitive (CGP40116; 100 and 300 μM) and (c) noncompetitive (MK801; 200 nM), antagonists of NMDA subtype of glutamate receptors. (d) 7-Nitroindazole (10 μM), a neuronal NOS preferring inhibitor, prevented death of SH-SY5Y cells induced by NMDA (1.0 mM). Each value in a, b, c and d is the mean±s.e.m. of 3–6 experiments. ***P<0.001 vs control; ###P<0.001 vs NMDA given alone (ANOVA followed by Tukey–Kramer multiple comparisons test). (e) NMDA does not increase caspase-3 activity in SH-SY5Y cell cultures. SH-SY5Y cells were exposed to 1 mM NMDA for the indicated periods of time, and then caspase-3 activity was determined in the supernatants of lysed cell suspensions by measuring cleavage of the fluorogenic substrate Ac-DEVD-AMC (see the Methods section). Results are expressed as relative fluorescence units (RFU) per min per mg of protein. Each value is the mean±s.e.m. of three experiments. *P<0.05 vs control (ANOVA followed by Dunnett multiple comparisons test). (f) Activation of calpain I after exposure of SH-SY5Y cells to NMDA. Representative Western blot showing the increase of the calpain-specific 150/145 kDa SBDP early after NMDA addition (1 mM). Note the lack of accumulation of 120 kDa α-spectrin fragment derived from caspase-mediated proteolysis. Histograms in lower panel show results of densitometric analysis of autoradiographic bands corresponding to 150/145 and 120 kDa SBDP. Data were normalized to the values for α-tubulin. Each value is the mean±s.e.m. of three experiments. *P<0.05 vs control (ANOVA followed by Dunnett multiple comparisons test).
Preincubation with 7-nitroindazole (7-NI), a neuronal NOS preferring inhibitor (Babbedge et al., 1993), concentration dependently reduced NMDA-elicited cell death (Figure 1d), suggesting that exposure of SH-SY5Y cells to NMDA triggers NO accumulation and this contributes to cell demise.
Under our experimental conditions, cell death triggered by NMDA was caspase-independent. In fact, fluorimetric caspase-3 activity assay revealed no activation of caspase-3, the main executioner caspase in neurones (see Yuan and Yankner, 2000) following exposure (2 min to 20 h) of SH-SY5Y cells to the excitotoxin (1 mM; Figure 1e). The latter effect is specific because, exposure of SH-SY5Y cells to a different stimulus, 50 μM etoposide for 6 h, increased caspase-3 activity by 837±84% (P<0.0001 vs control= 74902±2636 RFU per min per mg of protein; n=6). Interestingly, NMDA exposure caused a significant reduction of caspase-3 activity after 3 h of treatment, compared to control (Figure 1e).
Quite importantly, activation of the Ca2+-activated neutral protease calpain I was detectable early after exposure to NMDA. Activation of calpain was studied by Western-blot analysis of generation of α-spectrin cleavage fragments (150–145 kDa), characteristic of calpain-mediated proteolysis. The accumulation of the calpain-cleaved 150–145 kDa α-spectrin breakdown products (SBDP) was evident early after exposure to NMDA and it peaked at 5 min after NMDA addition (Figure 1f). In contrast, no accumulation of the α-spectrin fragment derived from caspase-mediated proteolysis (120 kDa) was detectable (Figure 1f) and this is in accordance with the lack of caspase-3 activation evaluated by fluorimetric assay (Figure 1e).
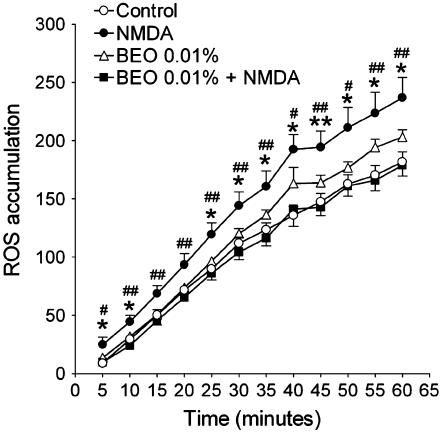
As shown in Figure 2, treatment of SH-SY5Y cultures with 1 mM NMDA triggers a significant increase of intracellular ROS generation.
Figure 2.
NMDA (1 mM) induced accumulation of intracellular ROS and this was prevented by preincubating SH-SY5Y cells with BEO (0.01%). ROS were measured by fluorescence assay and the data expressed as (Tx−T0/T0) × 100 as described in the Methods section. Each value is the mean±s.e.m. of eight wells per experimental group. Results were confirmed by three independent experiments. *P<0.05 and **P<0.01 vs control, respectively; #P<0.05 and ##P<0.01 vs BEO+NMDA, respectively (ANOVA followed by Tukey–Kramer multiple comparisons test).
