Abstract
Background and Purpose:
ATP-sensitive K+ channels (KATP) play a pivotal role in contractility of urinary bladder smooth muscle. This study reports the characterization of 4-methyl-N-(2,2,2-trichloro-1-(3-pyridin-3-ylthioureido)ethyl)benzamide (A-251179) as a KATP channel opener.
Experimental Approach:
Glyburide-sensitive membrane potential, patch clamp and tension assays were employed to study the effect of A-251179 in vitro. The in vivo efficacy of A-251179 was characterized by suppression of spontaneous contractions in obstructed rat bladder and by measuring urodynamic function of urethane-anesthetized rat models.
Key Results:
A-251179 was about 4-fold more selective in activating SUR2B-Kir6.2 derived KATP channels compared to those derived from SUR2A-Kir6.2. In pig bladder smooth muscle strips, A-251179 suppressed spontaneous contractions, about 27- and 71-fold more potently compared to suppression of contractions evoked by low-frequency electrical stimulation and carbachol, respectively. In vivo, A-251179 suppressed spontaneous non-voiding bladder contractions from partial outlet-obstructed rats. Interestingly, in the neurogenic model where isovolumetric contractions were measured by continuous transvesical cystometry, A-251179 at a dose of 0.3 μmol kg-1, but not higher, was found to increase bladder capacity without affecting either the voiding efficiency or changes in mean arterial blood pressure.
Conclusions and Implications:
The thioureabenzamide analog, A-251179 is a potent novel KATP channel opener with selectivity for SUR2B/Kir6.2 containing KATP channels relative to pinacidil. The pharmacological profile of A-251179 is to increase bladder capacity and to prolong the time between voids without affecting voiding efficiency and represents an interesting characteristic to be explored for further investigations of KATP channel openers for the treatment of overactive bladder.
Keywords: A-251179, ATP-sensitive K+ channels, overactive bladder, potassium channel opener, incontinence, potassium channel, bladder, SUR2A, SUR2B, Kir6.2
Introduction
ATP-sensitive K+ (KATP) channels play an important role in linking cellular energy metabolism to membrane potential and cellular excitability in a variety of tissues including pancreas, central nervous system, skeletal muscle, heart and in diverse smooth muscle tissues such as the bladder and peripheral vasculature (Quayle et al., 1997; Ashcroft, 2006). These channels function as hetero-octameric complexes derived from four inwardly rectifying K+ channel (Kir) subunits that form the K+ ion conducting pore, and four regulatory sulphonylurea receptor (SUR) subunits (Aguilar-Bryan et al., 1998; Seino, 1999). Distinct Kir (Kir6.1 and Kir6.2) and SUR (SUR1, SUR2A and SUR2B) subunits can assemble to generate tissue-specific KATP channels that exhibit unique biophysical and pharmacological properties. For example, SUR1/Kir6.2 KATP channels serve as regulators of pancreatic β-cell insulin release and as glucose sensors in the hypothalamic neurons (Inagaki et al., 1995; Lam et al., 2005; Pocai et al., 2005). Sarcolemmal cardiac KATP channels are constituted from SUR2A/Kir6.2 complexes, whereas smooth muscle KATP channels are derived from SUR2B assembled with either Kir6.1 or Kir6.2 subunits (Isomoto et al., 1996; Yamada et al., 1997). In addition, mitochondrial KATP channels also exists that may serve as targets for anti-ischemic agents (Grover and Garlid, 2000; Busija et al., 2004).
Although the majority of the initial work on KATP channel-based therapeutic agents focused on their potential as antidiabetic agents for which blockers are clinically used and as antihypertensive agents, more recently, efforts have shifted towards indications such as hyperinsulinemia, cardioprotection, ventricular arrhythmia and bladder overactivity (for a review see Mannhold, 2004; Carroll, 2006). Although achieving tissue selectivity remains a potential challenge, the quest for selective compounds has been facilitated by the identification of molecularly defined KATP channels, and indeed, SUR1/Kir6.2 openers for hyperinsulinemia and SUR2A/Kir6.2 blockers for ventricular fibrillation have emerged (Atwal et al., 1995; Sebille et al., 2006).
KATP channels, along with large and small conductance Ca2+-activated K+ channels, are critical to the control of myogenic tone and excitability in bladder smooth muscle cells (Foster et al., 1989a, 1989b; Buckner et al., 2002; Herrera and Nelson, 2002; Gopalakrishnan and Shieh, 2004). It is well established that KATP channels play a key role in regulating membrane potential and phasic spontaneous bladder smooth muscle contractility and, accordingly, openers are potentially useful in the treatment of urological conditions such as overactive bladder where heightened levels of disease-related spontaneous contractions have been observed (Brading, 1997; Hashitani and Brading, 2003; Gopalakrishnan and Shieh, 2004). In vitro studies have shown that KATP channel openers are effective in suppressing spontaneous myogenic contractility and do so more potently (about 15-fold) compared to inhibiting contractions evoked by electrical field stimulus, which are mediated through muscarinic and purinergic receptors (Buckner et al., 2002). Thus, channel openers that selectively modulate disease-related spontaneous contractions may have the potential to treat overactive bladder, without untoward or minimal cardiovascular effects.
Among ATP-sensitive K+ channel opener, a variety of structural classes including benzopyrans, cyanoguanidines, tertiary carbinols and dihydropyridines are known (Mannhold, 2006). However, the search for novel structures from which to explore bladder-selective KATP channel openers continues. Our in-house screening of compound libraries using membrane potential-based assays yielded a class of compounds, referred to as thioureabenzamides that were found to be potent openers of KATP channels (Perez-Medrano et al., 2004). In this study, we present in vitro and in vivo pharmacological properties of a prototypical compound 4-methyl-N-(2,2,2-trichloro-1-(3-pyridin-3-ylthioureido)ethyl)benzamide, A-251179. Some of these studies have previously been presented in an abstract form (Milicic et al., 2004).
Materials and methods
Animal experiments
Studies were carried out in accordance with guidelines outlined by the Animal Welfare Act, the Association for Assessment and Accreditation of Laboratory Animals (AAALAC) and the Institutional Animal Care and Use Committee of Abbott Laboratories.
