Abstract
Background and purpose:
Nitric oxide (NO) production and depression of neuromuscular transmission are closely related, but little is known about the role of L-citrulline, a co-product of NO biosynthesis, on neurotransmitter release.
Experimental approach:
Muscle tension recordings and outflow experiments were performed on rat phrenic nerve-hemidiaphragm preparations stimulated electrically.
Key results:
L-citrulline concentration-dependently inhibited evoked [3H]ACh release from motor nerve terminals and depressed nerve-evoked muscle contractions. The NO synthase (NOS) substrate, L-arginine, and the NO donor, 3-morpholinosydnonimine chloride (SIN-1), also inhibited [3H]ACh release with a potency order of SIN-1>L-arginine>L-citrulline. Co-application of L-citrulline and SIN-1 caused additive effects. NOS inactivation with No-nitro-L-arginine prevented L-arginine inhibition, but not that of L-citrulline. The NO scavenger, haemoglobin, abolished inhibition of [3H]ACh release caused by SIN-1, but not that caused by L-arginine. Inactivation of guanylyl cyclase with 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) fully blocked SIN-1 inhibition, but only partially attenuated the effects of L-arginine. Reduction of extracellular adenosine accumulation with adenosine deaminase or with the nucleoside transport inhibitor, S-(p-nitrobenzyl)-6-thioinosine, attenuated the effects of L-arginine and L-citrulline, while not affecting inhibition by SIN-1. Similar results were obtained with the selective adenosine A1 receptor antagonist, 1,3-dipropyl-8-cyclopentylxanthine. L-citrulline increased the resting extracellular concentration of adenosine, without changing that of the adenine nucleotides.
Conclusions and implications:
NOS catalyses the formation of two neuronally active products, NO and L-citrulline. While, NO may directly reduce transmitter release through stimulation of soluble guanylyl cyclase, the inhibitory action of L-citrulline may be indirect through increasing adenosine outflow and subsequently activating inhibitory A1 receptors.
Keywords: neuromuscular junction, acetylcholine release, adenosine, A1 receptor, nucleoside transport, nitric oxide, nitric oxide synthase, L-citrulline, L-arginine, NO donor
Introduction
Nitric oxide synthases (NOS) are a family of enzymes capable of oxidizing the amino acid, L-arginine, to form L-citrulline and nitric oxide (NO). NO is a freely membrane-permeant molecule with a short half-life. In the brain, NO acts as a neurotransmitter or neuromodulator-like substance during synaptic transmission (Garthwaite, 1991). In the peripheral nervous system, NO has been extensively studied and its involvement in a wide variety of functions has been demonstrated in the autonomic nervous system, though its functions in the motor system are less well defined. There is a close relationship between NOS activity and depression of neuromuscular transmission (Wang et al., 1995; Thomas and Robitaille, 2001). The most common mode of action of NO is by stimulating soluble guanylyl cyclase thereby increasing neuronal levels of cyclic GMP (Garthwaite, 1991; Boulton et al., 1994), but it also has several other biochemical effects of potential biological significance (reviewed by Stamler and Meissner, 2001). It has been proposed that NO acts through both cyclic GMP-dependant and -independent pathways at the amphibian neuromuscular junction, with the dominance of a particular pathway determined by the level of synaptic activity (Thomas and Robitaille, 2001).
L-citrulline, which is a ubiquitous amino acid in mammals, is closely related to arginine (reviewed by Curis et al., 2005). In hepatocytes, L-citrulline is locally synthesized by the enzyme ornithine carbamoyltransferase and metabolized by argininosuccinate synthetase (ASS, EC 6.3.4.5) for urea production. However, in NO-producing tissues, L-citrulline is the co-product in the biosynthesis of NO by NOS. Because L-citrulline can be easily converted into L-arginine by the successive action of ASS and argininosuccinate lyase (ASL), which are expressed in every cell examined including neurons (Wiesinger, 2001), some authors suggested that L-citrulline might be an indirect precursor of NO in NO-synthesizing cells (Mori and Gotoh, 2000). Previously, Ruiz and Tejerina (1998) advanced the possibility that L-citrulline was not merely a by-product of NO synthesis but might also play a role in cell signalling. In spite of this, little is known about the action of L-citrulline on synaptic transmission, particularly in the control of neurotransmitter release. This prompted us to investigate the role of L-citrulline on nerve-evoked skeletal muscle contractions and on [3H]acetylcholine ([3H]ACh) release from stimulated motor nerve terminals in comparison with the effects caused by the NOS substrate, L-arginine and by the NO donor, 3-morpholinosydnonimine chloride (SIN-1). To block L-citrulline conversion to L-arginine and subsequent formation of NO, we used the NOS inhibitor Nω-nitro-L-arginine (L-NOARG) (Moncada et al., 1991).
The depressant action of the NO pathway on synaptic transmission in the central nervous system (CNS) is, at least, partially mediated through increases in the release of endogenous adenosine acting on A1 receptors (Fallahi et al., 1996; Bon and Garthwaite, 2002). Controversy, however, exists over whether cyclic GMP is involved in NOS-dependant adenosine outflow (Boulton et al., 1994; Broad et al., 2000; Rosenberg et al., 2000; Bon and Garthwaite, 2002). At the rat neuromuscular junction, adenosine acts as a neuromodulator either inhibiting (via A1 receptors) or facilitating (via A2A receptors) the release of [3H]ACh from motor nerve terminals (Correia-de-Sá et al., 1991), depending on the concentration of the nucleoside at the synapse (Correia-de-Sá and Ribeiro, 1996). Interestingly, which adenosine receptor is predominantly activated is apparently determined by the differential contribution of the two main pathways leading to extracellular adenosine accumulation (Correia-de-Sá and Ribeiro, 1996; Cunha et al., 1996). Indeed, adenosine can either be released as such or can be formed upon the sequential extracellular dephosphorylation of ATP co-released with ACh in a frequency-dependant manner (Magalhães-Cardoso et al., 2003). The activity of the ecto-nucleotidase pathway is crucial in defining the pattern of formation of extracellular ATP-derived adenosine, which activates preferentially facilitatory A2A receptors (Cunha et al., 1996) overcoming the activation of A1 receptors restraining transmitter release at low synaptic adenosine levels (Correia-de-Sá and Ribeiro, 1996).
Due to the putative interactions between NOS activity and the adenosine system, we manipulated extracellular adenosine accumulation using either adenosine deaminase (ADA), the enzyme that inactivates endogenous adenosine by conversion to inosine, or the nucleoside transport inhibitor, S-(p-nitrobenzyl)-6-thioinosine (NBTI) (Correia-de-Sá and Ribeiro, 1996), to probe adenosine's role on NOS-induced depression of [3H]ACh release from nerve terminals of the motor endplate. We also investigated the ability of L-citrulline to cause the outflow of adenine nucleotides and adenosine from the rat phrenic nerve-hemidiaphragm. This work is the first to suggest that NOS catalyses the formation of two biologically active products, NO and L-citrulline, both capable of inhibiting neurotransmitter release from motor nerve terminals. Data are presented suggesting that while NO directly reduces transmitter release probability via a soluble guanylyl cyclase-dependant mechanism, the inhibitory action of L-citrulline is guanylyl cyclase-independent and results from the release of adenosine and subsequent activation of pre-synaptic inhibitory A1 receptors.
