Abstract
Background and Purpose:
Dipyrone is a potent analgesic drug that has been demonstrated to inhibit cyclooxygenase (COX). In contrast to classical COX-inhibitors, such as aspirin-like drugs, dipyrone has no anti-inflammatory effect and a low gastrointestinal toxicity, indicating a different mode of action. Here, we aimed to investigate the effects of dipyrone on COX.
Experimental approach:
The four major metabolites of dipyrone, including the two pharmacologically active metabolites, 4-methyl-amino-antipyrine (MAA) and amino-antipyrine (AA), were used to characterise their binding to COX and haem as well as their effects on the biochemical properties of COX. Mass spectrometry, UV and visible photometry were used to study binding and prostaglandin production. Levels of anti-oxidant enzymes were assessed by Western blotting.
Key results:
The pharmacologically active metabolites of dipyrone, MAA and AA, did not inhibit COX activity in vitro like classical COX inhibitors, but instead redirected the prostaglandin synthesis, ruling out inhibition of COX through binding to its active site. We found that MAA and AA formed stable complexes with haem and reacted with hydrogen peroxide in presence of haem, ferrous ions (Fe2+) or COX. Moreover, MAA reduced Fe3+ to Fe2+ and accordingly increased lipid peroxidation and the expression of anti-oxidant enzymes in cultured cells and in vivo.
Conclusions and implications:
Our data suggest that the pharmacologically active metabolites of dipyrone inhibit COX activity by sequestering radicals which initiate the catalytic activity of this enzyme or through the reduction of the oxidative states of the COX protein.
Keywords: dipyrone, prostaglandin E2, cyclooxygenase, prostaglandin H2 synthase, radical, haem
Introduction
Prostaglandin E2 (PGE2) is an important mediator of inflammation, pain and fever (Woolf and Costigan, 1999; Vanegas and Schaible, 2001). It is released at the site of an injury and, subsequently, in the dorsal horn of the spinal cord where it is involved in the development of central sensitization (Woolf and Costigan, 1999; Vanegas and Schaible, 2001). Prostaglandins are formed from arachidonic acid by the catalytic activity of the enzyme cyclooxygenase (COX; also referred to as prostaglandin H2 (PGH2) synthase; EC 1.14.99.1) that exists in two isoforms, COX-1 and -2, and several splice variants (Vanegas and Schaible 2001; Chandrasekharan et al., 2002). COX is a bifunctional enzyme that possesses two enzyme activities, a cyclooxygenase as well as a peroxidase activity. COX converts arachidonic acid into PGH2 by a two-step process both steps of which depend on haem as the prosthetic group: First, by reduction of a hydroperoxide to its corresponding hydroxy compound, the COX-peroxidase, oxidizes the haem from its resting state (ferric haem) to a ferryloxo-protoporphyrin radical cation analogous to compound I of classic peroxidases. By intramolecular electron transfer, this generates a tyrosyl radical in the COX-cyclooxygenase site that is required for oxygenation of arachidonic acid to PGG2. In the second COX-catalysed reaction, the peroxidase reaction, the hydroperoxy group of PGG2 is reduced by an electron transfer from the haem group, to the hydroxy compound PGH2 (Lambeir et al., 1985; Tsai et al., 1998; Marnett et al., 1999; Marnett, 2000).
Dipyrone is a potent analgesic and antipyretic drug that has been used clinically for more than 80 years. In aqueous solution, dipyrone is immediately hydrolysed to 4-methyl-amino-antipyrine (MAA; Figure 1a) which can then be further metabolized to 4-amino-antipyrine (AA), 4-formyl-amino-antipyrine (FAA) or 4-acetyl-amino-antipyrine (AAA). Of these four major dipyrone metabolites, MAA has been demonstrated to be the pharmacologically active compound, while AA has only a weak pharmacological effect and FAA or AAA are pharmacologically inactive (Vlahov et al., 1990; Levy et al., 1995). Over the last 20 years, several groups have demonstrated the inhibition of COX by dipyrone in vitro (Abbate et al., 1990; Campos et al., 1999; Chandrasekharan et al., 2002) and in vivo (Eldor et al., 1984; Geisslinger et al., 1996; Levy et al., 1998; Ayoub et al., 2004). Based on these findings, it has been proposed that the analgesic and antipyretic effects of dipyrone depend, at least in part, on COX inhibition and thus on decreased PGE2 synthesis (Carlsson et al., 1986; Gelgor et al., 1992; Tortorici and Vanegas 1995; Warner and Mitchell 2002; Wickelgren 2002). Hence, although the inhibitory effect of dipyrone on COX has long been known, the mechanism by which MAA inhibits COX activity remains unclear. Most COX inhibitors belong to the class of non-steroidal anti-inflammatory drugs (NSAIDs), which block the enzyme activity by competing with arachidonic acid for the cyclooxygenase active site (Marnett 2000; Smith et al., 2000). Our data suggest that MAA inhibits COX enzyme activity through an iron-dependant mechanism, resulting in the sequestration of radicals that are necessary to initiate the catalytic cycle of COX.
Figure 1.