Membrane potential assays
Functional activity of KATP channels in guinea-pig bladder smooth muscle cells was assessed as described previously (Gopalakrishnan et al., 1999) by evaluating changes in the membrane potential using the bis-oxonol dye, bis-(1,3-dibutylbarbituric acid)trimethine oxonol (DiBAC4(3)), in a 96-well fluorescent imaging plate reader (FLIPR). Briefly, urinary bladders were removed from anesthetized male guinea-pigs (Hartley, Charles River, Wilmington, MA, USA) weighing 250–300 g and cells were isolated by enzymic dissociation. The bladder was chopped into small sections and incubated in Dulbecco's phosphate-buffered saline (DPBS, Life Technologies, Gaithersburg, MD, USA) containing 1 mg ml−1 collagenase (type VIII, Sigma, St Louis, MO, USA) and 0.2 mg ml−1 pronase (Calbiochem, La Jolla, CA, USA) with continuous stirring at 37°C in a cell incubator for 30 min. The cells were harvested and resuspended in 5 ml growth media (Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 U ml−1 penicillin, 100 U ml−1 streptomycin and 0.25 mg ml−1 amphotericin B) and dissociated further by pipetting repeatedly through a flame-polished Pasteur pipette and passing it through a polypropylene mesh membrane (Spectrum, Houston, TX, USA). Cells were maintained in the incubator with 90% air:10% CO2 for 5–7 days before experiments. Confluent cells, cultured in black clear-bottomed 96-well plates (Packard ViewPlate-96), were rinsed twice with 200 μl assay buffer containing (mM): 20 HEPES, 120 NaCl, 2 KCl, 2 CaCl2, 1 MgCl2, 5 glucose (pH) 7.4 at 25°C and 5 μM DiBAC4(3) and then incubated with 180 μl of buffer in the incubator for 30 min to ensure dye distribution across the membrane. Assays were carried out at 37°C and were initiated by the addition of various concentrations of test compounds. Changes in DiBAC4(3) fluorescence were measured at excitation and emission wavelengths of 488 and 520 nm, respectively.
Whole-cell patch clamp
SUR2A/Kir6.2 and SUR2B/Kir6.2 KATP channels
The cDNA clones of rat Kir6.2 mouse SURs (SUR2A and SUR2B) were used (Isomoto et al., 1996; Shindo et al., 1998). The plasmid containing Kir6.2 was cotransfected with either SUR2A or SUR2B cDNA into HEK293T cells by using LipofectAMINE (Invitrogen Co, Carlsbad, CA, USA) according to manufacturer's instructions. Electrophysiological measurements were usually conducted 2–3 days after transfection. Electrophysiological studies were carried out at room temperature using the whole-cell patch-clamp configuration as described previously (Shindo et al., 1998). The tip of the electrodes was fire polished, coated with silicon and had a resistance of 3–5 MΩ upon filling with an internal solution containing (in mM): 140 KCl, 2 MgCl2, 5 EGTA–KOH and 5 HEPES–KOH (pH 7.3). ATP (3 mM) was added to the internal solutions with concentration of free Mg2+ adjusted to 1.4 mM (Dawson et al., 1986). The bath solution contained (in mM): 121.9 NaCl, 20.0 KCl, 1.8 CaCl2, 0.53 MgCl2, 5.5 glucose and 5.0 HEPES–NaOH (pH 7.4). The membrane currents were amplified with Axopatch 200A (Molecular Device Co, Sunnyvale, CA, USA) and monitored throughout the experiments with an analog-storage oscilloscope (Dual Beam Storage Oscilloscope, Tektronix, Inc., Beaverton, OR, USA). For subsequent analyses, the data were low-pass filtered at 1.0 kHz (−3 dB) with an 8-pole Bessel filter (Frequency Devices, Harverhill, MA, USA), and digitized at 3 or 5 kHz with an AD converter (ITC-16, Instrutech Corp., NY, USA). The whole-cell current response to compounds in cells expressing SUR2A/Kir6.2 or SUR2B/Kir6.2 channels was measured by subtracting the basal current from the evoked response. The subtracted current at each concentration of A-251179 was normalized to that induced by 100 μM pinacidil in each cell at −20 mV.
Urinary bladder smooth muscle cells
Whole-cell patch clamp technique was used to measure changes in ionic currents from bladder smooth muscle cells as described previously (Shieh et al., 2001). Urinary bladders were transferred directly into pre-oxygenated physiological saline solution containing (in mM): 137 NaCl, 5.4 KCl, 2 CaCl2, 2 MgCl2, 0.42 KH2PO4, 4.17 NaHCO3, 10 HEPES, 10 glucose (pH 7.4 with NaOH). Pieces of bladder smooth muscle were incubated with collagenase and single smooth muscle cells were obtained by triturating using a fire-polished large bore Pasteur pipette. The intracellular pipette solution contained the following (in mM): 107 KCl, 1.2 MgCl2, 1 CaCl2, 10 EGTA, 5 HEPES, 0.1 ATP (pH 7.2 with KOH; total K140 mM). The bath solution contained the following (in mM): 60 KCl, 80 NaCl, 2.6 CaCl2, 1.2 MgCl2, 5 HEPES (pH 7.4 with NaOH). Whole-cell currents were recorded at room temperature and were amplified using Axopatch-200B amplifier and low-pass filtered at 5 kHz (−3 dB, four pole Bessel filter) before digitization (Digidata 1200B) at a sampling rate of 10 kHz.
Smooth muscle relaxation
Urinary bladder
Bladder strip relaxation studies were performed as described previously (Buckner et al., 2000). Briefly, female Landrace pigs (Wilson's Prairie View Farm, Burlington, WI, USA) weighing 9–25 kg were killed with an intraperitoneal injection of pentobarbital (150–200 mg kg−1; Somlethol, JA Webster Inc., Sterling, MA, USA). The entire urinary bladder was removed and placed in Krebs–Ringer bicarbonate solution containing (mM): 120 NaCl, 20 NaHCO3, 11 dextrose, 4.7 KCl, 2.5 CaCl2, 1.5 MgSO4 and 1.2 KH2PO4 (equilibrated with 5% CO2: 95% O2, pH 7.4 at 37°C). The bladder was sectioned after discarding the top dome portion and the lower trigonal area. Approximately 3–5 20-mm strips were prepared from the remaining tissue adjacent to the trigonal area and cut in a circular fashion. The mucosal layer was removed and strips were mounted in 10 ml tissue baths maintained at 37°C with one end fixed to a stationary rod and the other to a Grass FT03 transducer at a basal preload of 1.0 g. Tissues were rinsed at 10 min intervals and allowed to equilibrate for at least 70 min. Spontaneous myogenic phasic activity manifested as transient spikes that varied in frequency, duration and amplitude was observed in many tissues. Tissue strips were exposed to varying concentrations of the test agents for 15 min and changes in contractility were assessed. For electrical field stimulation studies, two parallel platinum electrodes were included and tissues were stimulated using a frequency of 0.05 Hz, 0.5 ms at 20 V. In the presence of 100 nM tetrodotoxin (TTX), complete cessation of field-stimulated contraction was observed within 25 min, which returned to control values within 60 min after rinsing with Krebs–Ringer solution (data not shown). For carbachol-stimulated tissues, the protocol was noncumulative with rinse cycles between each concentration of test compound because the contractile response tended to wane over time. Tissues were pretreated with test compounds for 15 min, exposed to a fixed concentration of carbachol (300 nM, which is approximately an EC75 concentration of carbachol) and changes in tension assessed. The tissue was then rinsed for 15 min, and the cycle repeated with another concentration of test compound. Glyburide (10 μM) was added at the conclusion of each concentration response curve to assess reversibility of effects.
