Abstract
Background and purpose:
Adrenaline has been implicated in the pathogenesis of atherosclerosis. However, little is known regarding the role of adrenaline in glucose transport in VSMC.
Experimental approach:
In this study, we examined the effects of adrenaline on glucose uptake in rat VSMC. We also examined the downstream signaling pathway from the β-adrenoceptor to glucose uptake, using a pharmacological approach. To investigate the downstream action of adenylate cyclase, we studied the effects of GGTI-298, an inhibitor of geranylgeranylation of GTPases, including Rap1. To confirm the involvement of Rap1, we silenced Rap1 by siRNA.
Key results:
Adrenaline induced glucose uptake in a dose-dependent manner. The adrenaline-induced glucose uptake was inhibited by L-propranolol, (a selective β-adrenoceptor antagonist), but not by prazosin (a selective α1-adrenoceptor antagonist) or UK14304 (a selective α2-adrenoceptor antagonist), suggesting the involvement of β-adrenoceptors in glucose transport. Long-term treatment with cholera toxin, which resulted in sequestration of Gs proteins, prevented the adrenaline-induced glucose uptake. Forskolin, a direct activator of adenylate cyclase, was found to mimic the effects of adrenaline. Adrenaline-induced glucose uptake was inhibited by GGTI-298, not by H89 (a selective inhibitor of PKA). Silencing of Rap1 by siRNA attenuated the adrenaline-induced glucose uptake. Adrenaline-induced glucose uptake was inhibited by SB203580 (a selective inhibitor of p38MAPK) and adrenaline-induced p38MAPK activation was inhibited by GGTI-298 and siRNA against Rap1.
Conclusions and implications:
These findings suggest that adrenaline-induced glucose transport is mediated by β-adrenoceptors, Gs, adenylate cyclase, Rap1, and p38MAPK in vascular smooth muscle cells.
Keywords: adrenaline, Gs, adenylate cyclase, Rap1, p38MAPK, vascular smooth muscle cells, glucose uptake
Introduction
Hypertension, smoking and stress are among the independent risk factors for atherosclerosis (Goldstein, 1981) and are known to be associated with increased levels of catecholamines such as adrenaline and noradrenaline in plasma (Dimsdale and Moss, 1980). In addition, catecholamines have been shown to aggravate atherosclerosis in animals and humans (Kukreja et al., 1981). Since vascular smooth muscle cells (VSMC) play a key role in the pathogenesis of atherosclerosis and restenosis after percutaneous transluminal coronary angioplasty (Ross, 1999), adrenaline might play a role in the pathogenesis of atherosclerosis in VSMC.
Adrenaline exerts many responses through adrenoceptors. Adrenoceptors are classified into three main subtypes: α1-, α2- and β-adrenoceptors, which couple to Gq (increase inositol 1,4,5-trisphosphate and diacylglycerol levels), Gi (inhibit cyclic AMP formation) and Gs (increase cyclic AMP formation) G proteins, respectively. These adrenoceptor subtypes are reported to be expressed in VSMC.
Glucose provides metabolic energy for homeostasis and is transported via the glucose transporter (GLUT) family (Joost and Thorens, 2001). Among the GLUT family, GLUT1 and GLUT4 have been shown to be expressed in VSMC (Kanda and Watanabe, 2005; Park et al., 2005). Expression levels of GLUT1 have been reported to increase in rabbit VSMC after balloon injury, which is an experimental model of atherosclerosis (Hall et al., 2001). In addition, glucose transport has been shown to be regulated by growth factors or hormones in various cells. For example, we have already reported that thrombin stimulates glucose uptake in VSMC (Kanda and Watanabe, 2005). Adrenaline has been reported to induce glucose uptake in several cells other than VSMC (Nevzorova et al., 2002; Chernogubova et al., 2004). Based on these findings, there might be a possible role of adrenaline in glucose transport in VSMC.
In this study, we investigated the role of adrenaline in glucose transport in rat VSMC. We report here that adrenaline stimulates glucose uptake via a β-adrenoceptor, Gs and adenylate cyclase, and Rap1 in VSMC.
Methods
Cell culture
Rat aortic VSMC was prepared from 8-week-old Sprague–Dawley rats by using the explant method, as previously described (Nishio and Watanabe, 1997). The rats were individually housed in a temperature-controlled environment on a 12 h light:12 h dark cycle, with the lights on from 0700 to 1900 h and access to food and water ad libitum. All procedures involving animal preparation were approved by the National Defense Medical College Animal Committee. The isolated cells were confirmed by immunostaining with α-smooth muscle actin (data not shown). The cells were cultured at 37°C in a humidified atmosphere of 5% CO2/95% air in 100-mm dishes. The growth medium was Dulbecco's modified Eagle's medium (DMEM; Nissui Pharmaceutical Co. Ltd, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS; JRH Biosciences, Lenexa, KS, USA), 100 U ml−1 of penicillin (Gibco BRL, Gaitherburg, MD, USA) and 100 μg ml−1 of streptomycin (Gibco BRL). Medium was changed twice a week. Passages 2–6 were used for the experiments.