MAA inhibits PGE2 and TXB2 synthesis. (a) Structures of the four dipyrone metabolites. (b) LPS-stimulated RAW 264.7 were treated with the indicated MAA concentrations for 16 h. Human platelets were incubated with varying amounts of MAA for 30 min before induction of the release of intracellular arachidonic acid with A23187 for 10 min. PGE2 and TXB2 synthesis was determined as described in the ‘Materials and methods' section. The mean±s.e.m. of at least three experiments are shown. The control values (100%) correspond to 6 ng ml−1 for PGE2 and 70 ng ml−1 for TXB2. (c) Conditions as in (b) except that all dipyrone metabolites were used at 100 μM. The mean±s.e.m. of at least three experiments, each in triplicate, are shown. Students t-test *P<0.01, **P<0.001 metabolite vs control.
Materials and methods
Animals
In all experiments, the ethics guidelines for investigations in conscious animals were obeyed and the experimental procedures were approved by the local Ethics Committee. Male Sprague–Dawley rats weighing 250–300 g were purchased from Charles River Wiga GmbH (Sulzfeld, Germany).
COX-1 assay using Thromboxane B2 measurements
Platelets were purified from blood from healthy volunteers who had not taken acetylsalicylic acid or any other drug in the previous 2 weeks. Blood was collected using S Monocuvette – 9 NC syringes (Sarstedt, Nümbrecht, Germany). After an incubation of 20 min at room temperature, they were centrifuged at 210 g for 20 min. The platelet-rich plasma was mixed with 50% Hanks Balanced Salt Solution supplemented with 25 mM HEPES (HHBSS) and 30% anticoagulant solution (65 mM citric acid, 85 mM sodium citrate, 2% glucose) and again centrifuged at 1000 g for 10 min. The pellet was washed once more with HHBSS and 10% anticoagulant and then resuspended in the same buffer. A total of 4 × 106 platelets were preincubated with the dipyrone metabolites for 30 min at 37 °C. Then calcium ionophore A23187 (Sigma, St Louis, MO, USA) was added to a final concentration of 2 μM in a total volume of 200 μl. The reaction was terminated after 10 min by addition of 100 μl methanol. After centrifugation, thromboxane B2 (TXB2), the stable metabolite of TXA2, was determined in the supernatant using the enzyme immunoassay from Assay Designs (Ann Arbor, MI, USA) according to the instructions of the manufacturer.
COX-2 assay using PGE2 measurements
Murine RAW 264.7 (LGC Promochem, Wesel, Germany) (1 × 106 cells) were plated on 35 mm dishes and incubated in RPMI medium with 10% fetal calf serum, 1% penicillin–streptomycin. After 24 h, the cells were treated for 16 h in serum-free medium with 1 μg ml−1 bacterial lipopolysaccharide (LPS) in presence or absence of the dipyrone metabolites at the indicated concentrations. The PGE2 concentrations in the medium were determined using the PGE2 ELISA kit from Assay Designs (Ann Arbor, MI, USA) according to the instructions of the manufacturer.
Prostaglandin measurement by LC-MS/MS
Cell media (500 μl) were mixed with 500 μl 35 mM H3PO4, 20 μl methanol and 20 μl internal standard solution. Prostaglandins were extracted with solid-phase extraction using absolut Nexus cartridges (1 ml, Varian, Darmstadt, Germany), which were washed with 2 ml of hexane:ethyl acetate:isopropanol (35:60:5), dried for 20 s and conditioned with 1 ml of methanol and 1 ml of water. The prepared cell media was loaded onto the column and washed with 1 ml of water and 1 ml of methanol/water (40:60). The cartridges were then dried for 7 min and eluted with 1 ml of hexane:ethyl acetate:isopropanol (30:65:5). The organic phase was removed at a temperature of 45°C under a gentle stream of nitrogen. The residues were reconstituted with 50 μl of acetonitrile:water:formic acid (20:80:0.0025, pH 4.0), and injected into the LC-MS/MS system.
Sample analysis was performed by using liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). The LC-MS/MS system consisted of an API 4000 triple-mass spectrometer (Applied Biosystems, Darmstadt, Germany) equipped with a Turbo-V-source operating in negative ESI mode, an Agilent 1100 binary HPLC pump and degasser (Agilent, Böblingen, Germany). An inlet valve was used to truncate non-relevant signals (10-port, VICI Valco Instruments, Houston, TX, USA). For the chromatographic separation, a Synergi Hydro-RP column and precolumn were used (150 × 2 mm ID, 4 μm particle size and 80 Å pore size from Phenomenex, Aschaffenburg, Germany). A linear gradient was employed at a flow rate of 300 μl min−1. Mobile phase A was water:formic acid (100:0.0025, pH 4.0) and mobile phase B was acetonitrile:formic acid (100:0.0025). Total run time was 16 min and injection volume of samples was 45 μl. The mass spectrometer was operated in the negative ion mode with an electrospray voltage of −3300 V at 450°C. Multiple reaction monitoring was used for quantification. All quadrupoles were working at unit resolution. Quantitation was performed with Analyst Software V1.4 (Applied Biosystems, Darmstadt, Germany) using the internal standard method (isotope-dilution mass spectrometry). Ratios of analyte peak area and internal standard peak area (y axis) were plotted against concentration (x axis) and calibration curves for each prostaglandin were calculated by least-squares regression with concentration−2 weighting−1.
Metabolic labelling
Cells were incubated with 1 μM radioactive arachidonic acid [1–14C] (Moravek Biochemicals, Brea, CA, USA) for 8 h. Then the cells were washed with phosphate-buffered saline (PBS) and stimulated as indicated for 15 h. The medium (1 ml) was extracted with one volume chloroform containing 1% formic acid. After drying the extracts, eicosanoids were resuspended in 50 μl chloroform and subjected to thin layer chromatography (TLC) as described previously (Brenneis et al., 2006).