Thoracic aorta
The entire thoracic aorta from male Sprague–Dawley rats (200–350 g) was removed and immediately placed into Krebs–Ringer bicarbonate solution. The aorta was cleaned of extraneous tissue, endothelium removed, cut into 3–4 mm rings and mounted in 10 ml isolated tissue baths at 37°C. One end was fixed to a stationary glass rod and the other to a Grass FT03 transducer at a basal preload of 1.0 g. Tissues were rinsed every 10 min for a total of 45–60 min. The aorta was primed once with 80 mM KCl, washed to basal tension and stimulated with phenylephrine (10 μM) to test for receptor-mediated functional responses. Absence of functional endothelium was also confirmed by loss of the acetylcholine (10 μM)-induced relaxation. After an additional 60 min equilibration period, tension was established using 25 mM KCl, and cumulative concentration relaxation response curve was generated for test compounds.
Spontaneous contractions in obstructed rats
Female Sprague–Dawley rats (190–210 g) were anesthetized with halothane and the proximal urethra was ligated using a monofilament ligature as described previously from our laboratory (Fabiyi et al., 2003). At 4 weeks post-obstruction, rats were anesthetized with urethane and both femoral artery and vein were catheterized to measure arterial pressure and to administer test compounds, respectively. Intravesicular pressure was measured using a polyethylene catheter inserted into the apex of the bladder dome. Saline was infused at the rate of 0.1 ml min−1 until a volume was reached sufficient enough to trigger spontaneous non-voiding contractions, but below the threshold for voiding. These spontaneous contractions were allowed to stabilize, after which changes in bladder pressure and mean arterial pressure (MAP) were monitored simultaneously for 20 min before and after cumulative intravenous doses of test compound or vehicle.
Isovolumetric contractions in urethane-anesthetized rats
Female Wistar rats (280–330 g) were anesthetized with urethane (0.6 g kg−1 subcutaneously followed by 0.6 g kg−1 intraperitoneally). The left femoral artery and vein were cannulated with polyethylene (PE-50) tubing for the measurement of arterial pressure and compound administration, respectively. A third polyethylene catheter (PE-60) was inserted 3–4 mm into the apex of the bladder dome and secured using a 5-0 silk purse string suture. The bladder was emptied via this catheter and additionally by applying slight manual pressure on the lower abdomen. The urinary catheter was connected using a Y-tube connector to both a pressure transducer (Gould Statham P23ID) and a syringe pump (Harvard Model 22). Continuous transvesical cystometry was performed using a constant infusion of physiological saline at a rate of 0.1 ml min−1 at room temperature. Intravesical (bladder) pressure and arterial pressure were monitored continuously using a computerized data acquisition system (Modular Instruments Inc., Malvern, PA, USA). The time corresponding to each void and the volume of each void (determined by weighing the volume collected on a filter paper disk), the cystometric parameters of bladder capacity (bladder volume at micturition), residual volume (volume remaining in the bladder after micturition) and voiding efficiency (voided volume/capacity) were calculated. The lowest pressure after a void (basal pressure), the pressure at the onset of bladder contraction (threshold pressure), the pressure at which expelled fluid is first seen (bladder opening pressure) and the peak pressure during micturition were all determined from the intravesical pressure trace. Bladder compliance (capacity/(threshold pressure−basal pressure)) and the time between voids in seconds (inter-contraction interval) were also recorded. The various cystometric parameters together with mean arterial pressure and heart rate were assessed 30 min prior to and 30 min after, a single intravenous dose of the test compound. Data are presented as mean±s.e.m., with 4–7 rats tested at each dose.
Data analysis
The concentration dependence of maximal steady-state changes in fluorescence or tension responses of tissue strips was fitted by nonlinear regression analysis (GraphPad Prism, San Diego, CA, USA) to obtain EC50 or IC50 values as appropriate. Spontaneous phasic activity of bladder strips was analyzed for changes in the area under the curve (AUC) of the contractile response during a 15-min interval. For carbachol-stimulated responses, values were expressed as percentage of the precontraction responses produced by carbachol. In electrical field-stimulated tissues, concentration-dependent reduction in the peak amplitude (measured in grams) was used for calculating the EC50 values. In in vivo studies, data were acquired and analyzed using the Life Science Suite/Po-ne-mah Physiology Platform (Gould Instrument Systems, Valley View, OH, USA). Bladder contraction amplitude, frequency, duration and area under the bladder pressure curve (AUC) were determined using the Po-ne-mah CYS analysis module. Data were averaged over the entire 30 min postdosing period and expressed as percentage change from baseline values. In obstructed rats, an increase in bladder pressure over 1 cm H2O is considered a contraction, and data were averaged over the last 10 min of each period after dosing where bladder effects were maximal. Estimated intravenous doses of each compound required to reduce unstable contraction AUC by 35% values were estimated from the dose response relationships using GraphPad Prism. Data are expressed as mean±s.e.m. When comparing group means, a P-value <0.05 was considered statistically significant.
Materials
DiBAC4(3) was purchased from Molecular Probes (Eugene, OR, USA). Pinacidil, ATP and glibenclamide were purchased from Sigma Chemical Co. (St Louis, MO, USA). 4-methyl-N-(2,2,2-trichloro-1-(3-pyridin-3-ylthioureido)ethyl) benzamide (A-251179) was obtained from the Abbott Chemical Depository or synthesized in house.