Glucose uptake assay
Glucose uptake was determined using 2-[3H]deoxy-D-glucose (2-DG; Amersham Pharmacia Biotech, Buckinghamshire, UK) as previously reported (Kanda and Watanabe, 2005). In brief, VSMC grown in 24-well plates was incubated in serum-free DMEM for 48 h before the assay was performed. After exposure to agonists for 1 h, the cells were washed three times with prewarmed phosphate-buffered saline (PBS) and incubated with 2-DG (10 μM, 1 μCi ml−1) for 20 min. The uptake was terminated by washing the cells three times with ice-cold PBS. The cells were solubilized in 0.1 N NaOH/0.1% sodium dodecyl sulfate (SDS) for 1 h. Radioactivity was measured by liquid scintillation spectroscopy (Aloka, Tokyo, Japan). All experiments were performed in triplicate.
Cell lysis, immunoprecipitation and immunoblotting
Cell lysates were prepared and analyzed as described previously (Kanda et al., 2001a). Briefly, cells were lysed in a buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 10 mM Na4P2O7, 20 mM NaF, 2 mM EDTA, 1% Triton X-100, 1 mM phenylmethylsulfonyl flouride, 1 mM Na3VO4 and 10 μg ml−1 aprotinin) and centrifuged at 15 000 g for 20 min at 4°C. The supernatant was collected and assayed for protein concentration using a bicinchoninic acid protein assay reagent kit (Pierce, Rockford, IL, USA). For immunoprecipitation, the supernatant was precleared with protein G Sepharose beads (Amersham Pharmacia Biotech) and incubated with the appropriate antibody conjugated to sepharose beads for 3 h at 4°C. A 20 μg weight of protein was analyzed on 10% SDS-polyacrylamide gel electrophoresis and transferred electrophoretically to polyvinylidene difluoride membranes (15 V, 90 min; Millipore, Bedford, MA, USA). After blocking in 5% skimmed milk in PBS-T (0.2% Tween 20), the membranes were reacted with specific antibodies for 1.5 h at room temperature. The blots were then washed and then incubated with peroxidase-conjugated secondary antibodies for 1 h at room temperature. Peroxidase activity was detected by enhanced chemiluminescence (ECL detection kit; Amersham Pharmacia Biotech).
Rap1 activation assay
Rap1 activity was determined using a Rap1 activation kit (Upstate Biotechnology, Charlottesville, VA, USA). Briefly, cell lysates were incubated with glutathione-S-transferase-RalGDS Rap1 binding domain precoupled to glutathione beads. Following a 1-h incubation at 4°C, beads were rinsed three times with ice-cold lysis buffer and protein was eluted from the beads in Laemmli buffer. Proteins were analyzed by immunoblotting with anti-Rap1 polyclonal antibody.
PKA assay
Protein kinase A (PKA) activity was performed using the ProFluor PKA assay kit (Promega, Madison, WI, USA). Briefly, cell lysates were added to the reaction buffer containing a bisamide rhodamine 110 peptide substrate and were incubated for 30 min at 25°C. The reaction was stopped by adding the termination buffer, which contained a protease that removes amino acids specifically from the non-phosphorylated substrate and results in the production of highly fluorescent rhodamine 110. Thus, the fluorescence intensity is inversely correlated with kinase activity. PKA activity was determined from the fluorescence intensity of the non-phosphorylated substrate using a Fusion multilabel reader (PerkinElmer Life Sciences, Wellesley, MA, USA).
p38MAPK activity assay
p38MAPK activity was measured as previously reported (Kanda et al., 2001b). Briefly, p38MAPK was immunoprecipitated from 1 mg of cell lysates using 2 μg of anti-p38MAPK antibody conjugated to sepharose beads. After washing, the immunoprecipitates were suspended in 50 μl of the kinase buffer (20 mM Tris, pH 7.4, 20 mM MgCl2, 20 mM NaCl, 0.1 mM Na3VO4 and 2 mM dithiothreitol) containing 2 μg GST-ATF-2, 20 μM ATP and incubated at 30°C for 30 min. Reactions were stopped by heating for 5 min. Phosphorylation of ATF-2 was analyzed by immunoblotting using anti-phospho-specific ATF-2 antibody (Cell Signaling Technology, Beverly, MA, USA).