Cell-free prostaglandin synthesis
Murine RAW macrophages were treated for 16 h in serum-free medium with 1 μg ml−1 LPS. Cells were collected in 100 mM Tris-HCl, pH 7.5, sonicated and the final cell protein concentration adjusted at 1 mg ml−1 with Tris buffer. In a total volume of 200 μl, 100 μg protein was incubated with the indicated drugs for 15 min. Alternatively, 20 units of reconstituted COX-1 were used. The reaction was started with the addition of arachidonic acid [1–14C] (Moravek Biochemicals, Brea, CA, USA) to a final concentration of 0.25 μM. After 10 min incubation at room temperature, the reaction was stopped by adding 1.4 ml chloroform:methanol (1:1) containing 1% formic acid. Cell debris was removed by centrifugation with 13 000 g and 0.6 ml of chloroform, and 0.32 ml of 0.88% formic acid were added to the supernatant. The organic phase was dried under N2, redissolved in 50 μl of chloroform and spotted on a Silica Gel 60 TLC plate. TLC plates were run for 1 h benzene:dioxane:formic acid:acetic acid (82:14:1:1) at 4°C. After drying, the TLC plates were analysed by a phosphoimager reader (Bio-imaging Analyzer System BAS-1500, Fujifilm, Düsseldorf, Germany).
MS–MS
The four dipyrone metabolites were incubated with or without haem under the same conditions as described for the spectrophotometric analysis. Detection was performed using a PE Sciex API 3000 triple quadrupole mass spectrometer (Applied Biosystems, Langen, Germany) equipped with a turbo ion spray interface. Nitrogen (high purity) was supplied by a Whatman nitrogen generator (Parker Hannifin GmbH, Kaarst, Germany).
UV-visible absorbance spectra
The dipyrone metabolites (100 μM) were incubated for 10 min at room temperature with 10 μM H2O2 and 10 μM haem. Where indicated, ferrous chloride (FeCl2) or ferric chloride (FeCl3) was used instead of haem. COX-1 was reconstituted with 10 μM haem prior to incubation with MAA. To allow photometric analysis of the dipyrone metabolites, after incubation of 100 μM MAA with 40 units COX-1 (65 nM), the metabolites were separated from COX-1 by extraction with chloroform/1% formic acid. After evaporation of the chloroform, the metabolites were resuspended in water and analysed. The reactions were carried out with identical results using either no reagents to stabilize the pH or 25 mM Tris (pH 7.4). The spectra were recorded using the Biowave S2100 diode array spectrophotometer (Walden Precision Apparatus Ltd, Cambridge, UK).
Fe2+ measurements
The concentrations of Fe2+ ions were measured photometrically using two different methods.
Bathophenanthroline
Bathophenanthroline is a highly selective photometric reagent for Fe2+ determination. Bathophenanthroline was dissolved in 128 mM NaCl to yield a final concentration of 100 μM. The indicated dipyrone metabolites and FeCl3 were added to a final concentration of 100 and 500 μM, respectively. Reduction of Fe3+ to Fe2+ induced a colorimetric change that was measured after 10 min at 540 nm in a multiwell reader spectrophotometer (SpectraFluor Plus, Tecan, Crailsheim, Germany).
Potassium ferricyanide
The dipyrone metabolites were added at the indicated concentrations to a solution of 1 mM FeCl3, 1 mM potassium ferricyanide. Ferricyanide ions react with the Fe2+ ions to form a dark blue compound (KFe(III)Fe(II)(CN)6). Thus, reduction of Fe3+ to Fe2+ induced a colorimetric change that was determined at 690 nm in the multiwell reader spectrophotometer.
Lipid peroxidation
RAW 264.7 (3 × 106 cells per 35 mm dish) were incubated with 100 μM MAA or Fe2+ for 24 h in presence or absence of 100 μM deferoxamine before harvesting. Lipid peroxidation was detected as described previously (Wong et al., 2001).
Expression studies in spinal cords
Male adult rats were given 1 g kg−1 dipyrone intraperitoneally. After 4 or 24 h, they were killed and the spinal cord was removed. Spinal cords were lysed in PBS by sonication. The insoluble fraction was removed by centrifugation at 18 000 g for 20 min. Aliquots of the supernatant were stored at −80°C until further use. Western blots for superoxide dismutase and peroxiredoxin V were performed using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. About 30 μg protein was loaded per lane and an anti-Hsp70 antibody was used to control for equal loading.
Data analysis
Results are shown as means±s.e.m. Statistical analysis was performed using Student's t-test and P-values of less than 0.05 were considered to be significant.
Materials
Dipyrone, MAA, AA, FAA and AAA were a generous gift from Aventis (Frankfurt, Germany). RPMI medium and fetal bovine serum were purchased from Invitrogen (Karlsruhe, Germany). Purified COX isoforms and prostaglandins used as substrate or standards for TLC and LC-MS/MS experiments were purchased from Cayman (Ann Arbor, MI, USA). All other chemicals were purchased from Sigma (St. Louis, MO, USA) unless otherwise specified. Arachidonic acid [1–14C] (53 mCi/mmol, 0.05 mCi ml−1, 287 μg ml−1) was purchased from Moravek Biochemicals (Brea, CA, USA).