Results
Effects on DiBAC4(3) fluorescence responses
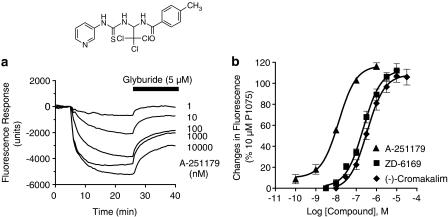
Screening of the in-house compound library yielded several thioureabenzamide analogs, exemplified by the prototypical compound, A-251179. A-251179 evoked concentration-dependent changes in membrane hyperpolarization when measured using DiBAC4(3) fluorescence changes in guinea-pig bladder smooth muscle cells. The evoked changes in membrane hyperpolarization were reversed by subsequent addition of glyburide (5 μM). A representative trace is shown in Figure 1a. The −log EC50 value for A-251179 to evoke membrane hyperpolarization was 7.87±0.07 (n=6), which was 16- and 24-fold more potent than ZD6169 (−log EC50=6.56±0.1) and (−)-cromakalim (−log EC50=6.47±0.03) (Figure 1b).
Figure 1.
Effect of A-251179 on membrane potential responses in guinea-pig bladder smooth muscle cells. (a) The traces shown are fluorescence responses to DiBAC4(3) triggered by application of varying concentrations of A-251179 and its reversal by glyburide (5 μM) as indicated. Inset: chemical structure of A-251179. (b) Concentration-response relationship of the membrane potential effects in comparison with ZD-6169 and (−)-cromakalim. Data are normalized to fluorescence responses evoked by a reference KATP channel opener, P1075 (10 μM).
Effect on SUR2B/Kir6.2 and SUR2A/Kir6.2 KATP channels
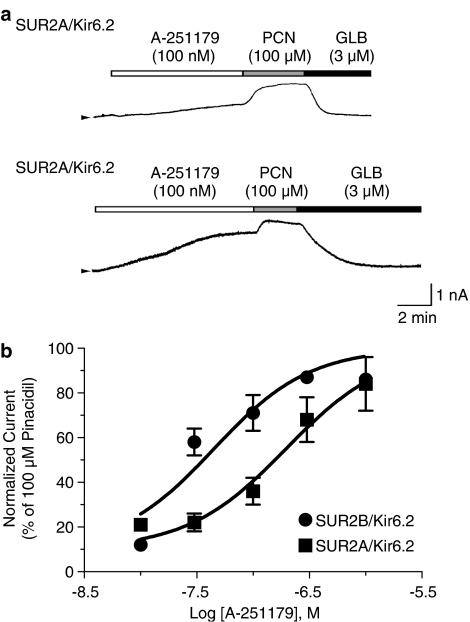
Direct interaction of A-251179 with KATP channels was studied by whole-cell patch clamp in HEK293T cells expressing SUR2B/Kir6.2 or Sur2A/Kir6.2 channels (Figure 2). In cells expressing SUR2B/Kir6.2, the threshold concentration of A-251179 to evoke a glyburide-sensitive current was approximately 10 nM and the plateau currents was obtained at approximately 100 nM, at which concentration the evoked current amplitude was 85±2% (n=6) of that evoked by 100 μM pinacidil. The concentration-dependent curve was generated by plotting the relative channel activity (normalized current with reference to the current amplitude evoked by 100 μM pinacidil) versus the concentration tested. The EC50 value for A-251179 to activate SUR2B/Kir6.2 channels was 36.5 nM with a Hill coefficient value of 0.9. In contrast, the threshold concentration to evoke a glyburide-sensitive current in HEK293T cells expressing SUR2A/Kir6.2 was approximately 30 nM and the maximum current evoked by 1 μM A-251179 was 83±12% (n=3) of that induced by 100 μM pinacidil. The EC50 value for A-251179 to evoke SUR2A/Kir6.2 was 138.5 nM with a Hill coefficient value of 0.73.
Figure 2.
Effect of A-251179 on activating KATP currents in HEK293T cells transfected with SUR2A/Kir6.2 and SUR2B/Kir6.2 subunits. (a) Activation of membrane currents by A-251179 (100 nM) and pinacidil (PCN, 100 nM) in HEK293 cells transfected with SUR2A/Kir6.2 and SUR2B/Kir6.2 is depicted. The evoked currents were inhibited by 3 μM glyburide (GLB). (b) A-251179 evoked concentration-dependent increases in SUR2A/Kir6.2 (solid squares) and SUR2B/Kir6.2 (solid circles) currents. The peak currents evoked by A-251179 at each concentration was normalized to that evoked by 100 μM pinacidil.
Effect on guinea-pig bladder smooth muscle KATP currents
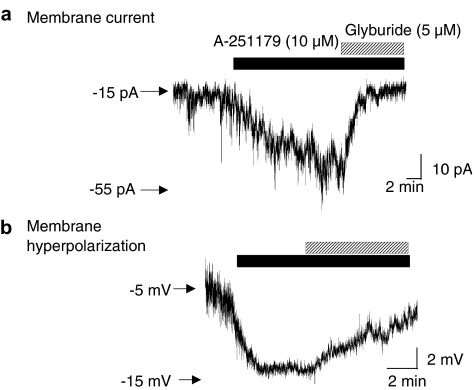
To examine effects of A-251179 at KATP channels, whole-cell patch clamp recording was performed in guinea-pig bladder smooth muscle cells. Application of 10 μM A-251179 evoked an increase in inward current under the conditions where the bath solution contained 60 mM K+ and cells were voltage clamped at −80 mV with patch pipette containing 140 mM K+ and 0.1 mM ATP. Addition of glyburide (5 μM) suppressed the A-251179-evoked increases in currents (Figure 3a). Under whole-cell current clamp recording conditions, A-251179 also hyperpolarized membrane potential from −5.5 to −14 mV in guinea-pig bladder smooth muscle cells in a glyburide-sensitive manner (Figure 3b). The changes in membrane potential is within the range of K+ equilibrium potential of −20 mV under the current-clamp recording conditions where the intracellular and extracellular K+ concentration are 140 and 60 mM, respectively. Taken together, these results suggest that A-251179 can stabilize excitability of smooth muscle cells by opening KATP channels.
Figure 3.