RNA interference
Small interfering RNA (siRNA) oligonucleotides against rat Rap1 (5′-GCATTCCAGACTTCAAAAA-3′) and control siRNA (#AM4611) were provided by Ambion (Austin, TX, USA). Amaxa Nucleofector device (Amaxa Biosystems, Gaithersburg, MD, USA) was used for transfection according to the manufacturer's directions. After transfection with 200 pmol of siRNA into 1 × 106 cells, the cells were incubated in DMEM for 48 h.
Statistics
Values are expressed as the arithmetic means±s.d. Statistical analysis of the data was performed by the use of one-way analysis of variance followed by the Scheffe test when F ratios were significant (P<0.05).
Materials
L-Propranolol, prazosin and UK14304 were from Wako Pure Chemicals (Osaka, Japan). PD98059 and GGTI-298 were from Sigma-Aldrich (St Louis, MO, USA). U0126 was from Promega. Anti-p38MAPK polyclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA), SB203580 was from Calbiochem (La Jolla, CA, USA), anti-Rac polyclonal antibody was from Cell Signaling Technology, dibutyryl cAMP (dbcAMP) was from Funakoshi (Tokyo, Japan) and 8-pCPT-2′-O-Me-cAMP was from BIOLOG Life Science Institute (Bremen, Germany). All other reagents were of analytical grades and obtained from commercial sources.
Results
Adrenaline-induced glucose transport in VSMC
To examine whether adrenaline stimulates glucose uptake in VSMC, cells were exposed to various concentrations of adrenaline for 1 h. As shown in Figure 1a, adrenaline stimulates glucose uptake in a dose-dependent manner, with the maximum response observed at 1 μM (from 120±20 to 216±34 pmol mg−1 min−1). To confirm that the effect was mediated by a receptor-dependent mechanism, we treated the cells with adrenoceptor antagonists. As shown in Figure 1b, L-propranolol (a selective β-adrenoceptor antagonist) inhibited the adrenaline-induced glucose uptake to the basal level (from 211±12 to 118±20 pmol mg−1 min−1). In contrast, prazosin (a selective α1-adrenoceptor antagonist) and UK14304 (a selective α2-adrenoceptor antagonist) failed to inhibit the glucose uptake (from 210±30 to 204±24pmol mg−1 min−1). In addition, isoprenaline, a selective β-adrenoceptor agonist, induced glucose uptake (from 118±30 to 252±57 pmol mg−1 min−1) and L-propranolol inhibited the isoprenaline-induced glucose uptake (from 252±57 to 124±31 pmol mg−1 min−1) (Figure 1c). These data suggest that adrenaline stimulates glucose uptake through β-adrenoceptors in VSMC.
Figure 1.
Effects of adrenaline on 2-DG uptake in VSMC. VSMC grown in 24-well plates were serum-starved for 24 h. (a) The cells were stimulated for 1 h with various concentrations of adrenaline (Adr). (b) The cells were pretreated with adrenoceptor antagonists (L-propranolol (prop), prazosin (praz), UK14304 (UK) all at 10 μM) or vehicle and then stimulated with adrenaline (10 μM) for 1 h. (b) The cells were pretreated with L-propranolol (10 μM) or vehicle and then stimulated for 1 h with isoprenaline (Iso, 10 μM). After stimulation, uptake of 2-DG by the VSMC was measured. Each value represents the mean±s.d. of three independent experiments in triplicate. *P<0.05.