Results
The dipyrone metabolites MAA and AA inhibit COX-1 and -2
We determined the effect of the four main metabolites of dipyrone (MAA, AA, FAA and AAA) on COX-2 activity by monitoring the release of PGE2 from LPS-stimulated RAW 264.7 cells. To determine the effects of the metabolites on COX-1 activity, TXB2 synthesis in human platelets was measured. MAA inhibited both COX isoforms, with COX-2 being more sensitive to inhibition by MAA (Figure 1b). Similarly, AA inhibited COX-2 effectively at 100 μM but not COX-1 (Figure 1c). In contrast, FAA and AAA did not decrease COX-1 or COX-2 activity (Figure 1c). As plasma concentrations in human subjects after a single dose of 1 g dipyrone are about 100 μM for MAA and 5–8 μM for AA, FAA and AAA (Damm 1989; Geisslinger et al., 1996), concentrations over 100 μM were not tested.
MAA and AA react with PGG2 in a cell-free but not in a cellular environment
To investigate whether MAA and AA inhibit COX activity by competing with arachidonic acid for the active site, we first incubated lysates from LPS-stimulated RAW 264.7 cells with [14C]arachidonic acid and determined the prostaglandin synthesis by TLC. Surprisingly, while aspirin abolished prostaglandin synthesis, MAA and AA did not block prostaglandin synthesis, but instead redirected the prostaglandin synthesis from PGE2 and PGD2 towards PGF2α and other unidentified prostaglandins (Figure 2a). Also incubation of purified COX-1 with [14C]arachidonic acid (Figure 2b) caused a redirection of prostaglandin synthesis towards PGF2α and other prostaglandins in presence of MAA.
Figure 2.
MAA does not inhibit COX activity by competitive binding to the active site. (a) LPS-stimulated RAW 264.7 were lysed and prostaglandin synthesis was measured in the presence of 100 μM MAA, AA, FAA, AAA or 1.25 mM aspirin, as described in the ‘Methods' section. Asterisks indicate the prostaglandins that were found only in MAA and AA treated samples. (b) Purified COX-1 (20 U) was incubated with [1–14C]-arachidonic acid (a.a.) in the presence or absence of 100 μM MAA and 1 μM haem and the products analysed by TLC. (c) 1.3 μM PGG2 was incubated with the indicated concentrations of MAA for 30 min and analysed by LC-MS/MS as described in the ‘Materials and methods' section. (d–h) 13 μM PGG2 alone (d) or incubated with 100 μM MAA (e), AA (f), AAA (g) or FAA (g) were incubated at room temperature for 30 min prior to analysis with LC-MS/MS. The arrows indicate the positions of the PGG2-specific signals. (i) RAW 264.7 cells were metabolically labelled as described in the ‘Methods' section. Cells were stimulated with 5 μg/ml−1 LPS 100 μM MAA and the prostaglandins in the medium (m) or in the cells (c) were analysed by TLC. (j) RAW 264.7 cells were incubated with LPS for 16 h in presence and absence of 100 μM MAA. PGF2α levels were analysed with LC-MS/MS as described under the ‘Methods' section. Students t-test *P<0.001 MAA vs LPS-treatment without MAA.
We hypothesized that MAA may react with PGG2 or other prostaglandins and determined, by mass spectroscopy, the effect of MAA on several prostaglandins. Our results showed that MAA concentration-dependently decreased PGG2 concentrations in vitro (Figure 2c). This reaction was specific for MAA and AA, since FAA and AAA did not alter PGG2 concentrations (Figure 2d–h). In contrast PGE2, PGD2, PGF2α or PGH2 concentrations were not altered by MAA (Supplementary data 1). Since PGG2 differs from the other tested prostaglandins by possessing a free hydroperoxide group and MAA is known to be oxidized by radical donors (Iogannsen et al., 1993), MAA may first react with this peroxide which then could lead to a non-specific oxidation of the endoperoxide and the generation of prostaglandins, such as PGF2α. Indeed nuclear magnetic resonance (NMR) spectrometry indicated that MAA oxidized PGG2 rapidly to PGF2α, 5-trans PGF2α and possibly further to 15-keto PGF2α and 13,14-dihydro-15-keto PGF2α (data not shown). However this redirection of the prostaglandin synthesis after MAA treatment to PGF2α and its metabolites was not observed in cultured LPS-stimulated RAW 264.7 cells (Figure 2i and j; Supplementary data 2) or spinal cord neurons (Supplementary data 3), indicating that the reaction between PGG2 and MAA does not occur in a cellular environment.
Taken together, the data show that competition of MAA with arachidonic acid for the catalytic site of COX, as known to occur with traditional NSAIDs, is highly unlikely, since no decrease of the prostaglandin synthesis was seen in the cell-free settings. Instead in the cell-free assays arachidonic acid is converted into either PGG2 or PGH2 by COX, which then seems to be oxidized by MAA to a number of prostaglandins, such as PGF2α. However in a cellular environment, the observed reaction between PGG2 and MAA does not take place and an alternative mechanism must be responsible for the inhibition of COX.