Methods
Preparation and experimental conditions
Rats (Wistar, 150–200 g) of either sex (Charles River, Barcelona, Spain) were kept at a constant temperature (21°C) and a regular light (0630–1930) dark (1930–0630) cycle, with food and water ad libitum. The animals were killed after stunning followed by exsanguination. Animal handling and experiments followed the guidelines of the International Council for Laboratory Animal Science. The experiments were performed on left phrenic nerve-hemidiaphragm preparations (4–6 mm width). Each muscle was superfused with gassed (95% O2 and 5% CO2) Tyrode's solution (pH 7.4) containing (mM): NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 1, NaH2PO4 0.4, NaHCO3 11.9, glucose 11.2 and choline 0.001, at 37°C.
Nerve stimulation conditions
The left phrenic nerve was stimulated with an extracellular glass-platinum suction electrode placed near its first division branch, to avoid direct stimulation of muscle fibres (indirect stimulation). To evaluate drug effects on muscle contractile properties, direct stimulation of muscle fibres was delivered through a pair of platinum electrodes placed at each side of the diaphragm near its costal insertion (field stimulation). Supramaximal intensity (current strength of 8 mA) rectangular pulses of 0.04 ms (indirect stimulation) or 1 ms (field stimulation) duration were used to achieve firing synchronization, thus reducing the number of silent units (motoneurons and/or muscle fibres) that might make interpretation of data difficult. The pulses were delivered by a Grass S48 (Quincy, MA, USA) stimulator coupled to a stimulus isolation unit (Grass SIU5) operating in a constant current mode. The stimulation parameters were continuously monitored on an oscilloscope (Meguro, MO-1251A, Japan) and were within the same range used in previous studies with this preparation (e.g., Wessler and Kilbinger, 1986; Correia-de-Sá et al., 2000).
[3H]acetylcholine release experiments
The procedures used for labelling the preparations and measuring-evoked [3H]ACh release were as previously described (Correia-de-Sá et al., 1991), with minor modifications. Phrenic nerve-hemidiaphragm preparations were mounted in 3 ml capacity Perspex chambers heated to 37°C. Nerve terminals were labelled for 40 min with 1 μM [3H]choline (specific activity 2.5 μCi nmol−1) under electrical stimulation at a 1 Hz frequency. Washout of the preparations was performed for 60 min, by superfusion (15 ml min−1) with Tyrode solution supplemented with the choline uptake inhibitor, hemicholinium-3 (10 μM). Tritium outflow was evaluated by liquid scintillation spectrometry (% counting efficiency 40±2%) after appropriate background subtraction using 2 ml bath samples collected automatically every 3 min. After the loading and washout periods, the preparation contained (5542±248) × 103 disintegrations per minute per gram (d.p.m. g−1) wet weight of tissue and the resting release was (132±12) × 103 d.p.m. g−1 (n=8). The fractional release was calculated to be 2.38±0.14% of the radioactivity present in the tissue at the first collected sample.
Unless otherwise stated, [3H]ACh release was evoked by electrical stimulation of the phrenic nerve with trains of 750 supramaximal intensity pulses of 0.04 ms duration delivered at a frequency of 5 Hz. Two stimulation periods were used, starting respectively at twelfth (S1) and thirty-ninth (S2) minutes after the end of washout (zero time). Electrical stimulation increased only the release of [3H]ACh in a Ca2+- and tetrodotoxin-sensitive manner (Correia-de-Sá et al., 2000), while the output of [3H]choline remained unchanged (Wessler and Kilbinger, 1986), thus indicating that ACh comes mainly from vesicle exocytosis from depolarized nerve terminals. Therefore, evoked [3H]ACh release was calculated by subtracting the basal tritium outflow from the total tritium outflow during the stimulation period (cf. Correia-de-Sá et al., 1991).
Test drugs were added 15 min before S2 and were present up to the end of the experiments (see Figure 2). The percentage change in the ratio between the evoked [3H]ACh release during the two stimulation periods (S2/S1) relative to that observed in control situations (in the absence of test drugs) was taken as a measure of the effect of the tested drugs. When we evaluated changes in the effect of test drugs induced by a modifier (e.g., enzymatic inhibitor, receptor antagonist, transport inhibitor), these compounds were applied 15 min before starting sample collection and hence were present during S1 and S2. When present during S1 and S2, none of the modifiers significantly altered (P>0.05) the S2/S1 ratio as compared to the S2/S1 ratio obtained in the absence of the modifiers (0.81±0.03, n=8). None of the drugs changed significantly (P>0.05) basal tritium outflow.
Figure 2.
Effect of the extracellular NO scavenger, haemoglobin, on L-arginine- and SIN-1-induced inhibition of [3H]ACh release from stimulated motor nerve terminals. The time course of tritium outflow from phrenic nerve terminals shown here is taken from typical experiments in the absence (control, filled squares) and in the presence of (a) L-arginine (L-Arg, 47 μM) or (b) SIN-1 (10 μM), used either alone (filled circles) or in the presence (open circles) of haemoglobin (Hb, 10 μM). Tritium outflow (ordinates) is expressed as a percentage of the total radioactivity present in the tissue at the beginning of the collection period and was measured in samples collected every 3 min. [3H]ACh release was elicited by stimulating the phrenic nerve trunk with 750 pulses delivered with a frequency of 5 Hz at the indicated times (S1 and S2). L-arginine (47 μM) and SIN-1 (10 μM) were applied 15 min before S2; haemoglobin (10 μM) was present throughout the assay, including S1 and S2 (horizontal bars). None of the drugs changed spontaneous tritium outflow.
Release of adenine nucleotides and adenosine
To follow the release of adenine nucleotides and adenosine, the preparations were incubated as for the release of [3H]ACh, except that no [3H]choline was added to the Tyrode's solution. The preparations were superfused (3 ml min−1) for 30 min with gassed Tyrode solution containing the ADA inhibitor, erythro-9(2-hydroxy-3-nonyl) adenine (EHNA, 0.3 μM), which was present from then on. After stopping superfusion, the preparations were incubated with 2 ml oxygenated Tyrode solution that was automatically changed every 3 min by emptying and refilling the organ bath with the solution in use. As for the release of [3H]ACh, the preparations were also stimulated twice using similar nerve stimulating conditions (750 pulses of 0.04 ms duration delivered at a frequency of 5 Hz), starting respectively at 12th (S1) and 39th (S2) min after starting sample collection (zero time). In these experiments, only the four samples collected before stimulus application and the three samples collected immediately after stimulation were retained for analysis.
Bath aliquots were frozen in liquid nitrogen immediately after collection, stored at −20°C and analysed by high performance liquid chromatography (HPLC) (see Cunha and Sebastião, 1991) within 1 week of collection. To measure adenine nucleotides and adenosine, we used 200 μl aliquots from collected samples. Test drugs were added 15 min before S2 and were present throughout the assay. The effects of test drugs were expressed by the ratios S2/S1, that is the ratio between the evoked release of adenine nucleotides and adenosine during the second stimulation period (in the presence of the test drug) and the corresponding release during the first stimulation period (without the test drug). To evaluate the effect of test drugs on the basal outflow of adenine nucleotides and adenosine, we calculated the B2−n/B1−n ratios (see Figure 6). B1−n and B2−n correspond to the content of adenine nucleotides and adenosine in bath samples collected n minutes before the first (without the test drug) and the second (in the presence of the test drug) stimulation periods, respectively.