Glucose uptake by adrenaline is mediated via Gs proteins in VSMC
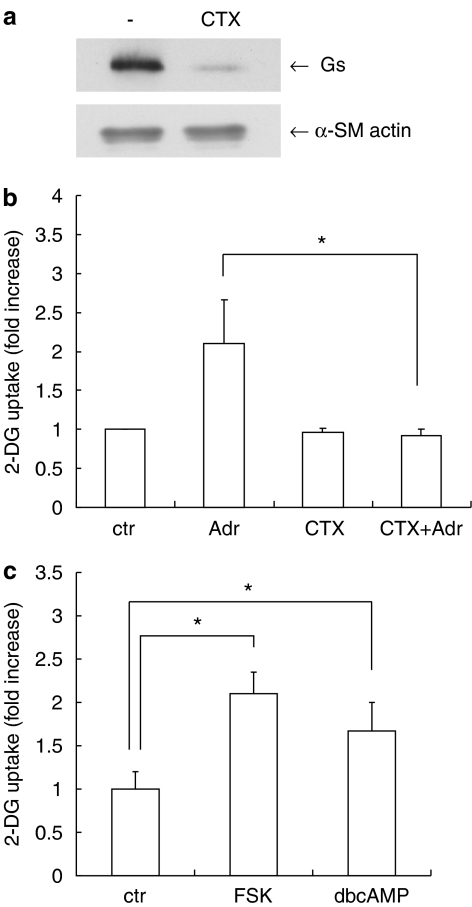
We next examined the signaling pathways from the β-adrenoceptor to glucose uptake in VSMC. β-Adrenoceptors are known to couple to the Gs class of heterotrimeric G proteins. To examine the involvement of Gs in glucose uptake, we used cholera toxin (CTX). We have previously reported that long-term treatment with CTX dramatically decreased immunoreactive Gs protein in 3T3-L1 cells (Mizuno et al., 2002). As shown in Figure 2a, long-term treatment with CTX decreased Gs protein in VSMC. CTX did not affect the expression of α-smooth muscle actin, confirming the selectivity of CTX. Under this condition, CTX inhibited the adrenaline-induced glucose uptake (from 259±58 to 116±10 pmol mg−1 min−1) (Figure 2b). Furthermore, glucose uptake was stimulated by forskolin, which directly activates adenylyl cyclase (Seamon et al., 1981) (from 115±23 to 242±28 pmol mg−1 min−1) and dbcAMP, which is a membrane-permeable cAMP analog (from 115±23 to 193±39pmol mg−1 min−1) (Figure 2c). These results suggest that Gs and adenylyl cyclase mediate adrenaline-induced glucose uptake in VSMC.
Figure 2.
The role of Gs in adrenaline-induced 2-DG uptake in VSMC. VSMC were incubated with or without cholera toxin (CTX, 100 ng ml−1) for 72 h in DMEM. (a) Western blot analysis with anti-Gs antibody or anti-α-smooth muscle (SM) actin antibody. (b) The cells were stimulated with adrenaline (Adr, 10 μM) for 1 h and then uptake of 2-DG was measured. (c) VSMC were incubated with forskolin (FSK, 50 μM), dibutyryl cAMP (dbcAMP, 100 μM) or vehicle for 30 min. Then uptake of 2-DG by the VSMC was measured. Each value represents the mean±s.d. of three independent experiments in triplicate. *P<0.05.
Effects of PKA inhibition on adrenaline-induced glucose uptake in VSMC
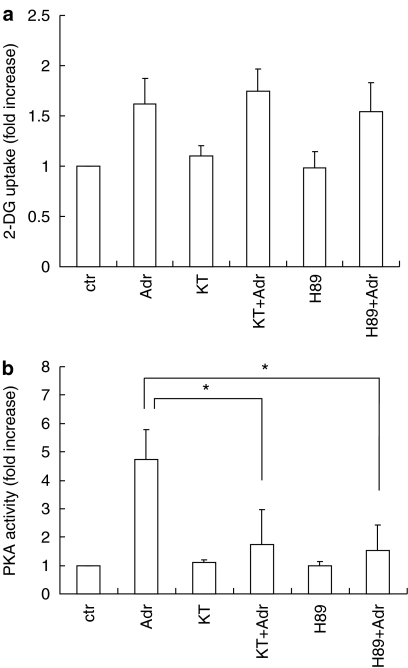
We further examined the signaling pathways from Gs to glucose uptake in VSMC. Since PKA acts downstream from adenylate cyclase, we examined whether PKA regulates adrenaline-induced glucose uptake in VSMC. As shown in Figure 3a, H89, a selective inhibitor for PKA, failed to block adrenaline-induced glucose uptake (from 194±30 to 210±26 pmol mg−1 min−1). KT5720, another PKA inhibitor, produced similar results (185±35 pmol mg−1 min−1). The effectiveness of these inhibitors was confirmed by inhibition of adrenaline-induced PKA activation (Figure 3b). These data suggest that adrenaline-induced glucose uptake is independent of PKA.
Figure 3.
Effects of PKA inhibitors on adrenaline-induced 2-DG uptake and PKA activation in VSMC. Serum-starved VSMC were incubated with H89 (1 μM), KT5720 (1 μM) or vehicle for 30 min. (a) The cells were stimulated with adrenaline (Adr, 10 μM) for 1 h and then uptake of 2-DG by the VSMC was measured. (b) The cells were stimulated with adrenaline (10 μM) for 10 min and PKA activity was analyzed. Each value represents the mean±s.d. of three independent experiments in triplicate. *P<0.05.