Dipyrone metabolites can form complexes with haem
Recently it was reported that at high concentrations, paracetamol inhibits COX activity by reducing the higher oxidative states of the protein that are part of the catalytic cycle of COX (Aronoff et al., 2003; Lucas et al., 2005). Since aminopyrine, a close structural homolog of dipyrone, is known to reduce peroxide in the presence of Fe2+ ions (Iogannsen et al., 1993), we hypothesized that MAA may interact with the haem group of COX, as described for paracetamol. To study whether a molecular interaction between MAA and haem can be observed, we used mass spectrometry to identify possible MAA–haem complexes. A full spectrum scan in the negative ion mode of haem alone yielded a signal with the expected m/z of 632.6 [M–H]- (Figure 3a). Haem was not detectable using the positive ion mode. After incubation of haem with MAA (100 μM), a new substance with an m/z of 833.6 [M+H]+ was detected in the positive ion mode (Figure 3b) that was not present in the spectrum derived from haem or MAA alone. In the negative ion mode, a matching signal at m/z 831.2 [M–H]− appeared which was considerably weaker (data not shown). The precursor ion spectrum of the 833.6- and the 831.5-substances matched the one of the haem alone and suggests the formation of a new product that contains haem (Figure 3a and b). The calculated mass of 832.6 equals the mass of a haem–MAA adduct, assuming that MAA substitutes the iron bound hydroxyl group with elimination of a molecule of water. Accordingly, fragmentation of the newly formed substance yielded a major fragment with m/z of 615.1 in the negative ion mode that corresponds to the mass of haem lacking a hydroxyl group (Figure 3c). However, the formation of this complex alone cannot be correlated with the inhibitory effect of MAA on the COX activities, since haem formed similar complexes with AA, FAA and AAA (Figure 3d, Supplementary data 4). Instead it seems more likely that the metabolite–haem complexes are reaction intermediates common to all four dipyrone metabolites.
Figure 3.
Dipyrone metabolites form stable complexes with haem. (a) Full scan mass spectrometric analysis (ESI-MS) in the negative ion mode of haem alone. The inset shows a magnification of the indicated signals. (b) Full scan mass spectrometric analysis (ESI-MS) in the positive ion mode of haem and MAA. The inset shows a magnification of the indicated signals. (c) Fragmentation of the suspected haem–MAA complex with the m/z of 831.2 in the negative ion mode. (d) Full scan mass spectrometric analysis in the positive ion mode of haem with FAA. Arrows indicate the position of the proposed haem–metabolite adduct.
MAA and AA react with peroxide in presence of Fe ions
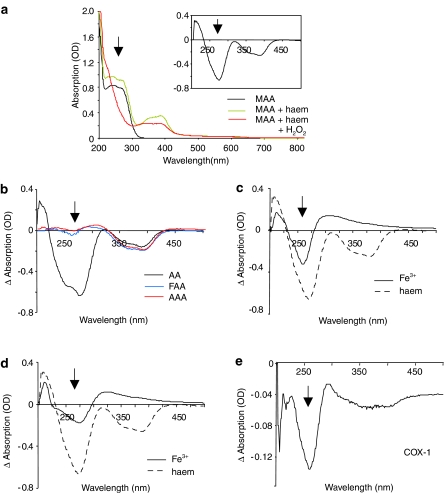
Next we monitored the absorption spectra of the dipyrone metabolites to study their interaction with haem and hydrogen peroxide, as the UV- and visible spectra of haem as well as dipyrone metabolites depend on their oxidation state (Damm 1989; Iogannsen et al., 1993; Uetrecht et al., 1995; Van Eps et al., 2003; Voegtle et al., 2003). Addition of 10 μM haem to the four metabolites slightly increased the absorption of all four metabolites (Figure 4a and b), while the haem spectrum itself was not altered by the metabolites (data not shown). Most interestingly, a dramatic decrease in the absorption spectrum for MAA and AA, but not FAA and AAA, was observed between 230 and 270 nm in presence of haem and hydrogen peroxide (Figure 4a and b). In contrast, addition of peroxide had no effect on the absorption spectra of the dipyrone metabolites (data not shown). A slight decrease in the absorption spectrum of haem occurred after hydrogen peroxide addition, independantly of the metabolites, and is believed to reflect a change in the redox state of the haem (Van Eps et al., 2003; Voegtle et al., 2003). To elucidate the relevance of the presence of the protoporphyric component of the haem, we repeated the experiments in presence of Fe3+ or Fe2+ and hydrogen peroxide. The MAA absorption spectrum was also altered by hydrogen peroxide in presence of Fe3+ or Fe2+, although the observed absorption changes after addition of hydrogen peroxide were significantly smaller as compared to the absorption changes seen in presence of haem (Figure 4c and d). In presence of 10 μM Fe3+, the decrease in the absorption was only 35% of the decrease seen with an equimolar amount of haem (Figure 4c). Fe2+ was even less effective and decreased the absorption only by 25%, compared to haem (Figure 4d). Most importantly, the same changes in the absorption spectrum of MAA occurred in presence of COX-1 and peroxide (Figure 4e). As we used significantly less COX (65 nM) compared to haem (10 μM) and an extraction step was necessary to remove the protein before the absorption measurements, a quantitative comparison of the absorption changes is not possible. Taken together, the data suggest that MAA and AA are able to react with electron donors, such as hydrogen peroxide in the presence of haem, Fe2+ or Fe3+ ions. In this way, MAA and AA can either prevent the start of the catalytic cycle of COX or, alternatively, reduce the already activated states of COX.
Figure 4.
MAA and AA react with hydrogen peroxide in presence of an iron source. (a) UV-visible absorption spectra of MAA in absence or presence of haem or haem and peroxide. The inset shows absorption changes of the metabolite–haem complexes after addition of hydrogen peroxide. The arrows indicate MAA specific changes. (b) As in (a) except that AA, AAA or FAA were used instead of MAA. (c–e) Changes in the absorption spectrum of MAA after addition of 100 μM hydrogen peroxide in presence of 10 μM, Fe3+ (c), Fe2+ (d) or 65 nM reconstituted COX-1. (e) For better comparison, the absorption changes in presence of haem (dotted line) were included in (c and d).