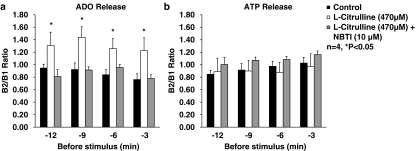
Figure 6.
Effect of L-citrulline (470 μM) on the resting outflow of (a) adenosine and (b) ATP (and related adenine nucleotides) from the rat innervated hemidiaphragm in the absence and in the presence of the nucleoside transport inhibitor, NBTI (10 μM). L-citrulline (470 μM) was applied 15 min before S2; NBTI (10 μM) was present throughout the assay, including S1 and S2. The ordinates represent the resting outflow of (a) adenosine and (b) ATP (and related adenine nucleotides) expressed by the B2−n/B1−n ratios; B1−n and B2−n correspond to the adenosine or adenine nucleotide content in bath samples collected n minutes before the first (without L-citrulline) and the second (in the presence of L-citrulline) stimulation periods, respectively. Each column represents pooled data from four experiments. The vertical bars represent s.e.m. *P<0.05 (one-way ANOVA followed by Dunnett's modified t-test), significant differences from the control.
The remaining incubation medium was used to quantify the lactate dehydrogenase (LDH, EC 1.1.1.27) activity. The negligible activity of LDH in bath samples collected before (0.14±0.02 mU ml−1, n=16) and after (0.15±0.01 mU ml−1, n=16) electrical nerve stimulation is an indicator of the integrity of cells during the experimental procedure.
Muscle contraction recordings
When tension responses were recorded, the innervated diaphragm strips were mounted in 10 ml capacity isolated organ bath chambers. The preparations were superfused (5 ml min−1, 37°C, pH 7.4) with gassed (95% O2+5% CO2) Tyrode's solution. Alternate (0.1 Hz frequency) direct- and nerve-induced responses were recorded isometrically at a resting tension of 50 mN with a force transducer and displayed on a Hugo-Sachs (Germany) recorder. After the initial stabilization period, these experimental conditions allowed a well-preserved contraction pattern for several hours in the absence of test drugs. The solutions were changed by transferring the inlet tube of the peristaltic pump (Gilson, Minipuls3, France) from one flask to another. Test drugs were allowed to be in contact with the preparations for at least 12 min. To reduce the safety margin of neuromuscular transmission (see, e.g., Wood and Slater, 2001), MgCl2 (6 mM) was added to the bath in some of the experiments. Osmolarity was maintained by equimolar substitution of NaCl. Elevation of magnesium ions to 6 mM decreased the amplitude of nerve-evoked responses from a control value of 40±2 to 17±1 mN (n=6), without significantly (P>0.05) changing the contractions induced by direct muscle stimulation.
Presentation of data and statistical analysis
The data are expressed as mean±s.e.m. from n observations. Statistical analysis of data was carried out using paired or unpaired Student's t-test or one-way analysis of variance (ANOVA) followed by Dunnett's modified t-test. A value of P<0.05 was considered to represent a significant difference.
Materials and solutions
ADA (type VI, 1803 U ml−1, EC 3.5.4.4), choline chloride, hemicholinium-3, haemoglobin from rat, D- and L-arginine, D- and L-citrulline, L-NOARG, NBTI (Sigma, St Louis, MO, USA); 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), EHNA (Research Biochemicals, Natick, MA, USA); 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), SIN-1, 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM 241385) (Tocris Cookson Inc., UK); [methyl-3H]choline chloride (ethanol solution, 80 Ci mmol−1) (Amersham, UK). All other reagents were of the highest purity available.
EHNA was made up in a 5 mM stock solution in ethanol. DPCPX was made up in a 5 mM stock solution in 99% dimethyl sulphoxide (DMSO)+1% NaOH 1 M (v/v). ZM 241385 and NBTI were made up in 5 and 50 mM stock solutions in DMSO, respectively. Other drugs were prepared in distilled water. All stock solutions were stored as frozen aliquots at −20°C. Dilutions of these stock solutions were made daily and appropriate solvent controls were done. No statistically significant differences between control experiments, made in the absence or in the presence of the solvents at the maximal concentrations used (0.5%, v/v), were observed. The pH of the superfusion solution did not change following addition of the drugs at the maximum concentrations applied to the preparations.
Results
Neuronal NOS catalyses the formation of two biologically active products, NO and L-citrulline, acting independently to reduce ACh release from motor nerve terminals
When applied in the physiological concentration range (see Griffith and Stuehr, 1995), the NOS substrate L-arginine (0.01–4.7 mM) concentration dependently decreased [3H]ACh release from stimulated phrenic nerve terminals (Figure 1). The NO donor, SIN-1 (1–100 μM) and the co-product of NO biosynthesis catalysed by NOS, L-citrulline (0.01–4.7 mM), mimicked the inhibitory effect of L-arginine on [3H]ACh release; the concentrations required to decrease neurotransmitter release by about 30% were 10 μM SIN-1, 47 μM L-arginine and 470 μM L-citrulline. The effects of L-arginine and L-citrulline were stereospecific, as their D-isomers (0.01–4.7 mM) were devoid of effect on [3H]ACh release; in the highest concentration (4.7 mM) tested, D-arginine and D-citrulline decreased transmitter release only by 7±3 (n=7) and 6±1% (n=4), respectively. The maximal inhibitory effects on [3H]ACh release of SIN-1 (100 μM) and L-citrulline (470 μM) were about half the magnitude of those exerted by L-arginine (4.7 mM) (Figure 1). Interestingly, SIN-1 (10 μM) applied 15 min before S2 to preparations incubated with L-citrulline (470 μM) during the whole assay, including S1 and S2, could still reduce evoked [3H]ACh release by a similar amount (27±5%, n=5) to that observed in control conditions (30±6%, n=4).
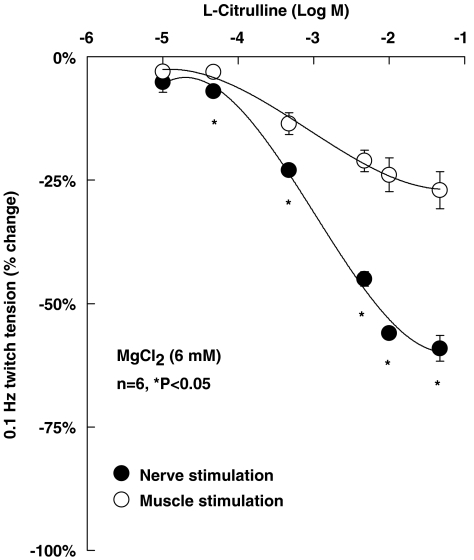
Figure 1.