Effects of Rap1 inhibition on adrenaline-induced glucose uptake in VSMC
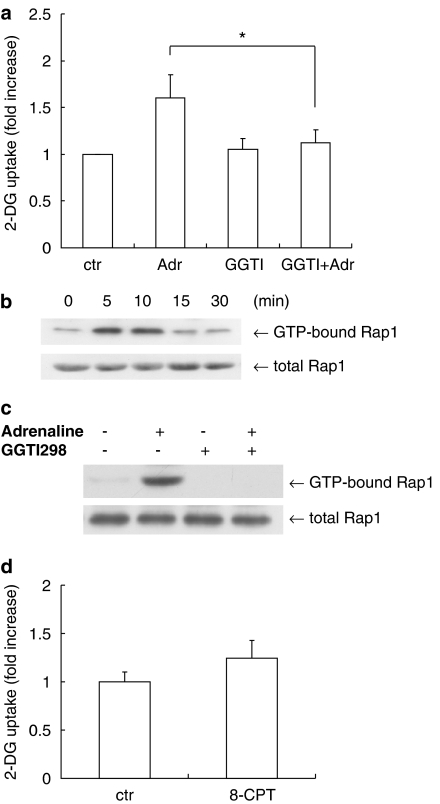
Recent studies have shown that cAMP binds to cAMP binding guanine nucleotide exchange factors (Epac), which in turn stimulate the small GTPase Rap, for which the pathway is independent from PKA (de Rooij et al., 1998; Bos, 2003). To investigate whether Rap1 participates in glucose uptake, we tested the effects of GGTI-298, an inhibitor of geranylgeranylation of GTPase, including Rap1 (Lerner et al., 1997). In contrast to the PKA inhibitors described above, GGTI-298 inhibited the adrenaline-induced glucose uptake to the basal level (from 192±30 to 134±17 pmol mg−1 min−1) (Figure 4a). Furthermore, adrenaline induced transient Rap1 activation in VSMC (Figure 4b) and GGTI-298 inhibited the adrenaline-induced Rap1 activation (Figure 4c). We next tested the effect of 8-pCPT-2′-O-Me-cAMP, which is a cAMP analog for Epac activation (Enserink et al., 2002). 8-pCPT-2′-O-Me-cAMP stimulated glucose uptake slightly but not significantly (from 112±11 to 139±26 pmol mg−1 min−1) (Figure 4d). To further confirm the involvement of Rap1, we tested the effects of siRNA against Rap1. siRNA against Rap1 decreased Rap1 protein, but did not inhibit the expression of Rac, which belongs to the small GTPase protein family (Figure 5a). siRNA against Rap1 inhibited the adrenaline-induced glucose uptake in VSMC (from 222±24 to 144±49 pmol mg−1 min−1) (Figure 5b). These data suggest that the adrenaline-induced glucose uptake is mediated by Rap1 and not PKA in VSMC.
Figure 4.
Effects of GGTI-298 in 2-DG uptake in VSMC. (a) Serum-starved VSMC were incubated with GGTI-298 (10 μM) or vehicle for 30 min. The cells were then stimulated with adrenaline (Adr, 10 μM) for 1 h and then uptake of 2-DG by the VSMC was measured. Each value represents the mean±s.d. of three independent experiments in triplicate. (b) VSMC were incubated with GGTI-298 (10 μM) or vehicle for 30 min and then stimulated with adrenaline (Adr, 10 μM) for 10 min. The cells were lysed and Rap1 activity was measured using GST-RalGDS Rap1 binding domain. (c) VSMC were incubated with GGTI-298 (10 μM) or vehicle for 30 min and then stimulated with adrenaline (10 μM) for10 min. The cells were lysed and Rap1 activity was measured as in (b). (d) Serum-starved VSMC were stimulated with 8-pCPT-2′-O-Me-cAMP (8-CPT, 1 mM) for 30 min and then uptake of 2-DG by the VSMC was measured. Each value represents the mean±s.d. of three independent experiments in triplicate. *P<0.05.
Figure 5.
The role of Rap1 in 2-DG uptake in VSMC. (a) Forty-eight hours after transfection with the siRNA against Rap1, cell lysates were prepared and subjected to SDS-PAGE and immunoblotting with and anti-Rap1 antibody, anti-Rac antibody or anti-α-smooth muscle (SM) actin antibody. (b) Rap1-silenced VSMC were serum-starved for 4 h and then stimulated with adrenaline (Adr, 10 μM) for 1 h. Then uptake of 2-DG by the VSMC was measured. Each value represents the mean±s.d. of three independent experiments in triplicate. *P<0.05.