Effect of A-251179 on membrane current and potential in guinea-pig bladder smooth muscle cells. (a) Application of 10 μM A-251179 evoked an increase in inward whole-cell current in guinea-pig bladder smooth muscle cells that was sensitive to the inhibition by 5 μM glyburide. Cells were voltage-clamped at −80 mV and changes in membrane currents were measured in bath solution containing 60 mM K+ with pipette solution containing 140 mM K+ and 0.1 mM ATP. (b) A-251179 also lowered membrane potential in bladder smooth muscle cells. In the trace shown above, 10 μM A-251179 hyperpolarized membrane potential, which was reversed to control values in the presence of 5 μM glyburide. The K+ equilibrium potential is −20 mV under the current-clamp recording conditions where the intracellular and extracellular K+ concentrations are 140 and 60 mM, respectively.
Smooth muscle relaxation
Rat thoracic aorta
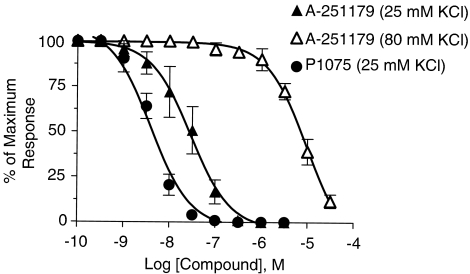
Effects of A-251179 on muscle strip relaxation were studied in rat thoracic aorta precontracted with 25 mM or 80 mM KCl. A-251179 suppressed 25 mM KCl evoked muscle contraction in a concentration-dependent manner (concentration and tension, respectively, 0.3 nM, 103.3±2.3 cg; 3 nM, 93.3±26.2 cg; 30 nM, 61.7±39.2; 300 nM, 1.0±0.0 cg). The −log EC50 was 7.53±0.05, which was sevenfold less potent than P1075 (−log EC50 value, 8.40±0.08; Figure 4), a known KATP channel opener (concentration and tension, respectively, 0.3 nM, 113.3±13.3 cg; 3 nM, 75.0±24.7 cg; 30 nM, 4.3±0.7 cg; 300 nM, 0 cg). In contrast, the capability of A-251179 to relax vascular smooth muscle was greatly attenuated when muscle contraction was evoked with 80 mM KCl depolarization (−log EC50 value of 5.01±0.04). These results indicated that the relaxation by A-251179 is mediated through K+ channel-dependent mechanisms.
Figure 4.
Inhibition of contractility of rat thoracic aorta strip. A-251179 suppressed contractility evoked by 25 mM K+ (solid triangles) and 80 mM K+ (open triangles) in a concentration-dependent manner. Inhibition of contractility evoked by 20 mM K+ by P1075 (solid circles) is shown for comparative purposes.
Urinary bladder smooth muscle
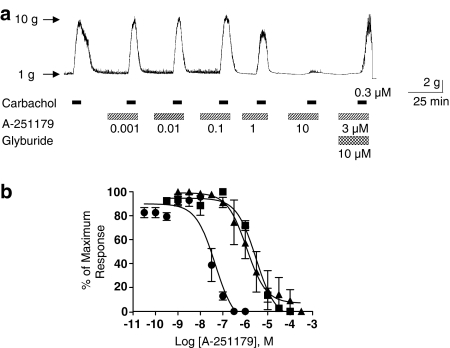
In pig bladder smooth muscle strips that showed spontaneous phasic contractions, A-251179 evoked a concentration-dependent suppression of spontaneous contractions in a glyburide sensitive manner with a −log EC50 value of 7.34±0.22. A-251179 also suppressed carbachol-stimulated pig bladder smooth muscle contractions with a −log EC50 value of 5.52±0.12. A representative trace is shown in Figure 5a. Contractions evoked by low-frequency electrical stimulation (0.05 Hz, 0.5 ms, 20 V) that reflect presynaptic release of neurotransmitters such as acetylcholine and ATP were also inhibited by A-251179 with −log EC50 value of 5.91±0.05 (Figure 5).
Figure 5.
Inhibition of contractility of pig bladder smooth muscle. (a) A-251179 suppressed carbachol (0.3 μM)-induced bladder smooth muscle contraction in a glyburide-sensitive manner. Note the intrinsic spontaneous activity of smooth muscle was reduced in the presence of A-251179. (b) Concentration-dependent inhibition curves demonstrating suppression of spontaneous contractions (solid circles) and that evoked by carbachol (solid squares) and low-frequency field stimulation (solid triangles).
Effects on unstable bladder contractions
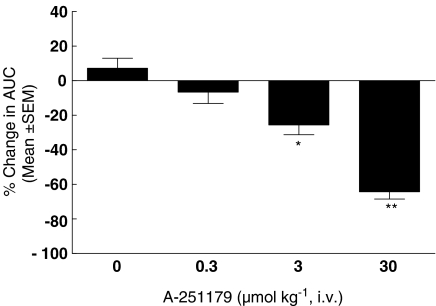
To examine efficacy in vivo, A-251179 was evaluated in a rat model of bladder hyperactivity with spontaneous nonvoiding myogenic contractions secondary to partial outlet obstruction described previously from our laboratory (Fabiyi et al., 2003). As shown in Figure 6, A-251179 was found to suppress unstable bladder contractions in a dose-dependent manner. At the three doses tested (0.3, 3 and 10 μmol kg−1 intravenous), A-251179 reduced total contractions AUC by 7±7, 26±6 and 64±4%, respectively (Figure 6).
Figure 6.
Effects of A-251179 on bladder contractions in the obstructed rat bladder model. A-251179 suppressed bladder contraction (AUC) concentration-dependent manner. Data represent mean±s.e.m. of five animals (*P<0.05, **P<0.01, paired t-test).