Concentration–response curves for the inhibitory effects of L-arginine (0.01–4.7 mM), L-citrulline (0.01–4.7 mM) and SIN-1 (1–100 μM) on [3H]ACh release from motor nerve terminals stimulated with 5 Hz-trains (750 pulses). Abscissa, log of the concentration (M) of L-arginine (0.01–4.7 mM, squares), L-citrulline (0.01–4.7 mM, circles) and SIN-1 (1–100 μM, triangles), applied 15 min before S2. Ordinate, percentage change in S2/S1 ratio as compared with the S2/S1 ratio in control experiments. Zero per cent represents identity between the two ratios, negative values indicate inhibition of evoked [3H]ACh release. Each point represents the mean±s.e.m. of 4–11 experiments.
Figure 2 illustrates the time course of tritium outflow in experiments where L-arginine (47 μM) and SIN-1 (10 μM) were applied 15 min before S2 in the absence and in the presence of the extracellular NO scavenger, haemoglobin (10 μM). As can be seen from these typical experiments, the inhibitory effect of the NO donor SIN-1 (10 μM), but not that of the NOS substrate L-arginine (47 μM), was completely prevented by haemoglobin (10 μM). It thus appears that the inhibitory effect of L-arginine on [3H]ACh release from stimulated motor nerve terminals did not require diffusion of NO throughout the extracellular space.
To investigate whether changes in neurotransmitter release caused by L-citrulline (0.01–47 mM) correspond to alterations in the contraction of hemidiaphragm preparations, we studied the effect of this compound on twitch tension induced either by phrenic nerve stimulation or by direct depolarization of muscle fibres. Because of the high-safety factor of neuromuscular transmission, depression of nerve-evoked muscle contractions due to prejunctional acting drugs might not always reflect the magnitude of transmitter release inhibition (for a review, see Wood and Slater, 2001). This might explain why L-citrulline (0.01–47 mM) was more potent to inhibit evoked [3H]ACh release (Figure 1) than to cause depression of nerve-evoked muscle contractions while keeping a high-transmission safety margin (data not shown). By decreasing the safety factor of synaptic transmission with high magnesium (MgCl2, 6 mM), L-citrulline (0.01–47 mM) reduced diaphragm twitch tension caused by electrical nerve stimulation in a concentration-dependent manner (Figure 3). The magnitude of inhibition was significantly (P<0.05) higher when the contractions were induced by phrenic nerve stimulation as compared with direct muscle depolarization, indicating that the inhibitory effect of L-citrulline on nerve-evoked contractions requires synaptic transmission rather than an action on excitation–contraction coupling or on the muscle contraction apparatus.
Figure 3.
Inhibitory effect of L-citrulline (0.01–47 mM) on diaphragm twitch tension induced by phrenic nerve stimulation (indirect stimulation, filled circles) or by direct muscle depolarization (direct stimulation, open circles) in conditions where the safety factor of neuromuscular transmission was reduced (high Mg2+, 6 mM). Twitch responses were induced alternating stimulus application to the phrenic nerve trunk or to muscle fibres at a frequency of 0.1 Hz. L-citrulline (0.01–47 mM) was applied in a cumulative manner; each concentration contacted the preparation at least 12 min before solution changeover. Ordinate, percentage change of the maximal twitch tension obtained in control conditions. Each point represents the mean±s.e.m. of six experiments. *P<0.05 (one-way ANOVA followed by Dunnett's modified t-test) compared with the effect of L-citrulline on twitch tension induced by direct muscle stimulation.
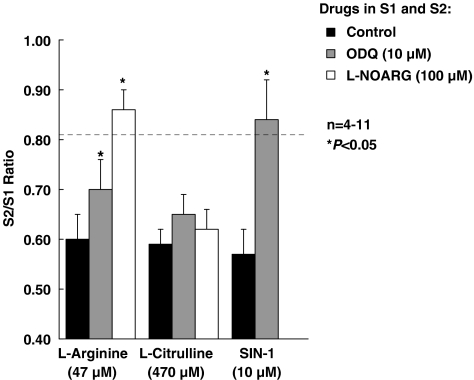
To block formation of NO by exogenous L-citrulline acting as a precursor of L-arginine, we used the NOS inhibitor, L-NOARG (100 μM). As shown in Figure 4, L-NOARG (100 μM, applied during the whole assay including S1 and S2) completely prevented the action of L-arginine (47 μM), but the inhibitory effect of L-citrulline (470 μM) remained virtually unchanged (P>0.05). Moreover, pre-treatment of the preparations with the soluble guanylyl cyclase inhibitor, ODQ (10 μM, applied during the whole assay including S1 and S2), partially attenuated L-arginine (47 μM) inhibition, but fully prevented the inhibitory effect of SIN-1 (10 μM). ODQ (10 μM) was unable to change the inhibitory action of L-citrulline (470 μM) even though this substance and the two NO-generating compounds, L-arginine (47 μM) and SIN-1 (10 μM), all reduced the release of [3H]ACh by a similar amount (∼30%) (Figure 4). L-NOARG (100 μM) and ODQ (10 μM) increased the evoked [3H]ACh release by 22±1% (n=4) and 34±7% (n=5), respectively, indicating that both NOS and guanylyl cyclase are tonically activated to control neurotransmitter release from stimulated phrenic nerve terminals.
Figure 4.
Influence of inhibition of soluble guanylyl cyclase (with ODQ) and NOS (with L-NOARG) on the reduction of evoked [3H]ACh release caused by L-arginine, L-citrulline and SIN-1. L-arginine (47 μM), L-citrulline (470 μM) and SIN-1 (10 μM) were applied 15 min before S2 in concentrations that caused about 30% inhibition of [3H]ACh release from stimulated motor nerve terminals. ODQ (10 μM) and L-NOARG (100 μM) were present throughout the assay, including S1 and S2; the S2/S1 ratios obtained under these conditions were not statistically different from the ratio obtained in control experiments (without any drug during S1 and S2) (dashed horizontal line, see ‘Methods' section). The ordinates represent evoked tritium outflow expressed by S2/S1 ratios. Each column represents pooled data from 4 to 11 experiments. The vertical bars represent s.e.m. *P<0.05 (one-way ANOVA followed by Dunnett's modified t-test) when compared with the effects of L-arginine, L-citrulline and SIN-1 applied alone, respectively.
L-Citrulline inhibition of [3H]ACh release is secondary to activation of pre-synaptic inhibitory A1 receptors by endogenous adenosine
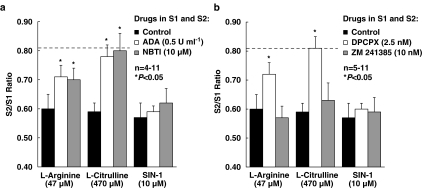
Inactivation of endogenous adenosine with ADA (0.5 U ml−1) or inhibition of the nucleoside transport system with NBTI (10 μM) partially attenuated the inhibitory role of L-arginine (47 μM) on [3H]ACh release (Figure 5a). Interestingly, ADA (0.5 U ml−1) or NBTI (10 μM) fully prevented the inhibitory effect of L-citrulline (470 μM), but did not affect the reduction of evoked [3H]ACh release induced by SIN-1 (10 μM) (Figure 5a).