Effects of MAPK inhibitors on adrenaline-induced glucose uptake in VSMC
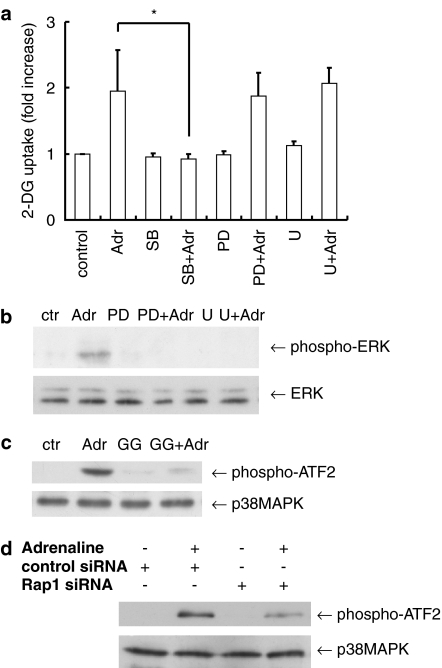
Since we have previously reported that p38MAPK plays a role in thrombin-induced glucose uptake in VSMC (Kanda and Watanabe, 2005), we examined the role of the mitogen-activated protein kinase (MAPK) family on adrenaline-induced glucose uptake. SB203580, a selective inhibitor of p38MAPK, attenuated the adrenaline-induced glucose uptake (from 230±73 to 109±9 pmol mg−1 min−1) (Figure 6a). In contrast, PD98059, a selective inhibitor of MEK (MAPK/ERK kinase), which is an upstream kinase of extracellular signal-regulated kinase (ERK), failed to affect the glucose uptake (222±41 pmol mg−1 min−1). The use of U0126, another MEK inhibitor, produced similar results (244±27 pmol mg−1 min−1). The effectiveness of MEK inhibitors was confirmed by its ability to inhibit adrenaline-induced ERK phosphorylation (Figure 6b). In addition, adrenaline-induced p38MAPK activation was blocked by GGTI-298 (Figure 6c) or by silencing of Rap1 using siRNA (Figure 6d), suggesting the signaling pathway from Rap1 to p38MAPK. Taken together, these results suggest that p38MAPK and not ERK, mediate adrenaline-induced glucose uptake via Rap1 in VSMC.
Figure 6.
The role of p38MAPK in adrenaline-induced 2-DG uptake in VSMC. (a) Serum-starved VSMC were incubated with SB203580 (SB, 10 μM), PD98059 (PD, 20 μM) and U0126 (U, 1 μM), or vehicle for 30 min and then stimulated with adrenaline (Adr, 10 μM). Then uptake of 2-DG by the VSMC was measured. Each value represents the mean±s.d. of three independent experiments in triplicate. (b) Serum-starved VSMC were incubated with PD98059 (20 μM), U0126 (1 μM) or vehicle for 30 min and then stimulated with adrenaline (10 μM) for 5 min. ERK phosphorylation was analyzed by immunoblotting with anti-phospho-specific ERK antibody. (c) Serum-starved VSMC were incubated with or without GGTI-298 (GG, 10 μM) for 30 min. The cells were then stimulated with adrenaline (10 μM) for 10 min. p38MAPK activity was measured as described in Methods. (d) Forty-eight hours after transfection with the siRNA against Rap1, VSMC were stimulated with adrenaline (10 μM) for 10 min. Then p38MAPK activity was measured as in (c). *P<0.05.
Discussion and conclusions
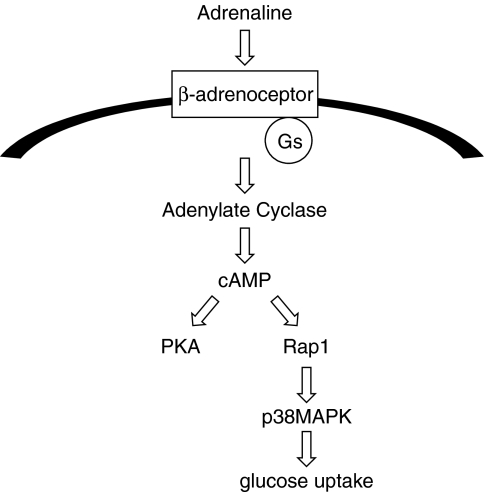
In the present study, we investigated the role of adrenaline in glucose uptake in VSMC. We showed that Gs, adenylate cyclase, Rap1 and subsequent p38 MAPK activation play a role in adrenaline-mediated glucose uptake in VSMC. A model of the pathways underlying adrenaline-induced glucose uptake, based on our present data, is shown in Figure 7.
Figure 7.
Schematic model summarizing our findings. Gs, cAMP, Rap1 and subsequent p38MAPK activation play a role in adrenaline-mediated glucose uptake in VSMC.