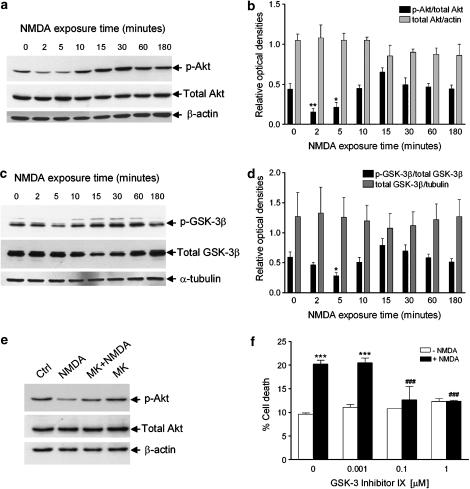
The kinase, Akt, is activated following phosphorylation at Thr308 and Ser473 (Alessi et al., 1996). Interestingly, exposure of SH-SY5Y cells to NMDA induced a fast and transient deactivation of Akt kinase as determined by Western-blot analysis of p-Akt levels, at Ser473 (Figure 3a). Reduced phosphorylation of Akt was evident at 2 and 5 min after NMDA exposure. It was then followed by a trend towards an increase of phosphorylation that peaks at 15–30 min and returned to control levels at 1–3 h after treatment (Figure 3a and b). Such a transient increase in p-Akt at Ser473 following 15 min exposure to NMDA is consistent with previous reports obtained in primary cortical and hippocampal neuronal cultures (Luo et al., 2003), except that the early decline has not been studied previously. At variance with modification of p-Akt levels, NMDA did not affect the expression of total Akt (Figure 3a and b). MK801 (200 nM) enhanced phosphorylation of Akt, typically reduced by 5 min exposure to NMDA (Figure 1e).
Figure 3.
Time course of NMDA effects on Akt and GSK-3β phosphorylation in SH-SY5Y cells and protection from NMDA-induced cell death by an inhibitor of GSK-3β kinase. SH-SY5Y cells were exposed to 1 mM NMDA for the indicated periods of time, then cellular proteins were extracted for subsequent analysis by Western blotting of Akt and GSK-3β phosphorylation by using a polyclonal antibody specific for (a) Akt phosphorylated at Ser473 and a polyclonal antibody specific for (c) GSK-3β phosphorylated at Ser9. To establish that changes in phospho-Akt (p-Akt) and phospho-GSK-3β (p-GSK-3β) levels are not due to changes in Akt and GSK-3β protein expression, nitrocellulose membranes were subsequently immunoblotted with an antibody specific for (a) total Akt and (c) total GSK-3β. Equal protein loading in each lane was confirmed by hybridization with an anti-β-actin (a) or anti α-tubulin antibody (c), for Akt anf GSK-3β blots, respectively. Histograms in (b) and (d) show the results of the densitometric analysis of autoradiographic bands; the data are reported as the ratio of (b) p-Akt/total Akt and (d) p-GSK-3β/total GSK-3β, whereas total Akt and total GSK-3β levels were normalized to the values yielded for β-actin and α-tubulin, respectively. Each value is the mean±s.e.m. of 3–6 experiments; *P<0.05 and **P<0.01 vs control, respectively (ANOVA followed by Dunnett multiple comparisons test). (e) MK801 (200 nM; MK), applied to neuroblastoma cultures 10 min before 1 mM NMDA, attenuated reduction of phospho(Ser473)-Akt (p-Akt) levels caused by 5 min exposure to the excitotoxin; a typical example, representative of three independent experiments, is shown. (f) GSK-3 inhibitor IX (0.1 and 1 μM) prevented death of SH-SY5Y cells induced by NMDA. GSK-3 inhibitor IX was added to SH-SY5Y cultures 30 min before NMDA application (1.0 mM) and cell death was evaluated 24 h later by trypan blue staining. No protection was afforded by the enzyme inhibitor applied at a lower concentration (0.001 μM). Each value is the mean±s.e.m. of 3–6 experiments. ***P<0.001 vs control; ###P<0.001 vs NMDA given alone (ANOVA followed by Tukey–Kramer multiple comparisons test).
Phosphorylation of GSK-3β at Ser9 by Akt inhibits GSK-3β kinase activity (Cross et al., 1995; van Weeren et al., 1998); therefore, we next investigated whether NMDA exposure would reduce levels of GSK-3β phosphorylation at Ser9. In these experiments, NMDA led to a significant reduction of p-GSK-3β levels at Ser9 at 5 but not 2 min after exposure (Figure 3c and d), indicating that in NMDA-stimulated SH-SY5Y cells, deactivation of Akt precedes reduction of p-GSK-3β levels.
To test whether or not NMDA-triggered decrease of phosphorylation at Ser9 was accompanied by an elevation of GSK-3β activity that underlies cell death, cells were incubated with NMDA in the presence of GSK-3 inhibitor IX, a selective inhibitor of GSK-3 (Meijer et al., 2003). As shown in Figure 3f, GSK-3 inhibitor IX concentration dependently reduced cell death triggered by NMDA.
BEO protects against NMDA-induced neuronal death in vitro
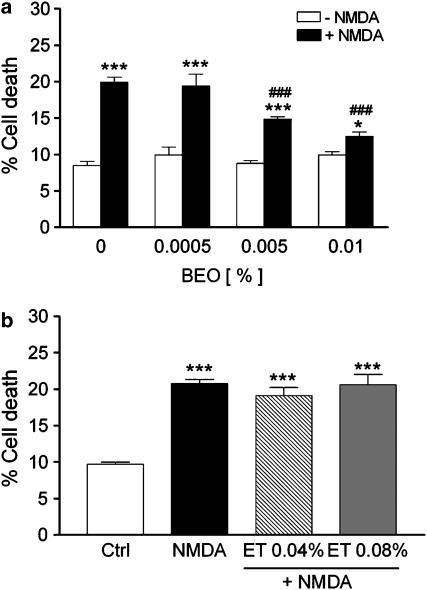
Preincubation of SH-SY5Y cultures with BEO for 60 min concentration dependently reduced cell death triggered by NMDA (Figure 4a). As shown in Figure 4b, the protective effects cannot be attributed to the vehicle used to dissolve the essential oil because ethanol, applied at the same concentrations present in 0.005 and 0.01% BEO, did not affect NMDA-induced cell death.