Effects on isovolumetric contractions
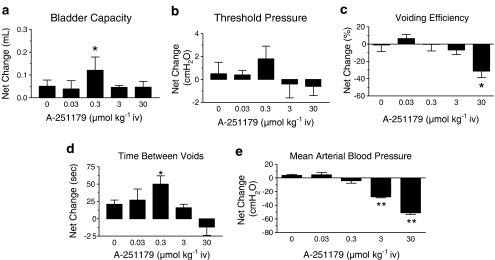
To study further the effects on urodynamic function, A-251179 was tested for effects on volume-induced neurogenic contractions in the anesthetized rat model (Figure 7). No substantial changes in cystometric parameters was observed following intravenous administration of 0.03 μmol kg−1 A-251179. On the other hand, administration of the 0.3 μmol kg−1 dose resulted in significant increases in bladder capacity and time between voids without compromising voiding efficiency. The reduction in voiding frequency could be attributed to increases in voided volume (vehicle, 0.028±0.009 ml; A-251179, 0.074±0.016 ml, n=5, P<0.05) with no significant changes in residual volume. A-251179 appeared to demonstrate the positive effect of inhibiting the voiding reflex without affecting the contractility of the bladder and without a decrease in arterial pressure. Increases in doses of A-251179 to 3 μmol kg−1 did not significantly change cytometric parameters, but did reduce mean arterial pressure (by 27.8±1.3 mm Hg; n=5), measured simultaneously. At the highest dose tested, 30 μmol kg−1, A-251179 reduced voiding efficiency resulting in increases in residual volume (0.083±0.01 ml, n=4, compared to vehicle 0.023±0.03 ml, P<0.01) with no significant improvement of bladder capacity and voiding frequency. At this dose, the mean arterial pressure was also significantly reduced by 51.1±2.4 mm Hg (n=6, P<0.01).
Figure 7.
Effects of A-251179 on urodynamic parameters of the neurogenic hyperactivity model. The figure shows changes in bladder capacity (a), threshold pressure (b), voiding efficiency (c), time between voids (d) and mean arterial pressure (e) evoked at various concentrations of A-251179. Values shown are means±s.e.m. of six separate determinations (*P<0.05, **P<0.01, paired t-test).
Discussion
Overactive bladder, also referred to as urgency/frequency syndrome with or without incontinence, remains an unmet medical condition that affects individuals, particular women and the elderly. Although muscarinic receptor antagonists are currently the therapeutic mainstay, the limited efficacy of these agents coupled with side effects such as constipation, blurred vision and dry mouth often lead to discontinuation of the therapy. Although a new generation of cholinergic antagonists such as darifencin and solifenacin have been developed with the promise of improved efficacy and/or tolerability (Cardozo et al., 2004; Chapple, 2004; Zinner et al., 2004), no clinically meaningful additional benefits have yet been demonstrated. A range of K+ channels including ATP-sensitive, large-conductance and small conductance K+ channels have been demonstrated to play important roles in regulating urinary bladder smooth muscle contractility, and accordingly, modulating distinct K+ channels with K+ channel openers could serve as an alternative approach for the treatment of overactive bladder (Kumar et al., 2003). Compared to other K+ channels, potent openers of KATP channels are known, including more recent compounds that exhibit reportedly enhanced bladder vs vascular selectivity such as WAY-133537 (Wojdan et al., 1999) and A-278637 (Brune et al., 2002; Gopalakrishnan et al., 2002).
The present study reports on the identification of A-251179 as an ATP-sensitive K+ channel opener derived from a chemotype distinct from those described previously (for reviews see Gopalakrishnan and Shieh, 2004; Mannhold, 2004; Carroll, 2006). A-251179 evoked membrane potential responses in bladder smooth muscle cells in a glyburide-sensitive manner like other KATP channel openers. The potency of A-251179 to evoke membrane hyperpolarization measured by fluorescence-based FLIPR assay is comparable to that of WAY-133537 (pD2=7.72) and is somewhat lower than A-278637 (pD2=7.04) and ZD6169 (pD2=6.56) (Gopalakrishnan et al., 2002). Consistent with these observations, A-251179 evoked a hyperpolarization and activated current responses in bladder smooth muscle cells in a glyburide-sensitive manner as measured by patch-clamp. A-251179 also evoked glyburide-sensitive currents derived from SUR2B/Kir6.2 channels. The compound was 3.8-fold more potent in activating SUR2B/Kir6.2 channels compared to SUR2A/Kir6.2 channels demonstrating that A-251179 is a KATP channel opener with selectivity for the smooth muscle type channels and is distinct from KATP openers such as pinacidil.
Functional studies revealed that A-251179 suppressed both spontaneous and neurogenically evoked contractions of the pig bladder smooth muscle. The potency of A-251179 to suppress spontaneous myogenic contraction is comparable with A-278637 (pD2=7.64) and is 2.2- and 4.7-fold more potent than that of WAY-133537 and ZD-6169, respectively (Gopalakrishnan et al., 2002). Interestingly, A-251179 was some 70-fold more potent in suppressing spontaneous myogenic contractions compared to contractions evoked by carbachol or low frequency electrical stimulations. Although the enhanced potency vs myogenic contractions is in line with that observed for other KATP channel openers, the magnitude of shift with A-251179 is somewhat greater (70-fold vs about 15-fold for other compounds (Buckner et al., 2002; Carroll et al., 2004). It remains to be elucidated why A-251179 is more efficacious in suppression of spontaneous contractions than those evoked by carbachol or low frequency electrical stimulation.
The in vivo efficacy profile of A-251179 was assessed in two rat bladder hyperactivity models: spontaneous nonvoiding myogenic contractions secondary to partial outlet obstruction and volume-induced neurogenic contractions. A-251179 caused dose-dependent suppression of spontaneous contractions in the obstructed model, with mean inhibition of the contraction area under the curve of 64% at 10 μmol kg−1. A-251179 also evoked dose-dependent decreases in mean arterial blood pressure at bladder-effective doses. In this regard, the dose-dependent decreases in mean arterial pressure for A-251179 after intravenous administration over the effective dose range for bladder effects is comparable to those reported previously with other KCOs such as WAY-133537 and ZD-6169 in this model (Fabiyi et al., 2003).
In the neurogenic model, A-251179 at a dose of 0.3 μmol kg−1 was found to increase bladder capacity without affecting voiding efficiency; however, the higher dose of 3 μmol kg−1 was ineffective at increasing bladder capacity and decreasing voiding frequency. The reason for this observation could be due to decreases in arterial pressure at higher doses, which is thought to decrease urethral resistance by a reduction in vascular filling of the lamina propria (Greenland and Brading, 1997). Interestingly, this dose of A-251179 (0.3 μmol kg−1), which decreased the reflex contractions, did not inhibit spontaneous nonvoiding contractions secondary to obstruction or resulted in alterations in mean arterial pressure. At 3 or 30 μmol kg−1, the reduction in spontaneous nonvoiding contractions was observed. This profile is distinct from compounds such as tolterodine, which significantly increases reflex contraction frequency and decreases contraction duration at doses of 0.1 or 1 μmol kg−1 (Fabiyi et al., 2003). The mechanism underlying the selective effects of A-251179 remains to be elucidated. It should be pointed out that A-251179 was found to have no significant interactions across a range of receptor-ion channel targets (CEREP, unpublished observations). Given the interesting profile of A-251179 in increasing bladder capacity without compromising voiding efficiency, additional studies to elucidate mechanisms underlying the selective effects are warranted. For example, it has been demonstrated that compounds such as ZD-6169 (Yu and de Groat, 1998) and KW-7158 (Sculptoreanu et al., 2004) can suppress hyperactivity of C-afferents and reduce bladder hyperactivity induced by chemical irritation. It remains to be determined whether similar neuronal sites of action exist for thioureabenzamide analogs such as A-251179.