The G protein Gq has been shown to mediate glucose uptake, induced by several Gq-coupled receptor agonists such as endothelin and thrombin (Imamura et al., 1999; Kanda and Watanabe, 2005). We found that β-adrenoceptors, which are Gs-coupled receptors, were involved in glucose transport in VSMC and that forskolin mimicked the effects of adrenaline. Therefore, in addition to Gq, Gs also might be a regulator of glucose uptake in VSMC.
Our further examination of the signal transduction pathways from cAMP to glucose uptake revealed the possible role of Rap1 in adrenaline-induced glucose uptake in VSMC. Adrenaline-induced glucose uptake was inhibited by GGTI-298, an inhibitor of geranylgeranylation of GTPases. As there is a possibility that GGTI-298 would inhibit other targets, which are modified by geranylgeranyltransferase, we confirmed the involvement of Rap1 by the silencing experiments using siRNA. Consistent with our data, G protein-coupled receptors (GPCRs) have been shown to have the ability to activate Rap1 in human embryonic kidney 293 (HEK293) cells (Schmitt and Stork, 2000). In contrast, from the data using H89, PKA did not appear to be involved in the glucose uptake that we have studied (Figure 4). This difference between PKA and Rap1 is supported by recent data indicating that PKA and Rap1 utilize different signaling pathways (Bos, 2003).
We have previously shown the possible role of p38MAPK in glucose uptake induced by thrombin (Kanda and Watanabe, 2005). Here, we found that p38MAPK was involved in adrenaline-induced, GPCR-mediated glucose uptake in VSMC. Furthermore, we found that this uptake is mediated via the Rap1-mediated p38MAPK pathway. This pathway is supported by the recent finding indicating p38MAPK as a downstream target of Rap in other cells (Sawada et al., 2001; Huang et al., 2004). The phosphorylation level of p38MAPK could be regulated by MKK3/6 and protein phosphatases (Kyriakis and Avruch, 2001), but a direct interaction between Rap1 and MKK3/6 or protein phosphatases has not yet been reported. There might be additional downstream effectors of Rap1 for p38MAPK activation. As Rap1-mediated ERK activation by B-Raf has been reported in neuronal cells, ERK might not be a downstream target of Rap1 in VSMC. It remains to be shown how Rap1 induced p38MAPK activation in VSMC.
As glucose is transported via GLUT, GLUT would act downstream of p38MAPK. Among the GLUT family, GLUT1 and GLUT4 are the predominant transporters in VSMC (Park et al., 2005), and p38MAPK has been shown to regulate activity of GLUT1 or GLUT4 in several cells (Barros et al., 1997; Sweeney et al., 1999). Further studies would be required to elucidate how p38MAPK regulates GLUT in VSMC.
In summary, our data suggest that adrenaline stimulates glucose uptake via β-adrenoceptor in VSMC. In addition, Rap1 activation and subsequent p38MAPK activation play a role in adrenaline-mediated glucose uptake. These findings may contribute to an improved understanding of the pathogenesis of atherosclerosis.
Acknowledgments
This work was supported in part by a grant from the Smoking Research Foundation to YW
Abbreviations
- CTX
cholera toxin
- 2-DG
2-[3H]deoxy-D-glucose
- dbcAMP
dibutyryl cAMP
- DMEM
Dulbecco's modified Eagle's medium
- ERK
extracellular signal-regulated kinase
- GPCR
G protein-coupled receptor
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- PKA
protein kinase A
- siRNA
small interfering RNA
- VSMC
vascular smooth muscle cells
Conflict of interest
The authors state no conflict of interest.