Figure 4.
BEO reduces cell death induced by NMDA in SH-SY5Y cultures. (a) BEO (final concentrations in medium: 0.0005, 0.005 and 0.01%) was added to SH-SY5Y cultures 60 min before application of 1 mM NMDA and cell death was assessed by trypan blue exclusion assay 24 h later; no protection against NMDA-induced cell death was produced by BEO at the lowest concentration (0.0005%). (b) Ethanol (ET, 0.04 and 0.08%), the vehicle used to dissolve BEO, does not affect 1.0 mM NMDA-induced cell death; these concentrations of ethanol correspond to those found in 0.005 and 0.01% BEO. Each value is the mean±s.e.m. of 4–12 experiments. *P<0.05 and ***P<0.001 vs control, respectively; ###P<0.001 vs NMDA given alone (ANOVA followed by Tukey–Kramer multiple comparisons test).
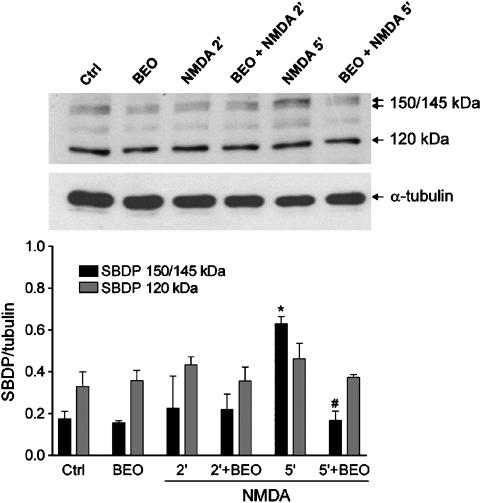
Interestingly, BEO (0.01%) prevented the accumulation of intracellular ROS caused by 1 mM NMDA (Figure 2). In addition, pretreatment with BEO (0.01%) prevented accumulation of calpain-specific 150–145 kDa SBDP caused by 5 min exposure to 1 mM NMDA (Figure 5).
Figure 5.
BEO prevented NMDA-induced activation of calpain I. Exposure of SH-SY5Y cells to 1 mM NMDA induced activation of calpain as assessed by Western-blotting analysis of generation of α-spectrin cleavage fragments (150/145 kDa) characteristic of calpain-mediated proteolysis. A significant accumulation of calpain-specific 150/145 kDa SBDP was reached at 5 min after NMDA exposure and this was prevented by a pretreatment (60 min beforehand) with BEO (0.01%). Exposure to NMDA and BEO, given alone or in combination, did not affect generation of 120 kDa α-spectrin fragment derived from caspase-mediated proteolysis. Histograms in lower panel show results of densitometric analysis of autoradiographic bands corresponding to 150/145 and 120 kDa SBDP. Data were normalized to the values yielded for α-tubulin. Each value is the mean±s.e.m. of three experiments. *P<0.05 vs control and #P<0.05 vs NMDA given alone (ANOVA followed by Tukey–Kramer multiple comparisons test).
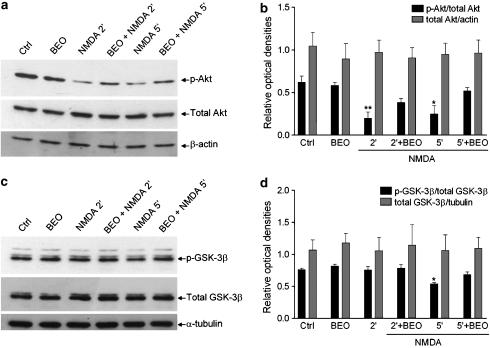
Exposure of SH-SY5Y cells to NMDA induces a fast and transient deactivation of Akt, which is associated with activation of GSK-3β. Therefore, we next evaluated whether or not BEO counteracts deactivation of Akt induced by NMDA. Interestingly, preincubation with a neuroprotective concentration of BEO (0.01%) reduced deactivation of Akt induced by 2 and 5 min exposure to NMDA (Figure 6a and b). Deactivation of Akt by NMDA is associated with a detrimental activation of GSK-3β (Figure 3); therefore, we next examined the effects of BEO on GSK-3β activation following exposure to NMDA. As shown in Figure 6c and d, BEO enhanced phosphorylation of GSK-3β, typically reduced by 5 min exposure to NMDA.
Figure 6.
BEO reduced NMDA-induced decrease of phospho-Akt and phospho-GSK-3β levels in SH-SY5Y cells. (a). Exposure of SH-SY5Y cells to 1 mM NMDA for 2 and 5 min induced deactivation of Akt kinase as determined by Western-blot analysis of phospho(Ser473)-Akt (p-Akt) levels and this was attenuated by BEO (0.01%) applied to neuroblastoma cultures 60 min before NMDA exposure. (b) Densitometric analysis of immunoreactive bands shows that the significant changes in phospho(Ser473)-Akt levels induced by BEO in NMDA-treated cells were not associated with changes in total Akt immunoreactivity. (c) Exposure of SH-SY5Y cells to 1 mM NMDA for 5 but not for 2 min induced activation of GSK-3β kinase as determined by Western-blot analysis of phospho(Ser9)-GSK-3β (p-GSK-3β) levels and this is attenuated by BEO (0.01%) applied to neuroblastoma cultures 60 min before NMDA addition. As confirmed by densitometric analysis of immunoreactive bands (d), BEO did not affect the expression of total GSK-3β in NMDA-treated cells. Each value in b and d is the mean±s.e.m. of 3–6 experiments; *P<0.05 and **P<0.01 vs control, respectively (ANOVA followed by Tukey–Kramer multiple comparisons test).