MAA and AA reduce Fe3+ to Fe2+ ions
Next, we studied the influence of the metabolites on the redox state of Fe3+, present in the resting state of haem in COX. We determined the Fe2+ formation in presence of the four dipyrone metabolites using two different detection methods, potassium ferricyanide and bathophenanthroline. Interestingly, only the pharmacologically active metabolites MAA and AA were able to reduce Fe3+ to Fe2+, while no reducing effect was seen with FAA and AAA (Figure 5a and b). Since MAA and AA interact with peroxide only in the presence of Fe ions and this interaction depends on the redox state of the iron, so far the data suggest that the oxidation of MAA and AA by Fe3+ is required for their reaction with peroxide. Next, we wanted to determine if the reaction between MAA and Fe3+ occurs also in a cellular environment. Since the free Fe pool in cells consists mainly of Fe3+ (Lipinski et al., 2000; Imlay 2003; Huang et al., 2004), an interaction of MAA with the free Fe would be expected to increase at least temporarily the Fe2+ concentration in cells and may result in the increased generation of reactive oxygen species (ROS) (Wang et al., 2002; Imlay 2003; Huang et al., 2004). Indeed an increased lipid oxidation was observed in MAA-treated RAW macrophages, which was prevented by the Fe2+-scavenger deferoxamine (Figure 5c). Furthermore, adult rats that received a single dose of dipyrone showed, in the spinal cord, an increased expression of superoxide dismutase and peroxiredoxin V, two enzymes that are necessary to neutralize superoxide and other ROS species. The expression levels of both enzymes were increased in spinal cords of dipyrone-treated animals 4 and 24 h after treatment (Figure 5d).
Figure 5.
MAA and AA reduce Fe3+ to Fe2+. (a and b) Determination of Fe2+ formation after addition of dipyrone metabolites or ascorbate to FeCl3 by potassium ferricyanide (a) or bathophenanthroline (b) as described in the ‘Materials and methods' section. The mean±s.e.m. of at least three experiments each carried out in duplicate are shown. Student's t-test **P<0.001 MAA or AA vs control. (c) Lipid oxidation in RAW 264.7 cells after incubation with 100 μM Fe2+ or MAA in presence or absence of 100 μM deferoxamine (DFO) as determined by levels of malondialdehyde (MDA). The mean±s.e.m. of at least four experiments is shown. Students t-test *P<0.05 MAA or Fe2+ vs control. (d) Western blot analysis of the expression of superoxide dismutase and peroxiredoxin V in spinal cords of adult rats. The spinal cords were excised 4 or 24 h after i.p. application of 1 g kg−1 dipyrone. Hsp 70 expression was used to control for even loading.
Discussion
Traditional NSAIDs inhibit COX enzyme activity by competing with arachidonic acid for binding to the cyclooxygenase active site (Smith and Dewitt 1996; Marnett et al., 1999). One exception is aspirin which inhibits PGG2 synthesis by acetylation of serine-530 in the arachidonic acid cavity (Marnett 2000; Smith et al., 2000). Our data clearly show that MAA does not compete with arachidonic acid for the catalytic site of COX. Instead it seems that MAA can oxidize radical donors thus blocking the initiation of the catalytic reaction and/or the further progress of the catalytic cycle by reduction of the higher oxidative states of the enzyme protein, in a mechanism similar to the one proposed for paracetamol (Aronoff et al., 2003; Lucas et al., 2005). This model would be in line with recent reports describing that dipyrone and its metabolites are effective scavengers for several ROS species (Costa et al., 2006a, 2006b).
A possible inhibitory mechanism of MAA could start with the binding of MAA to the Fe3+ in the haem. The mass determination of the MAA–haem complex by mass spectrometry suggests the formation of a bond between the 4-amino group of the dipyrone metabolites and the Fe with release of a water molecule. However, the complex formation by itself does not cause inhibition of COX activity, since all four metabolites form similar complexes with haem. In the next step, the Fe3+ is reduced and an MAA radical is formed. This assumption is in accordance with a previous report which shows that AA forms radicals after direct oxidation by Fe3+ (Iogannsen et al., 1993). Since these radicals are stabilized by alkyl groups (Iogannsen et al., 1993), the stabilization of a radical intermediate by the 4-methyl group of MAA would explain why MAA is the only metabolite that is active at pharmacologically relevant concentrations. The other three tested metabolites form either less stable radical intermediates, as in the case of AA, or even prevent formation of the radicals due to the destabilizing effect of a 4-acetyl (AAA) or a 4-formyl (FAA) group. The reaction of MAA and AA with Fe seems necessary for the reaction with hydrogen peroxide, since no effect of peroxide on the absorption spectra of MAA and AA was observed in the absence of haem or Fe ions Thus, MAA could block COX activity by sequestering the peroxide and thereby suppressing the generation of the tyrosyl radical in the COX-cyclooxygenase site that is required for oxygenation of arachidonic acid to PGG2. Notably, the structurally similar drug aminopyrine has also been demonstrated to be oxidized by COX to an aminopyrine cation radical in the presence of arachidonic acid and hydrogen peroxide (Lasker et al., 1981; Eling et al., 1985).