In summary, in the search of novel chemotypes that activate KATP channels, we have identified a structurally distinct and potent thioureabenzamide analog, A-251179 by screening the in-house compound library using the FLIPR-based membrane potential assay with bladder smooth muscle cells. Our studies show that A-251179 behaves as a prototypical KCO across cellular, tissue based assays of KATP channel/bladder function. Although the A-251179 contains thiourea and the trichloromethyl groups that might be associated with potential toxicity, A-251179 remains a potent KCO compared to other analogs in which the thiourea and trichloromethyl groups were substituted with other functional groups (Perez-Medrano et al., 2004). In vivo studies demonstrated that A-251179 was effective in suppressing unstable bladder contractions, and more interestingly, distinguished itself from other KCOs with selective effects in the neurogenic model, where significant increases in bladder capacity without affecting voiding efficiency was observed.
Abbreviations
- AUC
area under curve
- DIBAC4(3)
bis-(1,3-dibutylbarbituric acid)trimethine oxonol
- DMEM
Dulbecco's modified Eagle's medium
- FLIPR
fluorescent imaging plate reader
- GLB
glyburide
- KATP
ATP-sensitive K+ channel
- KCO
potassium channel opener
- Kir
inwardly rectifying K+ channel
- MAP
mean arterial pressure
- PCN
pinacidil
- SUR
sulphonylurea receptor
Conflict of interest
The authors state no conflict of interest.
References
- Aguilar-Bryan L, Clement JPT, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM. KATP channels and insulin secretion: a key role in health and disease. Biochem Soc Trans. 2006;34:243–246. doi: 10.1042/BST20060243. [DOI] [PubMed] [Google Scholar]
- Atwal KS, Grover GJ, Ferrara FN, Ahmed SZ, Sleph PG, Dzwonczyk S, et al. Cardioselective antiischemic ATP-sensitive potassium channel openers. 2. Structure-activity studies on benzopyranylcyanoguanidines: modification of the benzopyran ring. J Med Chem. 1995;38:1966–1973. doi: 10.1021/jm00011a016. [DOI] [PubMed] [Google Scholar]
- Brading AF.A myogenic basis for the overactive bladder Urology 19975057–67.discussion 68–73 [DOI] [PubMed] [Google Scholar]
- Brune ME, Fey TA, Brioni JD, Sullivan JP, Williams M, Carroll WA, et al. (−)-(9S)-9-(3-Bromo-4-fluorophenyl)-2,3,5,6,7,9-hexahydrothieno[3,2-b]quin olin-8(4H)-one 1,1-dioxide (A-278637): a novel ATP-sensitive potassium channel opener efficacious in suppressing urinary bladder contractions. II. in vivo characterization. J Pharmacol Exp Ther. 2002;303:387–394. doi: 10.1124/jpet.102.034553. [DOI] [PubMed] [Google Scholar]
- Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M. Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol. 2002;135:639–648. doi: 10.1038/sj.bjp.0704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner SA, Milicic I, Daza A, Davis-Taber R, Scott VE, Sullivan JP, et al. Pharmacological and molecular analysis of ATP-sensitive K+ channels in the pig and human detrusor. Eur J Pharmacol. 2000;400:287–295. doi: 10.1016/s0014-2999(00)00388-5. [DOI] [PubMed] [Google Scholar]
- Busija DW, Lacza Z, Rajapakse N, Shimizu K, Kis B, Bari F, et al. Targeting mitochondrial ATP-sensitive potassium channels-a novel approach to neuroprotection. Brain Res Brain Res Rev. 2004;46:282–294. doi: 10.1016/j.brainresrev.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Cardozo L, Lisec M, Millard R, van Vierssen Trip O, Kuzmin I, Drogendijk TE, et al. Randomized, double-blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol. 2004;172:1919–1924. doi: 10.1097/01.ju.0000140729.07840.16. [DOI] [PubMed] [Google Scholar]
- Carroll WA.Drugs active at ATP-sensitive K+ channels Voltage-Gated Ion Channels as Drug Targets 2006Wiley-VCH, Verlag GmBH & Co., KGaA, Weinheim; 335–354.In: Triggle DJ, Gopalakrishnan M, Rampe D, Zheng W (eds) [Google Scholar]
- Carroll WA, Altenbach RJ, Bai H, Brioni JD, Brune ME, Buckner SA, et al. Synthesis and structure-activity relationships of a novel series of 2,3,5,6,7,9-hexahydrothieno[3,2-b]quinolin-8(4H)-one 1,1-dioxide KATP channel openers: discovery of (−)-(9S)-9-(3-bromo-4-fluorophenyl)-2,3,5,6,7,9- hexahydrothieno[3,2-b]quinolin-8(4H)-one 1,1-dioxide (A-278637), a potent KATP opener that selectively inhibits spontaneous bladder contractions. J Med Chem. 2004;47:3163–3179. doi: 10.1021/jm030356w. [DOI] [PubMed] [Google Scholar]
- Chapple CR. Darifenacin: a novel M3 muscarinic selective receptor antagonist for the treatment of overactive bladder. Expert Opin Investig Drugs. 2004;13:1493–1500. doi: 10.1517/13543784.13.11.1493. [DOI] [PubMed] [Google Scholar]
- Dawson CM, Croghan PC, Scott AM, Bangham JA. Potassium and rubidium permeability and potassium conductance of the beta-cell membrane in mouse islets of Langerhans. Q J Exp Physiol. 1986;71:205–222. doi: 10.1113/expphysiol.1986.sp002979. [DOI] [PubMed] [Google Scholar]
- Fabiyi AC, Gopalakrishnan M, Lynch JJ, III, Brioni JD, Coghlan MJ, Brune ME. In vivo evaluation of the potency and bladder-vascular selectivity of the ATP-sensitive potassium channel openers (−)-cromakalim, ZD6169 and WAY-133537 in rats. BJU Int. 2003;91:284–290. doi: 10.1046/j.1464-410x.2003.03069.x. [DOI] [PubMed] [Google Scholar]