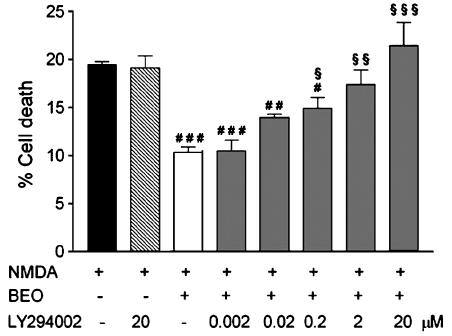
To investigate the intracellular pathways through which BEO reduces Akt deactivation, cells were preincubated with LY294002, a specific inhibitor of PI3K (Vlahos et al., 1994), for 30 min before addition of BEO and NMDA. Pretreatment with LY294002 concentration dependently reversed the neuroprotection (Figure 7) and prevented enhancement of p-Akt levels induced by BEO (data not shown), whereas NMDA-induced cell death was not affected by any concentration of the enzyme inhibitor (Figure 7 and data not shown).
Figure 7.
LY294002 concentration dependently reversed the neuroprotection afforded by BEO against NMDA-induced cell death. SH-SY5Y cells were treated with LY294002 (0.002–20 μM), a specific inhibitor of PI3K, for 30 min before the addition of BEO (0.01%, added 1 h before NMDA) and of 1 mM NMDA for 24 h and, then, cell death assessed by means of trypan blue staining. SH-SY5Y cells were treated with LY294002 (20 μM) for 30 min before exposure to 1 mM NMDA for 24 h. LY294002, given alone, did not affect NMDA-induced cell death. Each value is the mean±s.e.m. of 4–6 experiments. #P<0.05, ##P<0.01 and ###P<0.001 vs NMDA, respectively; $P<0.05, $$P<0.01 and $$$P<0.001 vs BEO+NMDA, respectively (ANOVA followed by Tukey–Kramer multiple comparisons test).
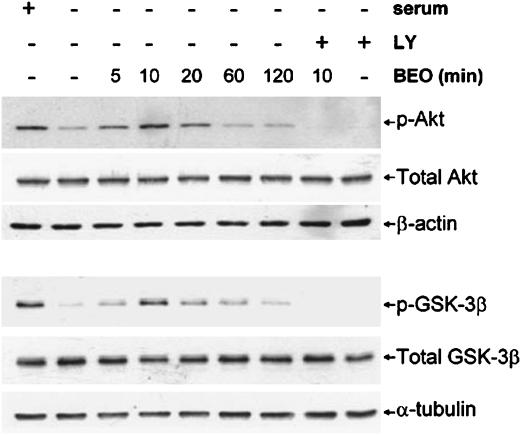
BEO activates Akt via PI3K in serum-starved cells
Serum-starved SH-SY5Y cells have reduced levels of p(Ser473)-Akt and p(Ser9)-GSK-3β (De Sarno et al., 2002). To examine whether or not BEO affects Akt and GSK-3β phosphorylation reduced by serum starvation, SH-SY5Y cells were cultured in serum-free medium for 1 h and then exposed to 0.01% BEO for 5, 10, 20, 60 and 120 min. Western-blot analysis showed that stimulation with BEO restored Akt and GSK-3β phosphorylation reduced by serum deprivation (Figure 8). To investigate the intracellular pathways involved, cells were pretreated with the PI3K inhibitor, LY294002, for 30 min before BEO stimulation. Inhibition of PI3K, which is upstream of Akt (see Brazil and Hemmings, 2001), prevented phosphorylation of Akt and GSK-3β otherwise enhanced by 10 min stimulation with BEO (Figure 8).
Figure 8.
BEO restored Akt and GSK-3β phosphorylation, reduced by serum withdrawal, via a PI3K-dependent mechanism. SH-SY5Y cells were maintained in serum-containing medium (+serum) or cultured in serum-free medium for 1 h (−serum); serum-starved cells were exposed to 0.01% BEO for 5, 10, 20, 60 and 120 min (min), then cellular proteins were extracted for subsequent analysis by Western blotting of Akt and GSK-3β phosphorylation by using polyclonal antibodies specific for Akt phosphorylated at Ser473 or for GSK-3β phosphorylated at Ser9. Serum deprivation caused a dramatic decline in phospho-Akt (p-Akt) and phospho-GSK-3β (p-GSK-3β) immunoreactivity. Application of BEO for 5–20 min enhanced phosphorylation of Akt and GSK-3β reduced by serum deprivation; this effect peaked at 10 min, then it progressively declined. A preincubation for 30 min with the specific PI3K inhibitor, LY294002 (LY; 20 μM), before exposure to BEO for 10 min, prevented the effects of the essential oil on Akt and GSK-3β phosphorylation. Representative immunoblots from three independent experiments are shown.