Figure 5.
Role of endogenous adenosine on the inhibitory effect of L-arginine, L-citrulline and SIN-1 on evoked [3H]ACh release from motor nerve terminals. L-arginine (47 μM), L-citrulline (470 μM) and SIN-1 (10 μM) were applied 15 min before S2 in concentrations that caused about 30% inhibition of [3H]ACh release from stimulated motor nerve terminals. (a) ADA (0.5 U ml−1), the nucleoside transport inhibitor (NBTI, 10 μM) and (b) the two adenosine antagonists exhibiting high-subtype selectivity for A1 (DPCPX, 2.5 nM) and A2A (ZM 241385, 10 nM) receptors were present throughout the assay, including S1 and S2. The S2/S1 ratios obtained under these conditions were not statistically different from the ratio obtained in control experiments (without any drug during S1 and S2) (dashed horizontal line, see ‘Methods' section). The ordinates represent evoked tritium outflow expressed by S2/S1 ratios. Each column represents pooled data from (a) 4–11 and (b) 5–11 experiments. The vertical bars represent s.e.m. *P<0.05 (one-way ANOVA followed by Dunnett's modified t-test) when compared with the effects of L-arginine, L-citrulline and SIN-1 applied alone, respectively.
We then investigated the adenosine receptor subtype involved in the control of [3H]ACh release by NOS activity. Selective blockade of A1 receptors with DPCPX (2.5 nM) completely prevented depression of release caused by L-citrulline (470 μM) (Figure 5b). In the presence of DPCPX (2.5 nM), the inhibitory action of L-arginine (47 μM) was only partially attenuated by a similar amount to that observed in the presence of ADA (0.5 U ml−1) or NBTI (10 μM) (see Figure 5a). The A1 receptor antagonist failed to affect inhibition of [3H]ACh release induced by SIN-1 (10 μM). Conversely, blockade of A2A receptors with ZM 241385 (10 nM) was unable to modify the inhibitory effects of L-arginine (47 μM), L-citrulline (470 μM) or SIN-1 (10 μM) (Figure 5b).
L-Citrulline increases adenosine outflow via the equilibrative nucleoside transport system
Stimulation of the phrenic nerve at a frequency of 5 Hz (750 pulses of 0.04 ms duration) led to an increased accumulation of ATP (and related adenine nucleotides) in the bath effluent from an average basal value of 7093±25 fmol (mg tissue)−1 to a total value of 11 363±57 fmol (mg tissue)−1 (n=4). Nerve-evoked release of ATP (and related adenine nucleotides) was dependant on extracellular Ca2+ and on neuronal activity, since omission of Ca2+ in the Tyrode's solution or application of 1 μM tetrodotoxin essentially abolished nucleotide outflow (Magalhães-Cardoso et al., 2003). Electrical nerve stimulation also led to significant (P<0.05) extracellular adenosine accumulation while the amounts of inosine and hypoxanthine remained virtually unchanged (data not shown) providing that ADA activity was inhibited with EHNA (0.3 μM); nerve-evoked adenosine outflow increased from an average basal value of 2343±26 fmol (mg tissue)−1 to a total value of 3525±88 fmol (mg tissue)−1 (n=4).
L-Citrulline (470 μM) decreased the nerve-evoked release of adenine nucleotides by a similar proportion to that observed when measuring [3H]ACh release, but did not increase the extracellular adenosine accumulation following phrenic nerve stimulation (Table 1). The inhibitory effect of L-citrulline (470 μM) on the release of [3H]ACh and adenine nucleotides from stimulated nerve terminals was prevented by the nucleoside transport blocker, NBTI (10 μM) (see also Figure 5a). Although these results indicate an essential role of the adenosine transport system in mediating L-citrulline-induced inhibition of evoked transmitter release, they do not clearly implicate L-citrulline as a promoter of extracellular adenosine production. Therefore, we investigated in more detail the outflow pattern of adenine nucleotides and adenosine during the resting period before stimulus application. As shown in Figure 6, L-citrulline (470 μM) increased the resting adenosine concentration above the control level, without significantly (P>0.05) affecting the release of adenine nucleotides. This increase in extracellular adenosine concentration caused by L-citrulline (470 μM) appeared to be controlled predominantly by the equilibrative nucleoside transport system, as it was blocked by NBTI (10 μM; Figure 6a).
Table 1.
Effect of L-citrulline on the release of [3H]ACh, adenine nucleotides (ATP) and adenosine (ADO) from stimulated motor nerve terminals in the absence and in the presence of the nucleoside transport inhibitor, NBTI (10 μM)
| S2/S1 ratios |
|||
|---|---|---|---|
| [3H]ACh release | ATP release | ADO release | |
| Control | 0.81±0.03 (8) | 0.95±0.02 (4) | 0.86±0.05 (4) |
| L-Citrulline (470 μM) | 0.59±0.03 (6)* | 0.66±0.05 (4)* | 0.91±0.12 (4) |
| L-Citrulline (470 μM)+NBTI (10 μM) | 0.80±0.06 (5) | 0.95±0.09 (4) | 0.76±0.03 (4) |
The release of [3H]ACh, ATP and ADO was elicited by two trains (S1 and S2) of electrical stimulation consisting of 750 pulses delivered at a 5 Hz frequency (0.04 ms pulse duration). Values for S2/S1 ratios are means±s.e.m. L-Citrulline (470 μM) was applied 15 min before S2. NBTI (10 μM) was present throughout the assay, including S1 and S2; the S2/S1 ratio obtained in the presence of NBTI (10 μM) alone was not statistically different from control ratios (without any drug during S1 and S2). The number of experiments is given in parentheses.
P<0.05 (one-way ANOVA followed by Dunnett's modified t-test), significant differences from the control.
Discussion and conclusions
NOS immunoreactivity has been localized in the sarcolemmal surface of muscle cells, intramuscular axons and neuromuscular synapses in a variety of vertebrate species including man. At the skeletal neuromuscular junction, constitutive neuronal NOS (nNOS) is considered to be the predominant isoform and seems to be localized mainly in the cytoplasm of pre-synaptic nerve terminals and terminal Schwann cells (Ribera et al., 1998; Rothe et al., 2005). Regional distribution and cellular density of nNOS may be altered during development and ageing (Blottner and Lück, 2001), and in some specific diseases (e.g., Duchenne muscular dystrophy, myasthenia gravis) (reviewed by Stamler and Meissner, 2001). Localization of endogenous nNOS and cyclic GMP-dependant protein kinase at the neuromuscular junction suggests that NO may be involved in the physiological modulation of ACh release. Alternatively, NO may act as a short-lived (milliseconds) diffusible (∼500 μm) messenger originated from Schwann cells and muscle fibres to regulate synaptic activity and synapse formation in severed neuromuscular junctions (Descarries et al., 1998; Thomas and Robitaille, 2001). The literature provides evidence that NO released from post-synaptic sources acts pre-synaptically (Moncada and Higgs, 1993), increasing the release of glutamate from neurons of the CNS (Garthwaite, 1991) or decreasing the release of both substance P and ACh from neurons of peripheral tissues (Gustafsson et al., 1990). Although this hypothesis is feasible, we failed to modify the inhibitory effect of L-arginine by incubating the preparations with the extracellular NO scavenger, haemoglobin, while inhibition of NOS activity with L-NOARG completely prevented L-arginine action. As haemoglobin does not easily enter cells (Hakim et al., 1996), but it was able to block the action of the NO donor, SIN-1 (Figure 2), the results suggested that L-arginine-induced NO formation catalysed by NOS occurred mainly inside nerve terminals directly leading to depression of [3H]ACh release at the rat motor endplate.