References
- Barros LF, Young M, Saklatvala J, Baldwin SA. Evidence of two mechanisms for the activation of the glucose transporter GLUT1 by anisomycin: p38(MAP kinase) activation and protein synthesis inhibition in mammalian cells. J Physiol. 1997;504:517–525. doi: 10.1111/j.1469-7793.1997.517bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- Chernogubova E, Cannon B, Bengtsson T. Norepinephrine increases glucose transport in brown adipocytes via β3-adrenoceptors through a cAMP, PKA and PI3-kinase-dependent pathway stimulating conventional and novel PKCs. Endocrinology. 2004;145:269–280. doi: 10.1210/en.2003-0857. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Moss J. Plasma catecholamines in stress and exercise. JAMA. 1980;243:340–342. [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- Goldstein DS. Plasma norepinephrine in essential hypertension: a study of the studies. Hypertension. 1981;3:48–52. doi: 10.1161/01.hyp.3.1.48. [DOI] [PubMed] [Google Scholar]
- Hall JL, Chatham JC, Elder-Finkelman H, Gibbons GH. Upregulation of glucose metabolism during intimal lesion formation is coupled to the inhibition of vascular smooth muscle cells. Role of GSK3β. Diabetes. 2001;50:1171–1179. doi: 10.2337/diabetes.50.5.1171. [DOI] [PubMed] [Google Scholar]
- Huang CC, You JL, Wu MY, Hsu KS. Rap1-induced p38 mitogen-activated protein kinase activation facilitates AMPA receptor trafficking via the GDI.Rab5 complex. Potential role in (S)-3,5-dihydroxyphenylglycene-induced long term depression. J Biol Chem. 2004;279:12286–12292. doi: 10.1074/jbc.M312868200. [DOI] [PubMed] [Google Scholar]
- Imamura T, Ishibashi K, Dalle S, Ugi S, Olefsky JM. Endothelin-1-induced GLUT4 translocation is mediated via Gq/11 protein and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. J Biol Chem. 1999;274:33691–33695. doi: 10.1074/jbc.274.47.33691. [DOI] [PubMed] [Google Scholar]
- Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature. Sequence characteristics, and potential function of its novel members (review) Mol Membr Biol. 2001;18:247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- Kanda Y, Mizuno K, Kuroki Y, Watanabe Y. Thrombin-induced p38 mitogen-activated protein kinase activation is mediated by epidermal growth factor receptor transactivation pathway. Br J Pharmcol. 2001a;132:1657–1664. doi: 10.1038/sj.bjp.0703952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda Y, Nishio E, Kuroki Y, Mizuno K, Watanabe Y. Thrombin activates p38 mitogen-activated protein kinase in vascular smooth muscle cells. Life Sci. 2001b;68:1989–2000. doi: 10.1016/s0024-3205(01)00990-0. [DOI] [PubMed] [Google Scholar]
- Kanda Y, Watanabe Y. Thrombin-induced glucose transport via Src-p38 MAPK pathway in vascular smooth muscle cells. Br J Pharmcol. 2005;146:60–67. doi: 10.1038/sj.bjp.0706293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukreja RS, Datta BN, Chakra Varti RN. Catecholamine-induced aggravation of aortic and coronary atherosclerosis in monkeys. Atherosclerosis. 1981;40:291–298. doi: 10.1016/0021-9150(81)90139-8. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lerner EC, Zhang TT, Knowles DB, Qian Y, Hamilton AD, Sebti SM. Inhibition of the prenylation of K-Ras, but not H- or N-Ras, is highly resistant to CAAX peptidomimetics and requires both a farnesyltransferase and a geranylgeranyltransferase I inhibitor in human tumor cell lines. Oncogene. 1997;15:1283–1288. doi: 10.1038/sj.onc.1201296. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Kanda K, Kuroki Y, Nishio M, Watanabe Y. Stimulation of β3-adrenoceptors causes phosphorylation of p38 mitogen-activated protein kinase via a stimulatory G protein-dependent pathway in 3T3-L1 adipocytes. Br J Pharmacol. 2002;135:951–960. doi: 10.1038/sj.bjp.0704537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevzorova J, Bengtsson T, Evans BA, Summers RJ. Characterization of the β-adrenoceptor subtype involved in mediation of glucose transport in L6 cells. Br J Pharmacol. 2002;137:9–18. doi: 10.1038/sj.bjp.0704845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio E, Watanabe Y. The involvement of reactive oxygen species and arachidonic acid in α1-adrenoceptor-induced smooth muscle cell proliferation and migration. Br J Pharmacol. 1997;121:665–670. doi: 10.1038/sj.bjp.0701171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JL, Loberg RD, Duquaine D, Zhang H, Deo BK, Ardanaz N, et al. GLUT4 facilitative glucose transporter specifically and differentially contributes to agonist-induced vascular reactivity in mouse aorta. Arterioscler Thromb Vasc Biol. 2005;25:1596–1602. doi: 10.1161/01.ATV.0000170137.41079.ab. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Nakamura K, Doi K, Takeda K, Tobiume K, Saitoh M, et al. Rap1 is involved in cell stretching modulation of p38 but not ERK or JNK MAP kinase. J Cell Sci. 2001;114:1221–1227. doi: 10.1242/jcs.114.6.1221. [DOI] [PubMed] [Google Scholar]
- Schmitt JM, Stork PJ. β2-adrenergic receptor activates extracellular signal-regulated kinases (ERKs) via the small G protein rap1 and the serine/threonine kinase B-Raf. J Biol Chem. 2000;275:25342–25350. doi: 10.1074/jbc.M003213200. [DOI] [PubMed] [Google Scholar]
- Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney G, Somwar R, Ramlal T, Volchuk A, Ueyama A, Klip A. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3-L1 adipocytes and L6 myotubes. J Biol Chem. 1999;274:10071–10078. doi: 10.1074/jbc.274.15.10071. [DOI] [PubMed] [Google Scholar]