Characterization of the fractions of BEO responsible for neuroprotection in vitro
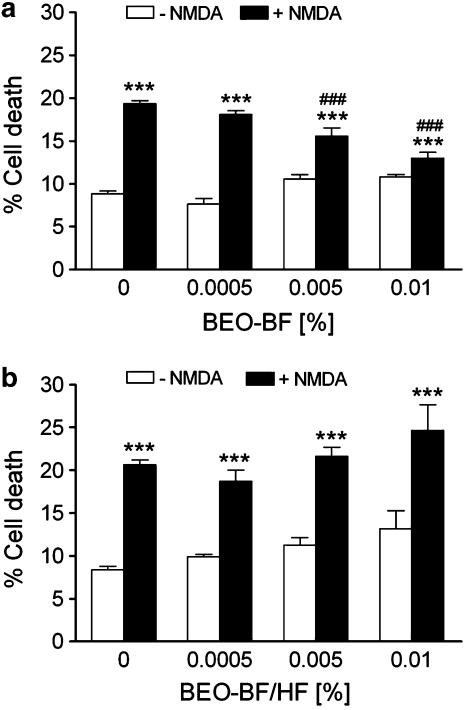
Preincubation of SH-SY5Y cultures with a bergapten-free extract of the essence (BEO-BF) also concentration dependently reduced cell death triggered by NMDA (Figure 9a). Quite importantly, a fraction of the oil deprived of bergapten and of monoterpene hydrocarbons (BEO-BF/HF) failed to rescue SH-SY5Y neuroblastoma cultures from NMDA-induced cell death (Figure 9b) thus suggesting that monoterpene hydrocarbons found in the volatile fraction of BEO may be responsible for neuroprotection.
Figure 9.
Monoterpene hydrocarbons could be involved in the neuroprotection afforded by BEO against NMDA-induced cell death. SH-SY5Y cultures were incubated with a bergapten-free extract of the essence (BEO-BF (a)) or with a fraction of the oil deprived of bergapten and of monoterpene hydrocarbons (BEO-BF/HF (b)) for 60 min before application of 1 mM NMDA and cell death was assessed by trypan blue exclusion assay 24 h later. (a) BEO-BF, but not (b) BEO-BF/HF, concentration dependently reduced NMDA-induced cell death. Each value is the mean±s.e.m. of 4–8 experiments. ***P<0.001 vs control; ###P<0.001 vs NMDA given alone (ANOVA followed by Tukey–Kramer multiple comparisons test).
Discussion
In the present study, we report the original observation that BEO protects against NMDA-induced cell death by affecting diverse death pathways triggered by the excitotoxin.
Human SH-SY5Y neuroblastoma cells have been shown to express both ionotropic and metabotropic glutamate receptors (Sun and Murali, 1998); however, no study has been until now carried out to characterize the effects on cell viability caused by abnormal stimulation of the NMDA subtype of glutamate receptors. The present experiments show that NMDA produces cell death when applied to human SH-SY5Y neuroblastoma cells in culture. Cytotoxicity elicited by NMDA is concentration-dependent and sensitive to blockade by selective, competitive (CGP40116) and noncompetitive (MK801), antagonists of the NMDA receptor complex. Calcium entry through NMDA receptor-associated cation channel activates calcium-dependent enzymes including NOS (Garthwaite et al., 1988; Kiedrowski et al., 1992). Sustained activation of NOS and the consequent generation of abnormal amounts of NO have been implicated in the mechanisms of excitotoxic cell death triggered in vitro by overactivation of glutamate receptors (Dawson et al., 1991; Corasaniti et al., 1992). Interestingly, 7-nitroindazole, a neuronal NOS preferring inhibitor (Babbedge et al., 1993), prevented cell death caused by the excitotoxin, thus suggesting that NO production may take part in the mechanisms of NMDA-evoked death of SH-SY5Y cells. These results are consistent with our previous observations obtained in other neuroectodermal cells, cultured human CHP100 cells, exposed to NMDA (Corasaniti et al., 1992). Collectively, these findings indicate that SH-SY5Y cells in culture represent a useful experimental model to begin evaluating the neuroprotective properties of selected compounds against excitotoxicity in vitro.
Akt is activated by phosphorylation of Ser473 and Thr308 by a signaling cascade involving PI3K and 3-phosphoinositide-dependent kinase-1 (PDK-1) (see Brazil and Hemmings, 2001), whereas de-phosphorylation is regulated by protein phosphatase 2A (Andjelkovic et al., 1996). Activated Akt promotes cell survival by phosphorylating and, thus, inactivating proteins implicated in promoting cell death such as Bad, caspase-9 and GSK-3β (Cardone et al., 1998; see Brazil and Hemmings, 2001). In particular, Akt inactivates GSK-3β by phosphorylating the enzyme at Ser9 (Cross et al., 1995; van Weeren et al., 1998).
Exposure of SH-SY5Y cells to NMDA resulted in a rapid deactivation of Akt kinase and this preceded activation of GSK-3β as monitored by Western-blot analysis of p-GSK-3β at Ser9. Quite importantly, a selective inhibitor of GSK-3 (Meijer et al., 2003) was able to rescue SH-SY5Y cells from death caused by NMDA, thus suggesting that reduction of p-GSK-3β levels was associated with an increase of kinase activity which underlies cell death. Consequently, the ability of BEO to maintain GSK-3β phosphorylation in NMDA-treated cells may well correlate with neuroprotection. The finding that BEO is able to prevent deactivation of Akt kinase and the downstream activation of GSK-3β induced by NMDA suggests an intriguing mechanism of neuroprotection. Indeed, pharmacological inhibition of GSK-3β, a critical activator of cell death in different models of neuronal insults (see Grimes and Jope, 2001; Bhat et al., 2004), has been recently reported to reduce neuronal death resulting from excitotoxicity in vitro (Facci et al., 2003; Kelly et al., 2004) and cerebral ischemia in vivo (Kelly et al., 2004), indicating that GSK-3β may represent a useful target for neuroprotective strategies.