In skeletal muscle, NO plays a role in the regulation of neural transmission (Wang et al., 1995). NO appears to influence both quantal and non-quantal ACh release from pre-synaptic terminals in several types of neuromuscular junctions, including the mammalian skeletal muscle (Ribera et al., 1998; Mukhtarov et al., 2000). It is unlikely that non-quantal transmitter release accounts for the total amount of [3H]ACh released upon electrical stimulation of the phrenic nerve. This assumption is based on findings indicating that the spontaneously releasable neuronal pool of ACh is not labelled with [3H]choline nor it is released by electrical nerve stimulation (Molenaar et al., 1987) and it is completely exhausted (within minutes) in the presence of hemicholinium-3 (Nikolsky et al., 1991). Whether nerve-induced glutamate secretion modulates non-quantal and spontaneous ACh release, directly via activation of neuronal metabotropic receptors or indirectly via activation of muscle N-methyl-D-aspartate (NMDA) receptors and retrograde diffusion of NO synthesized by skeletal muscle fibres, has been a matter of debate (see, e.g., Malomouzh et al., 2005). Although the glutamatergic modulation of neuromuscular transmission deserves further investigation, we failed to modify the nerve-evoked [3H]ACh release in the presence of D-(−)-2-amino-5-phosphonopentanoic acid (50 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (20 μM), which antagonize respectively ionotropic NMDA and non-NMDA glutamate receptors (unpublished observations).
The mechanism underlying NO suppression of transmitter exocytosis remains unclear. NO may regulate calcium-activated potassium currents by mechanisms independent of any effect on the calcium influx through voltage-sensitive calcium channels (Certiner and Bennett, 1993). Such channels modulate the release of ACh from motor nerve terminals of frogs (Zefirov et al., 2002), where NO may regulate transmitter release via cyclic GMP-dependant and -independent pathways (Thomas and Robitaille, 2001). In this work, we showed that the NO donor, SIN-1, decreased [3H]ACh release from stimulated motor nerve terminals. The inhibitory action of SIN-1 was prevented by the guanylyl cyclase inhibitor, ODQ, suggesting that NO triggered activation of soluble guanylyl cyclase and thereby raised cyclic GMP levels to cause depression of transmitter exocytosis. Many actions of cyclic GMP are elicited by cyclic GMP-dependant (G) kinase (Moncada et al., 1991). However, cyclic GMP can also directly gate certain ion channels and regulates the activity of cyclic adenosine monophosphate (AMP) phosphodiesterase, yielding increases in intracellular cyclic AMP (Ignarro, 1991). This pathway is highly unlikely to be involved here as activation of the cyclic AMP pathway increases, rather than decreasing, [3H]ACh release from stimulated motor nerve terminals of the rat (Correia-de-Sá and Ribeiro, 1994). At the amphibian neuromuscular junction, NO inhibition of neurotransmitter release cannot be solely explained by a reduction in calcium entry, suggesting that regulation occurs downstream of calcium entry (Thomas and Robitaille, 2001). Alternatively, NO has been shown to mediate post-transcriptional modifications of proteins through reactions with thiol and/or transition metal centres (identified in ion channels, receptors, enzymes, transcription factors and small G proteins) (Ignarro, 1991). These modifications may prevent normal interactions between proteins involved in synaptic vesicle pre-synaptic membrane-specific interactions occurring during exocytosis. Regulation via the frequency and the kinetics of vesicle fusion is, however, improbable as the frequency of miniature endplate potentials and the quantal content of neuromuscular transmission were not affected by NO donors at the rat diaphragm (Mukhtarov et al., 2000). It thus appears that there are important species differences concerning the mechanisms underlying control of neuromuscular transmission by NO (namely between mammalian and amphibian), which may be both structural and functional in nature (see, e.g., Wang et al., 1995; Thomas and Robitaille, 2001).
L-Arginine is a widespread amino acid involved in many physiological processes. The main importance of L-arginine is attributed to its role as a precursor for the synthesis of NO, and its effects on neuromuscular transmission were not observed when L-arginine was exchanged for D-arginine (see also Silva et al., 1999). Recently, data about specific targets of L-arginine action, independent of NO synthesis, have emerged (Mori and Gotoh, 2000). Here, we showed that L-arginine depressed the release of [3H]ACh from stimulated motor nerve terminals with a higher efficacy than the NO donor, SIN-1, while the soluble guanylyl cyclase inhibitor, ODQ (applied in a 10 μM concentration that fully blocked the effect of SIN-1), partially preserved L-arginine inhibition. This finding suggested that inhibition of electrically evoked transmitter release by L-arginine involved cooperation of NO-dependant and -independent mechanisms. The latter mechanism may be linked with an increased synthesis of L-citrulline, as this co-product of NO biosynthesis also reduced [3H]ACh release from stimulated motor nerve terminals in a way that was additive to the inhibitory action of SIN-1. Inhibition of transmitter release by L-citrulline was independent of soluble guanylyl cyclase activity, since it was not affected by ODQ. Although it has been reported that L-citrulline is capable of sustaining maximal rates of NO production in NO-synthesizing cells due to an extra supply of L-arginine (reviewed by Curis et al., 2005), our results showed that the inhibitory effect of L-citrulline was virtually unchanged in the presence of the NOS inhibitor, L-NOARG (Moncada et al., 1991). Thus, data suggest that NOS catalyses the formation of two biologically active products, NO and L-citrulline, which contribute independently to depress [3H]ACh release from stimulated motor nerve terminals. Recycling of L-citrulline into L-arginine (via the ASS and ASL pathways) might not occur at the rat motor endplate and, hence, L-citrulline could act by itself or strengthen the action of other substances inhibiting release, to depress neuromuscular transmission overall.