- Foster CD, Fujii K, Kingdon J, Brading AF. The effect of cromakalim on the smooth muscle of the guinea-pig urinary bladder. Br J Pharmacol. 1989a;97:281–291. doi: 10.1111/j.1476-5381.1989.tb11952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CD, Speakman MJ, Fujii K, Brading AF. The effects of cromakalim on the detrusor muscle of human and pig urinary bladder. Br J Urol. 1989b;63:284–294. doi: 10.1111/j.1464-410x.1989.tb05191.x. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Buckner SA, Whiteaker KL, Shieh CC, Molinari EJ, Milicic I, et al. (−)-(9S)-9-(3-Bromo-4-fluorophenyl)-2,3,5,6,7,9-hexahydrothieno[3,2-b]quin olin-8(4 H)-one 1,1-dioxide (A-278637): a novel ATP-sensitive potassium channel opener efficacious in suppressing urinary bladder contractions. I. In vitro characterization. J Pharmacol Exp Ther. 2002;303:379–386. doi: 10.1124/jpet.102.034538. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Shieh CC. Potassium channel subtypes as molecular targets for overactive bladder and other urological disorders. Expert Opin Ther Targets. 2004;8:437–458. doi: 10.1517/14728222.8.5.437. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Whiteaker KL, Molinari EJ, Davis-Taber R, Scott VE, Shieh CC, et al. Characterization of the ATP-sensitive potassium channels KATP expressed in guinea pig bladder smooth muscle cells. J Pharmacol Exp Ther. 1999;289:551–558. [PubMed] [Google Scholar]
- Greenland JE, Brading AF. The in vivo and in vitro effects of hypoxia on pig urethral smooth muscle. Br J Urol. 1997;79:525–531. doi: 10.1046/j.1464-410x.1997.00068.x. [DOI] [PubMed] [Google Scholar]
- Grover GJ, Garlid KD. ATP-Sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol. 2000;32:677–695. doi: 10.1006/jmcc.2000.1111. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003;140:159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JPt, Namba N, Inazawa J, Gonzalez G, et al. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, et al. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- Kumar V, Templeman L, Chapple CR, Chess-Williams R. Recent developments in the management of detrusor overactivity. Curr Opin Urol. 2003;13:285–291. doi: 10.1097/00042307-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- Mannhold R. KATP channel openers: structure-activity relationships and therapeutic potential. Med Res Rev. 2004;24:213–266. doi: 10.1002/med.10060. [DOI] [PubMed] [Google Scholar]
- Mannhold R. Structure-activity relationships of KATP channel openers. Curr Top Med Chem. 2006;6:1031–1047. doi: 10.2174/156802606777323647. [DOI] [PubMed] [Google Scholar]
- Milicic I, Buckner SA, Daza A, Shieh CC, Whiteaker KL, Carroll WA, et al. Aminal analogs as K+ channel openers: in vitro pharmacological characterization. FASEB J. 2004;18:A169.3. [Google Scholar]
- Perez-Medrano A, Buckner SA, Coghlan MJ, Gregg RJ, Gopalakrishnan M, Kort ME, et al. Design and synthesis of novel cyanoguanidine ATP-sensitive potassium channel openers for the treatment of overactive bladder. Bioorg Med Chem Lett. 2004;14:397–400. doi: 10.1016/j.bmcl.2003.10.063. [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, et al. Hypothalamic KATP channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A, Yoshimura N, de Groat WC. KW-7158 [(2S)-(+)-3,3,3-trifluoro-2-hydroxy-2-methyl-N-(5,5,10-trioxo-4,10-dihydrothieno[3,2-c][1]benzothiepin-9-yl)propanamide] enhances A-type K+ currents in neurons of the dorsal root ganglion of the adult rat. J Pharmacol Exp Ther. 2004;310:159–168. doi: 10.1124/jpet.104.065409. [DOI] [PubMed] [Google Scholar]
- Sebille S, Gall D, de Tullio P, Florence X, Lebrun P, Pirotte B. Design, synthesis, and pharmacological evaluation of R/S-3,4-dihydro-2,2-dimethyl- 6-halo-4-(phenylaminocarbonylamino)-2H-1-benzopyrans: toward tissue-selective pancreatic beta-cell KATP channel openers structurally related to (+/−)-cromakalim. J Med Chem. 2006;49:4690–4697. doi: 10.1021/jm060161z. [DOI] [PubMed] [Google Scholar]
- Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol. 1999;61:337–362. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- Shieh CC, Feng J, Buckner SA, Brioni JD, Coghlan MJ, Sullivan JP, et al. Functional implication of spare ATP-sensitive K+ channels in bladder smooth muscle cells. J Pharmacol Exp Ther. 2001;296:669–675. [PubMed] [Google Scholar]
- Shindo T, Yamada M, Isomoto S, Horio Y, Kurachi Y. SUR2 subtype (A and B)-dependent differential activation of the cloned ATP-sensitive K+ channels by pinacidil and nicorandil. Br J Pharmacol. 1998;124:985–991. doi: 10.1038/sj.bjp.0701927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojdan A, Freeden C, Woods M, Oshiro G, Spinelli W, Colatsky TJ, et al. Comparison of the potassium channel openers, WAY-133537, ZD6169, and celikalim on isolated bladder tissue and in vivo bladder instability in rat. J Pharmacol Exp Ther. 1999;289:1410–1418. [PubMed] [Google Scholar]
- Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, et al. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol. 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, de Groat WC. Effects of ZD6169, a KATP channel opener, on bladder hyperactivity and spinal c-fos expression evoked by bladder irritation in rats. Brain Res. 1998;807:11–18. doi: 10.1016/s0006-8993(98)00707-0. [DOI] [PubMed] [Google Scholar]
- Zinner N, Gittelman M, Harris R, Susset J, Kanelos A, Auerbach S. Trospium chloride improves overactive bladder symptoms: a multicenter phase III trial. J Urol. 2004;171:2311–2315. doi: 10.1097/01.ju.0000127742.73136.0c. [DOI] [PubMed] [Google Scholar]