The mechanism through which NMDA receptor activation deactivates Akt in SH-SY5Y cells remains to be elucidated; however, the finding that this effect is prevented by the use-dependent NMDA receptor channel blocker, MK801, suggests that calcium influx may be implicated. Activation of NMDA receptors affects the activity of protein phosphatases 1 and 2 (Mulkey et al., 1994). Of interest, in cerebellar granule cells, protein phosphatase inhibitors such as okadaic acid and calyculin A prevent glutamate-induced reduction of Akt phosphorylation and activity (Chalecka-Franaszek and Chuang, 1999). Similarly, okadaic acid prevented the dephosphorylation of Akt induced in retinal ganglion and amacrine cells by injection of NMDA into the vitreous cavity of rats (Nakazawa et al., 2005). Altogether, these observations suggest that NMDA-induced reduction of Akt phosphorylation in SH-SY5Y cells might be caused by activation of protein phosphatase 2A. Under our experimental conditions, cell death induced by NMDA was not affected by LY294002, a specific PI3K inhibitor (Vlahos et al., 1994), suggesting that inhibition of Akt phosphorylation does not facilitate death caused by NMDA. Interestingly, the protection afforded by BEO against NMDA-induced cell death is concentration dependently antagonized by LY294002, indicating that neuroprotection is mediated by PI3K. The latter deduction is further supported by the finding that LY294002 inhibited the ability of BEO to restore Akt phosphorylation reduced by serum deprivation. Therefore, increased Akt phosphorylation by BEO does not seem to be the consequence of inhibition of a phosphatase dephosphorylating Akt, as the level of p-Akt in the presence of an inhibitor of PI3K is unaltered by BEO. It has been previously shown that withdrawal of serum from cultured rat cortical neurons reduces phosphorylation of Akt on Ser473 and enhances GSK-3β activity and that addition of brain-derived neurotrophic factor (BDNF) restores Akt phosphorylation and suppresses GSK-3β activation (Hetman et al., 2000). Here, we have provided evidence that BEO restores Akt and GSK-3β phosphorylation decreased by serum deprivation. In addition, treatment with LY294002 inhibited BEO-induced phosphorylation of GSK-3β on Ser9, indicating that BEO negatively regulates GSK-3β activity in SH-SY5Y cells via a PI3K-dependent mechanism that involves Akt.
Treatment of SH-SY5Y cells with NMDA causes a significant accumulation of intracellular ROS and a rapid activation of the calcium-activated neutral protease calpain I. The latter observation is in line with our previous finding that calpain I is implicated in death of human CHP100 neuroblastoma cells caused by NMDA (Corasaniti et al., 1996).
Under the present experimental conditions, death of SH-SY5Y cells by NMDA was not preceded nor it is accompanied by activation of caspase-3. Despite reports of caspase-dependent excitotoxic neuronal death, lack of caspase-3 activation has been previously observed in primary rat hippocampal neurones treated with NMDA (Lankiewicz et al., 2000; Luo et al., 2003), suggesting that activation of caspases is not mandatory for expression of excitotoxicity. Calpain I has been reported to inhibit processing of procaspase-3 and -9 into their active subunits (Lankiewicz et al., 2000) and to downregulate caspase activity (Neumar et al., 2003). Therefore, it is conceivable that, under our experimental conditions, lack of caspase-3 activation following exposure to NMDA was owing early and robust activation of calpain I induced by the excitotoxin.
BEO prevents accumulation of calpain-specific 150–145 kDa SBDP caused by 5 min exposure of SH-SY5Y cells to NMDA, indicating that inhibition of calpain activation may be implicated in neuroprotection. In addition to activation of Ca2+-dependent enzymes, such as NOS and calpain I, Ca2+ entry through the NMDA receptor-gated cation channel triggers mitochondrial Ca2+ overload and dysfunction leading to generation of ROS which contribute to cell demise (Lafon-Cazal et al., 1993; Dugan et al., 1995; Stout et al., 1998). Interestingly, pretreatment of SH-SY5Y cultures with BEO prevented accumulation of intracellular ROS induced by NMDA. Collectively, these observations suggest that BEO affects diverse death pathways triggered by alteration of Ca2+ homeostasis induced by the excitotoxin.
Protection afforded by BEO against NMDA-induced cell death is reproduced by BEO-BF supporting the suggestion that bergapten was not involved in the protective actions. Quite importantly, BEO-BF/HF failed to rescue SH-SY5Y neuroblastoma cultures from cell death induced by NMDA, thus suggesting that monoterpene hydrocarbons found in the volatile fraction of BEO may be responsible for neuroprotection. The most abundant monoterpene hydrocarbons found in the volatile fraction of BEO are limonene, γ-terpinene, and β-pinene (Mondello et al., 1995; Verzera et al., 1996, 2003). Several reports on limonene show it to interfere with mevalonate metabolism (Elson, 1995) and protein isoprenylation (Crowell et al., 1994; Kawata et al., 1994), although no study has addressed its neuroprotective potential. Further investigation is needed to identify the monoterpene hydrocarbon/s responsible for neuroprotection afforded by BEO.