The real novelty of the present study is the observation that endogenous adenosine mediates L-citrulline-induced inhibition of [3H]ACh release at the rat motor endplate. Previous reports have implicated endogenous adenosine accumulation as a key player operating the effects of the NOS pathway in the nervous system (Fallahi et al., 1996; Rosenberg et al., 2000). In keeping with this hypothesis, we showed that the inhibitory effect of L-arginine was partially attenuated by reducing endogenous adenosine accumulation with ADA, the enzyme that inactivates adenosine into its inactive metabolite inosine, or with the nucleoside transport inhibitor, NBTI (Correia-de-Sá and Ribeiro, 1996). Inactivation of extracellular adenosine with ADA prevented the ability of L-citrulline to inhibit [3H]ACh release, while keeping unchanged the effect of the NO donor, SIN-1. Because NBTI also blocked L-citrulline inhibition of transmitter release, as well as L-citrulline-induced adenosine outflow without causing a comparable change in the release of ATP (and related adenine nucleotides), it seems reasonable to assume that adenosine is transported as such across the plasma membrane via the equilibrative nucleoside transport system (Griffith and Jarvis, 1996). The adenosine A1 receptor seems to be the receptor underlying L-citrulline-induced inhibition, since pre-treatment with the selective A1 antagonist, DPCPX, but not with the A2A antagonist, ZM 241385, completely prevented the inhibitory effect of L-citrulline. It remains, however, to be elucidated whether L-citrulline-induced adenosine outflow results from inhibition of intracellular adenosine kinase, the primary metabolic pathway regulating both intra- and extracellular levels of the nucleoside (Lloyd and Fredholm, 1995), because most adenosine kinase inhibitors also compete with the nucleoside for the uptake system. Alternatively, intracellular adenosine may be generated from the hydrolysis of AMP produced as consequence of ATP consumption during the metabolism of L-citrulline catalysed by ASS. Unfortunately, there are no inhibitors of this enzyme responsible for the rate-limiting step in the utilization of L-citrulline, but its activity is genetically impaired in citrullinaemia, a rare autosomal recessive disorder leading to the accumulation (low millimolar range) of citrulline and ammonia in tissues and body fluids (Curis et al., 2005). Whether abnormalities of adenosine signalling contribute to the neurochemical imbalance (glutamate-mediated excitation) underlying neurological sequelae of citrullinaemic patients and animal models (e.g., convulsions, tremor, seizures, extensor rigidity, coma, brain oedema) (Dodd et al., 1992) deserve to be investigated in the future.
To our knowledge, this is the first demonstration that enzymatic conversion of L-arginine by NOS generates two neuronally active products, NO and L-citrulline, that act independently to depress-evoked [3H]ACh release from motor nerve endings. Physiologically, the NO pathway (and the formation of L-citrulline) is activated by nerve stimulation. While NO may directly reduce the probability of transmitter release through stimulation of soluble guanylyl cyclase, L-citrulline acts by enhancing the inhibitory effects of adenosine A1 receptors, subsequent to increased adenosine transport into the synaptic cleft. Because of the inhibitory role of NO on evoked transmitter exocytosis, tonic activation of facilitatory A2A receptors by adenosine generated from the breakdown of ATP co-released with ACh may become weaker. On the other hand, reduced formation of adenosine from ATP hydrolysis may be counterbalanced by the action of L-citrulline generated during nerve stimulation, which facilitates adenosine release from cells, via equilibrative nucleoside transporters, thereby changing the receptor activation balance towards the inhibitory A1 receptor (Correia-de-Sá and Ribeiro, 1996) (see Table 1). These findings gain pathophysiological relevance since systemic L-arginine administration has been recently shown to provide therapeutic benefit in Duchenne dystrophic patients by decreasing muscle degeneration (Barton et al., 2005) and adenosine-mediated changes in transmitter release, as described here, may contribute to the neurological signs of citrullinaemia.
Acknowledgments
This research was partially supported by FCT projects (POCTI/CVT/43368/2001 and UMIB-215/94). We also thank Mrs M Helena Costa e Silva, Suzete Liça and Belmira Silva for their technical assistance.
Abbreviations
- ADA
adenosine deaminase
- ASL
argininosuccinate lyase
- ASS
argininosuccinate synthetase
- DPCPX
1,3-dipropyl-8-cyclopentylxanthine
- EHNA
erythro-9(2-hydroxy-3-nonyl) adenine
- L-NOARG
Nω-nitro-L-arginine
- NBTI
S-(p-nitrobenzyl)-6-thioinosine
- NOS
nitric oxide synthase
- OCT
ornithine carbamoyltransferase
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- SIN-1
3-morpholinosydnonimine hydrochloride
- ZM 241385
4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol
Conflict of interest
The authors state no conflict of interest.
References
- Barton ER, Morris L, Kawana M, Bish LT, Toursel T. Systemic administration of L-arginine benefits mdx skeletal muscle function. Muscle Nerve. 2005;32:751–760. doi: 10.1002/mus.20425. [DOI] [PubMed] [Google Scholar]
- Blottner D, Lück G. Just in time and place: NOS/NO system assembly in neuromuscular junction formation. Microsc Res Tech. 2001;55:171–180. doi: 10.1002/jemt.1168. [DOI] [PubMed] [Google Scholar]
- Bon CLM, Garthwaite J. Adenosine acting on A1 receptors protects NO-triggered rebound potentiation and LTP in rat hippocampal slices. J Neurophysiol. 2002;87:1781–1798. doi: 10.1152/jn.00630.2001. [DOI] [PubMed] [Google Scholar]
- Boulton CL, Irving AJ, Southam E, Potier B, Garthwaite J, Collingridge GL. The nitric oxide-cyclic GMP pathway and synaptic depression in rat hippocampal slices. Eur J Neurosci. 1994;6:1528–1535. doi: 10.1111/j.1460-9568.1994.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Broad RM, Fallahi N, Fredholm BB. Nitric oxide interacts with oxygen free radicals to evoke the release of adenosine and adenine nucleotides from rat hippocampal slices. J Auton Nerv Sys. 2000;81:82–86. doi: 10.1016/s0165-1838(00)00124-7. [DOI] [PubMed] [Google Scholar]
- Certiner M, Bennett MR. Nitric oxide modulation of calcium-activated potassium channels in postganglionic neurons of avian cultured ciliary ganglia. Br J Pharmacol. 1993;110:995–1002. doi: 10.1111/j.1476-5381.1993.tb13912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia-de-Sá P, Ribeiro JA. Evidence that the presynaptic A2A-adenosine receptor of the rat motor nerve endings is positively coupled to adenylate cyclase. Naunyn-Schmiedebergs Arch Pharmacol. 1994;350:514–522. doi: 10.1007/BF00173021. [DOI] [PubMed] [Google Scholar]
- Correia-de-Sá P, Ribeiro JA. Adenosine uptake and deamination regulate tonic A2A receptor facilitation of evoked [3H]acetylcholine release from the rat motor nerve terminals. Neuroscience. 1996;73:85–92. doi: 10.1016/0306-4522(96)00028-0. [DOI] [PubMed] [Google Scholar]