This model for MAA-mediated COX inhibition suggests a reversible inhibition of COX as removal of MAA would allow newly formed radicals to initiate the catalytic activity of COX. Indeed, dipyrone inhibition of COX activity has been found to be reversible (Eldor et al., 1983) and the COX inhibitory actions of dipyrone correlate strongly with MAA concentrations in the plasma (Eldor et al., 1984; Geisslinger et al., 1996; Levy et al., 1998). Furthermore, the finding that dipyrone treatment leads to an increased lipid peroxidation in RAW 264.7 cells and an increased expression of antioxidative enzymes such as superoxide dismutase in the spinal cord supports the notion that MAA reduces Fe3+ in vivo. Thus, besides the actual MAA concentrations in the target cell, it seems likely that also the concentrations of ROS as well as the general redox environment in a specific cell will determine the inhibitory potential of MAA towards COX, as described previously for paracetamol (Boutaud et al., 2002). Such a cell- or tissue-specific influence on the ability of MAA to inhibit COX activity may explain the absence of the anti-inflammatory properties of dipyrone.
In summary, our results would suggest that the pharmacologically active dipyrone metabolite MAA targets the initiation of the catalytic reaction of both COX isoforms by reducing the higher oxidation states of COX or sequestering activating peroxides. Since the use of specific COX-2 inhibitors has recently been compromised by the discovery of severe cardiovascular side effects (Tegeder and Geisslinger, 2006), our finding of an alternative mechanism for the inhibition of COX activity might lead to the development of a new and safer class of COX inhibitors.
External data objects
Acknowledgments
We thank K Birod and J Häusler for excellent technical assistance and L. Brautigam for MS/MS analysis. This work was supported by an unrestricted grant from Sanofi-Aventis, Frankfurt, Germany.
Abbreviations
- AA
amino-antipyrine
- AAA
4-acetyl-amino-antipyrine
- COX
cyclooxygenase
- FAA
4-formyl-amino-antipyrine
- MAA
4-methyl-amino-antipyrine
- NSAID
non-steroidal anti-inflammatory drug
- PGE2
prostaglandin E2
- TXB2
Thromboxan B2
- TLC
thin layer chromatography
Conflict of interest
One of the authors (MM) is an employee of Sanofi-Aventis who also funded the research.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Abbate R, Gori AM, Pinto S, Attanasio M, Paniccia R, Coppo M, et al. Cyclooxygenase and lipoxygenase metabolite synthesis by polymorphonuclear neutrophils: in vitro effect of dipyrone. Prostaglandins Leukot Essent Fatty Acids. 1990;41:89–93. doi: 10.1016/0952-3278(90)90059-t. [DOI] [PubMed] [Google Scholar]
- Aronoff DM, Boutaud O, Marnett LJ, Oates JA. Inhibition of prostaglandin H2 synthases by salicylate is dependent on the oxidative state of the enzymes. J Pharmacol Exp Ther. 2003;304:589–595. doi: 10.1124/jpet.102.042853. [DOI] [PubMed] [Google Scholar]
- Ayoub SS, Botting RM, Goorha S, Colville-Nash PR, Willoughby DA, Ballou LR. Acetaminophen-induced hypothermia in mice is mediated by a prostaglandin endoperoxide synthase 1 gene-derived protein. Proc Natl Acad Sci USA. 2004;101:11165–11169. doi: 10.1073/pnas.0404185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutaud O, Aronoff DM, Richardson JH, Marnett LJ, Oates JA. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H(2) synthases. Proc Natl Acad Sci USA. 2002;99:7130–7135. doi: 10.1073/pnas.102588199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneis C, Maier TJ, Schmidt R, Hofacker A, Zulauf L, Jakobsson PJ, et al. Inhibition of prostaglandin E2 synthesis by SC-560 is independent of cyclooxygenase 1 inhibition. FASEB J. 2006;20:1352–1360. doi: 10.1096/fj.05-5346com. [DOI] [PubMed] [Google Scholar]
- Campos C, de Gregorio R, Garcia-Nieto R, Gago F, Ortiz P, Alemany S. Regulation of cyclooxygenase activity by metamizol. Eur J Pharmacol. 1999;378:339–347. doi: 10.1016/s0014-2999(99)00477-x. [DOI] [PubMed] [Google Scholar]
- Carlsson KH, Helmreich J, Jurna I. Activation of inhibition from the periaqueductal grey matter mediates central analgesic effect of metamizol (dipyrone) Pain. 1986;27:373–390. doi: 10.1016/0304-3959(86)90161-2. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa D, Marques AP, Reis RL, Lima JL, Fernandes E. Inhibition of human neutrophil oxidative burst by pyrazolone derivatives. Free Radic Biol Med. 2006a;40:632–640. doi: 10.1016/j.freeradbiomed.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Costa D, Vieira A, Fernandes E. Dipyrone and aminopyrine are effective scavengers of reactive nitrogen species. Redox Rep. 2006b;11:136–142. doi: 10.1179/135100006X116637. [DOI] [PubMed] [Google Scholar]