Acknowledgments
Partial financial support by the Italian Ministry for University and Scientific Research (PRIN 2004055288_1) and by Calabria Region (POR Calabria 2000–2006) to MTC is gratefully acknowledged. J Maiuolo and S Maida have contributed equally. We are particularly grateful to the referee of this MS for valuable suggestions that have enhanced the impact of the data presented in the present paper.
Abbreviations
- BEO
bergamot essential oil
- GSK-3β
glycogen synthase kinase-3β
- PI3K
phosphatidylinositol 3 kinase
- ROS
reactive oxygen species
Conflict of interest
The authors state no conflict of interest.
References
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52:119–132. doi: 10.1016/j.phrs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Jakubowicz T, Cron P, Ming XF, Han JW, Hemmings BA. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci USA. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood-Smith MJ, Poulton GA, Barker M, Mildenberger M. 5-Methoxypsoralen, an ingredient in several suntan preparations, has lethal, mutagenic and clastogenic properties. Nature. 1980;285:407–409. doi: 10.1038/285407a0. [DOI] [PubMed] [Google Scholar]
- Babbedge RC, Bland-Ward PA, Hart SL, Moore PK. Inhibition of rat cerebellar nitric oxide synthase by 7-nitro indazole and related substituted indazoles. Br J Pharmacol. 1993;110:225–228. doi: 10.1111/j.1476-5381.1993.tb13796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, LeFeuvre R, Rothwell NJ, Naldini L, et al. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Budd Haeberlein SL, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem. 2004;89:1313–1317. doi: 10.1111/j.1471-4159.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Chalecka-Franaszek E, Chuang D-M. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci USA. 1999;96:8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corasaniti MT, Bilotta A, Strongoli MC, Navarra M, Bagetta G, Di Renzo G. HIV-1 coat protein gp120 stimulates interleukin-1β secretion from human neuroblastoma cells: evidence for a role in the mechanisms of cell death. Br J Pharmacol. 2001;134:1344–1350. doi: 10.1038/sj.bjp.0704382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corasaniti MT, Navarra M, Catani MV, Melino G, Nisticò G, Finazzi-Agrò A. NMDA and HIV-1 coat protein, gp120, produce necrotic but not apoptotic cell death in human CHP100 neuroblastoma cultures via a mechanism involving calpain. Biochem Biophys Res Commun. 1996;229:299–304. doi: 10.1006/bbrc.1996.1796. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Tartaglia RL, Melino G, Nisticò G, Finazzi-Agrò A. Evidence that CHP100 neuroblastoma cell death induced by N-methyl-D-aspartate involves L-arginine-nitric oxide pathway activation. Neurosci Lett. 1992;147:221–223. doi: 10.1016/0304-3940(92)90600-c. [DOI] [PubMed] [Google Scholar]
- Croall DE, DeMartino GN. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991;71:813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovic M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Crowell PL, Ren Z, Lin S, Vedejs E, Gould MN. Structure–activity relationships among monoterpene inhibitors of protein isoprenylation and cell proliferation. Biochem Pharmacol. 1994;47:1405–1415. doi: 10.1016/0006-2952(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical culture. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3β phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, et al. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugo P, Mondello L, Dugo L, Stancanelli R, Dugo G. LC-MS for the identification of oxygen heterocyclic compounds in citrus essential oils. J Pharm Biomed Anal. 2000;24:147–150. doi: 10.1016/s0731-7085(00)00400-3. [DOI] [PubMed] [Google Scholar]
- Elson CE. Suppression of mevalonate pathway activities by dietary isoprenoids: protective roles in cancer and cardiovascular disease. J Nutr. 1995;125:1666S–1672S. doi: 10.1093/jn/125.suppl_6.1666S. [DOI] [PubMed] [Google Scholar]
- Facci L, Stevens DA, Skaper SD. Glycogen synthase kinase-3 inhibitors protect central neurons against excitotoxicity. Neuroreport. 2003;14:1467–1470. doi: 10.1097/00001756-200308060-00012. [DOI] [PubMed] [Google Scholar]
- Farmacopea Ufficiale Italiana 1991. Droghe Vegetali e Preparazioni (IX Edizione). Istituto Poligrafico e Zecca dello Stato, p. 75
- Fukunaga K, Kawano T. Akt is a molecular target for signal transduction therapy in brain ischemic insult. J Pharmacol Sci. 2003;92:317–327. doi: 10.1254/jphs.92.317. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean. Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3β in neuronal apoptosis induced by trophic withdrawal. J Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata S, Hagase T, Yamasaki E, Ishiguro H, Matsuzawa Y. Modulation of the mevalonate pathway and cell growth by pravastatin and d-limonene in a human hepatoma cell line (Hep G2) Br J Cancer. 1994;69:1015–1020. doi: 10.1038/bjc.1994.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Zhao H, Hua Sun G, Cheng D, Qiao Y, Luo J, et al. Glycogen synthase kinase 3beta inhibitor Chir025 reduces neuronal death resulting from oxygen-glucose deprivation, glutamate excitotoxicity, and cerebral ischemia. Exp Neurol. 2004;188:378–386. doi: 10.1016/j.expneurol.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L, Costa E, Wroblewski JT. Glutamate receptor agonists stimulate nitric oxide synthase in primary cultures of cerebellar granule cells. J Neurochem. 1992;58:335–341. doi: 10.1111/j.1471-4159.1992.tb09315.x. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- Lankiewicz S, Luetjens CM, Bui NT, Krohn AJ, Poppe M, Cole GM, et al. Activation of calpain I converts excitotoxic neuron death into a caspase-independent cell death. J Biol Chem. 2000;275:17064–17071. doi: 10.1074/jbc.275.22.17064. [DOI] [PubMed] [Google Scholar]