- Correia-de-Sá P, Sebastião AM, Ribeiro JA. Inhibitory and excitatory effects of adenosine receptor agonists on evoked transmitter release from phrenic nerve endings of the rat. Br J Pharmacol. 1991;103:1614–1620. doi: 10.1111/j.1476-5381.1991.tb09836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia-de-Sá P, Timóteo MA, Ribeiro JA. Influence of stimulation on Ca2+ recruitment triggering [3H]acetylcholine release from the rat motor-nerve endings. Eur J Pharmacol. 2000;406:355–362. doi: 10.1016/s0014-2999(00)00686-5. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Correia-de-Sá P, Sebastião AM, Ribeiro JA. Preferential activation of excitatory adenosine receptors at the rat hippocampal and neuromuscular synapses by adenosine formed from released adenine nucleotides. Br J Pharmacol. 1996;119:253–260. doi: 10.1111/j.1476-5381.1996.tb15979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Sebastião AM. Extracellular metabolism of adenine nucleotides and adenosine in the innervated skeletal muscle of the frog. Eur J Pharmacol. 1991;197:83–92. doi: 10.1016/0014-2999(91)90368-z. [DOI] [PubMed] [Google Scholar]
- Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, et al. Almost all about citrulline in mammals. Amino Acids. 2005;29:177–205. doi: 10.1007/s00726-005-0235-4. [DOI] [PubMed] [Google Scholar]
- Descarries LM, Cai S, Robitaille R, Josephson EM, Morest DK. Localization and characterization of nitric oxide synthase at the frog neuromuscular junction. J Neurocytol. 1998;27:829–840. doi: 10.1023/a:1006907531778. [DOI] [PubMed] [Google Scholar]
- Dodd PR, Williams SH, Gundlach AL, Harper PA, Healy PJ, Dennis JA, et al. Glutamate and gamma-aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves. J Neurochem. 1992;59:582–590. doi: 10.1111/j.1471-4159.1992.tb09409.x. [DOI] [PubMed] [Google Scholar]
- Fallahi N, Broad RM, Jin S, Fredholm BB. Release of adenosine from rat hippocampal slices by nitric oxide. J Neurochem. 1996;67:186–193. doi: 10.1046/j.1471-4159.1996.67010186.x. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell–cell signalling in the nervous system. Trends Neurosci. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Griffith DA, Jarvis SM. Nucleoside and nucleobase transport systems of mammalian cells. Biochim Biophys Acta. 1996;1286:153–181. doi: 10.1016/s0304-4157(96)00008-1. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- Gustafsson LE, Wiklund CU, Wiklund NP, Persson MG, Moncada S. Modulation of autonomic neuroefector transmission by nitiric oxide in guinea pig ileum. Biophys Res Comm. 1990;173:106–110. doi: 10.1016/s0006-291x(05)81028-9. [DOI] [PubMed] [Google Scholar]
- Hakim TS, Sugimori K, Camporesi EM, Anderson G. Half-life of nitric oxide in aqueous solutions with and without haemoglobin. Physiol Measurem. 1996;17:267–277. doi: 10.1088/0967-3334/17/4/004. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991;41:485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- Lloyd HG, Fredholm BB. Involvement of adenosine deaminase and adenosine kinase in regulating extracellular adenosine concentration in rat hippocampal slices. Neurochem Int. 1995;26:387–395. doi: 10.1016/0197-0186(94)00144-j. [DOI] [PubMed] [Google Scholar]
- Magalhães-Cardoso MT, Pereira MF, Oliveira L, Ribeiro JA, Cunha RA, Correia-de-Sá P. Ecto-AMP deaminase blunts the ATP-derived adenosine A2A receptor facilitation of acetylcholine release at rat motor nerve endings. J Physiol. 2003;549.2:399–408. doi: 10.1113/jphysiol.2003.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malomouzh AI, Nikolsky EE, Lieberman EM, Sherman JA, Lubischer JL, Grossfeld RM, et al. Effect of N-acetylaspartylglutamate (NAAG) on non-quantal and spontaneous quantal release of acetylcholine at the neuromuscular synapse of rat. J Neurochem. 2005;94:257–267. doi: 10.1111/j.1471-4159.2005.03194.x. [DOI] [PubMed] [Google Scholar]
- Molenaar PC, Oen BS, Polak RL, van der Laaken AL. Surplus acetylcholine and acetylcholine release in the rat diaphragm. J Physiol. 1987;385:147–167. doi: 10.1113/jphysiol.1987.sp016489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RMJ, Higgs A. Nitiric oxide: physiology, pathophysiology and pharmacology. Pharm Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Mori M, Gotoh T. Regulation of nitric oxide production by arginine metabolic enzymes. Biochem Biophys Res Commun. 2000;275:715–719. doi: 10.1006/bbrc.2000.3169. [DOI] [PubMed] [Google Scholar]
- Mukhtarov MR, Urazaev AK, Nikolsky EE, Vyskocil F. Effect of nitric oxide and NO synthase inhibition on nonquantal acetylcholine release in the rat diaphragm. Eur J Neurosci. 2000;12:980–986. doi: 10.1046/j.1460-9568.2000.00992.x. [DOI] [PubMed] [Google Scholar]
- Nikolsky EE, Voronin VA, Oranska TL, Vyskocil F. The dependence of non-quantal acetylcholine release on the choline-uptake system in the mouse diaphragm. Pflügers Archiv. 1991;418:74–78. doi: 10.1007/BF00370454. [DOI] [PubMed] [Google Scholar]
- Ribera J, Marsal J, Casanovas A, Hukkanen M, Tarabal O, Esquerda JE. Nitric oxide synthase in rat neuromuscular junctions and in nerve terminals of Torpedo electric organ: its role as regulator of acetylcholine release. J Neurosci Res. 1998;51:90–102. doi: 10.1002/(SICI)1097-4547(19980101)51:1<90::AID-JNR10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA, Ya L, Le M, Zhang Y. Nitric oxide-stimulated increase in extracelular adenosine accumulation in rat forebrain neurons in culture is associated with ATP hydrolysis and inhibition of adenosine kinase activity. J Neurosci. 2000;20:6294–6301. doi: 10.1523/JNEUROSCI.20-16-06294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe F, Langnaese K, Wolf G. New aspects of the location of neuronal nitric oxide synthase in the skeletal muscle: a light and electron microscopic study. Nitric Oxide. 2005;13:21–35. doi: 10.1016/j.niox.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Ruiz E, Tejerina T. Relaxant effects of L-citrulline in rabbit vascular smoth muscle. Br J Pharmacol. 1998;125:186–192. doi: 10.1038/sj.bjp.0702051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva HMV, Ambiel CR, Alves-do-Prado W. The neuromuscular transmission fade (Wedensky inhibition) induced by L-arginine in neuromuscular preparations from rats. Gen Pharmacol. 1999;32:705–712. doi: 10.1016/s0306-3623(98)00230-4. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- Thomas S, Robitaille R. Differential frequency dependent regulation of transmitter release by endogenous nitric oxide at the amphibian neuromuscular junction. J Neurosci. 2001;21:1087–1095. doi: 10.1523/JNEUROSCI.21-04-01087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Xie Z, Lu B. Nitric oxide mediates activity dependent synaptic suppression at developing neuromuscular synapses. Nature. 1995;374:262–266. doi: 10.1038/374262a0. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kilbinger H. Release of [3H]-acetylcholine from a modified rat phrenic nerve-hemidiaphragm preparation. Naunyn-Schmiedebergs Arch Pharmacol. 1986;334:357–364. doi: 10.1007/BF00569370. [DOI] [PubMed] [Google Scholar]
- Wiesinger H. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Prog Neurobiol. 2001;64:365–391. doi: 10.1016/s0301-0082(00)00056-3. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Prog Neurobiol. 2001;64:393–429. doi: 10.1016/s0301-0082(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Zefirov AL, Khaliullina RR, Anuchin AA, Yakovlev AV. The effects of exogenous nitric oxide on the function of neuromuscular synapses. Neurosci Behav Physiol. 2002;32:583–588. doi: 10.1023/a:1020449425703. [DOI] [PubMed] [Google Scholar]