- Luo HR, Hattori H, Hossain MA, Hester L, Huang Y, Lee-Kwon W, et al. Akt as a mediator of cell death. Proc Natl Acad Sci USA. 2003;100:11712–11717. doi: 10.1073/pnas.1634990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DR, Guttmann RP. Excitotoxicity: perspectives based on N-methyl-D-aspartate receptor subtypes. J Pharmacol Exp Ther. 2002;300:717–723. doi: 10.1124/jpet.300.3.717. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled. Trends Pharmacol Sci. 2004;25:577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Barger SW, Begley JG, Mark RJ. Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Methods Cell Biol. 1995;46:187–216. doi: 10.1016/s0091-679x(08)61930-5. [DOI] [PubMed] [Google Scholar]
- Meijer L, Skaltsounis A-L, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Moncada S, Bolanos JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- Mondello L, Stagno d'Alcontres I, Del Duce R, Crispo F. On the genuineness of citrus essential oils. Part XL. The composition of the coumarins and psoralens of calabrian bergamot essential oil (Citrus bergamia Risso) Flav Fragr J. 1993;8:17–24. [Google Scholar]
- Mondello L, Dugo P, Bartle KD, Dugo G, Cotroneo A. Automated HPLC-HRGC: a powerful method for essential oils analysis. Part V. Identification of terpene hydrocarbons of bergamot, lemon, mandarin, sweet orange, bitter orange, grapefruit, clementine and mexican lime oils by coupled HPLC-HRGC-MS(ITD) Flav Fragr J. 1995;10:33–42. [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Shimura M, Endo S, Takahasci H, Mori N, Tamai M. N-Methyl-D-Aspartic acid suppresses Akt activity through protein phosphatase in retinal ganglion cells. Mol Vis. 2005;11:1173–1182. [PubMed] [Google Scholar]
- Neumar RW, Xu YA, Gada H, Guttmann RP, Siman R. Cross-talk between calpain and caspase proteolytic systems during neuronal apoptosis. J Biol Chem. 2003;278:14162–14167. doi: 10.1074/jbc.M212255200. [DOI] [PubMed] [Google Scholar]
- Noshita N, Lewen A, Suguwara T, Chan PH. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21:1442–1450. doi: 10.1097/00004647-200112000-00009. [DOI] [PubMed] [Google Scholar]
- Roberts-Lewis JM, Savage MJ, Marcy VR, Pinsker LR, Siman R. Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J Neurosci. 1994;14:3934–3944. doi: 10.1523/JNEUROSCI.14-06-03934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Navarra M, Maiuolo J, Rotiroti D, Bagetta G, Corasaniti MT. 17β-Estradiol protects SH-SY5Y cells against HIV-1 gp120-induced cell death: evidence for a role of estrogen receptors. Neurotoxicology. 2005;26:905–913. doi: 10.1016/j.neuro.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Sauer D, Allegrini PR, Cosenti A, Pataki A, Amacker H, Fagg GE. Characterization of the cerebroprotective efficacy of the competitive NMDA receptor antagonist CGP40116 in a rat model of focal cerebral ischemia: an in vivo magnetic resonance imaging study. J Cereb Blood Flow Metab. 1993;13:595–602. doi: 10.1038/jcbfm.1993.77. [DOI] [PubMed] [Google Scholar]
- Siman R, Noszek JC, Kegerise C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J Neurosci. 1989;9:1579–1590. doi: 10.1523/JNEUROSCI.09-05-01579.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout AK, Raphael HM, Kantere BI, Klann E, Reynolds IJ. Glutamate-induced neuron death requires mitochondrial calcium uptake. Nat Neurosci. 1998;1:366–373. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- Sun D, Murali SG. Stimulation of Na+-K+-2Cl− cotransporter in neuronal cells by excitatory neurotransmitter glutamate. Am J Physiol. 1998;275:C772–C779. doi: 10.1152/ajpcell.1998.275.3.C772. [DOI] [PubMed] [Google Scholar]
- van Weeren PC, de Bruyn KM, de Vries-Smits AM, van Lint J, Burgering BM. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J Biol Chem. 1998;273:13150–13156. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- Verzera A, Lamonica G, Mondello L, Trozzi A, Dugo G. The composition of bergamot oil. Perfumer Flavorist. 1996;21:19–34. [Google Scholar]
- Verzera A, Trozzi A, Gazea F, Cicciarello G, Cotroneo A. Effects of rootstock on the composition of bergamot (Citrus bergamia Risso et Poiteau) essential oil. J Agric Food Chem. 2003;51:206–210. doi: 10.1021/jf0206872. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Wu D. Neuroprotection in experimental stroke with targeted neurotrophins. NeuroRx. 2005;2:120–128. doi: 10.1602/neurorx.2.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zaynoun ST, Johnson BE, Frain-Bell W. A study of oil of bergamot and its importance as a phototoxic agent. I. Characterization and quantification of the photoactive component. Br J Dermatol. 1977;96:475–482. doi: 10.1111/j.1365-2133.1977.tb07149.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]