- Damm D. Simultaneous determination of the main metabolites of dipyrone by high-pressure liquid chromatography. Arzneimittelforschung. 1989;39:1415–1417. [PubMed] [Google Scholar]
- Eldor A, Polliack G, Vlodavsky I, Levy M. Effects of dipyrone on prostaglandin production by human platelets and cultured bovine aortic endothelial cells. Thromb Haemost. 1983;49:132–137. [PubMed] [Google Scholar]
- Eldor A, Zylber-Katz E, Levy M. The effect of oral administration of dipyrone on the capacity of blood platelets to synthesize thromboxane A2 in man. Eur J Clin Pharmacol. 1984;26:171–176. doi: 10.1007/BF00630282. [DOI] [PubMed] [Google Scholar]
- Eling TE, Mason RP, Sivarajah K. The formation of aminopyrine cation radical by the peroxidase activity of prostaglandin H synthase and subsequent reactions of the radical. J Biol Chem. 1985;260:1601–1607. [PubMed] [Google Scholar]
- Geisslinger G, Peskar BA, Pallapies D, Sittl R, Levy M, Brune K. The effects on platelet aggregation and prostanoid biosynthesis of two parenteral analgesics: ketorolac tromethamine and dipyrone. Thromb Haemost. 1996;76:592–597. [PubMed] [Google Scholar]
- Gelgor L, Cartmell S, Mitchell D. Intracerebroventricular micro-injections of non-steroidal anti-inflammatory drugs abolish reperfusion hyperalgesia in the rat's tail. Pain. 1992;50:323–329. doi: 10.1016/0304-3959(92)90038-D. [DOI] [PubMed] [Google Scholar]
- Huang X, Moir RD, Tanzi RE, Bush AI, Rogers JT. Redox-active metals, oxidative stress, and Alzheimer's disease pathology. Ann N Y Acad Sci. 2004;1012:153–163. doi: 10.1196/annals.1306.012. [DOI] [PubMed] [Google Scholar]
- Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- Iogannsen MG, Petrov AS, Kachurin AM. Aminopyrazolone free radicals in the hydrogen peroxide oxidation reaction. Vopr Med Khim. 1993;39:9–13. [PubMed] [Google Scholar]
- Lambeir AM, Markey CM, Dunford HB, Marnett LJ. Spectral properties of the higher oxidation states of prostaglandin H synthase. J Biol Chem. 1985;260:14894–14896. [PubMed] [Google Scholar]
- Lasker JM, Sivarajah K, Mason RP, Kalyanaraman B, Abou-Donia MB, Eling TE. A free radical mechanism of prostaglandin synthase-dependent aminopyrine demethylation. J Biol Chem. 1981;256:7764–7767. [PubMed] [Google Scholar]
- Levy M, Brune K, Zylber-Katz E, Cohen O, Caraco Y, Geisslinger G. Cerebrospinal fluid prostaglandins after systemic dipyrone intake. Clin Pharmacol Ther. 1998;64:117–122. doi: 10.1016/S0009-9236(98)90029-7. [DOI] [PubMed] [Google Scholar]
- Levy M, Zylber-Katz E, Rosenkranz B. Clinical pharmacokinetics of dipyrone and its metabolites. Clin Pharmacokinet. 1995;28:216–234. doi: 10.2165/00003088-199528030-00004. [DOI] [PubMed] [Google Scholar]
- Lipinski P, Drapier J.C, Oliveira L, Retmanska H, Sochanowicz B, Kruszewski M. Intracellular iron status as a hallmark of mammalian cell susceptibility to oxidative stress: a study of L5178Y mouse lymphoma cell lines differentially sensitive to H(2)O(2) Blood. 2000;95:2960–2966. [PubMed] [Google Scholar]
- Lucas R, Warner TD, Vojnovic I, Mitchell JA. Cellular mechanisms of acetaminophen: role of cyclo-oxygenase. FASEB J. 2005;19:635–637. doi: 10.1096/fj.04-2437fje. [DOI] [PubMed] [Google Scholar]
- Marnett LJ. Cyclooxygenase mechanisms. Curr Opin Chem Biol. 2000;4:545–552. doi: 10.1016/s1367-5931(00)00130-7. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J Biol Chem. 1999;274:22903–22906. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]
- Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Geisslinger G. Cardiovascular risk with cyclooxygenase inhibitors: general problem with substance specific differences. Naunyn Schmiedebergs Arch Pharmacol. 2006;12:1269–1277. doi: 10.1007/s00210-006-0044-7. [DOI] [PubMed] [Google Scholar]
- Tortorici V, Vanegas H. Anti-nociception induced by systemic or PAG-microinjected lysine-acetylsalicylate in rats. Effects on tail-flick related activity of medullary off- and on-cells. Eur J Neurosci. 1995;7:1857–1865. doi: 10.1111/j.1460-9568.1995.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Tsai A, Palmer G, Xiao G, Swinney DC, Kulmacz RJ. Structural characterization of arachidonyl radicals formed by prostaglandin H synthase-2 and prostaglandin H synthase-1 reconstituted with mangano protoporphyrin IX. J Biol Chem. 1998;273:3888–3894. doi: 10.1074/jbc.273.7.3888. [DOI] [PubMed] [Google Scholar]
- Uetrecht JP, Ma HM, MacKnight E, McClelland R. Oxidation of aminopyrine by hypochlorite to a reactive dication: possible implications for aminopyrine-induced agranulocytosis. Chem Res Toxicol. 1995;8:226–233. doi: 10.1021/tx00044a007. [DOI] [PubMed] [Google Scholar]
- Van Eps N, Szundi I, Einarsdottir O. pH dependence of the reduction of dioxygen to water by cytochrome c oxidase. 1. The P(R) state is a pH-dependent mixture of three intermediates, A, P, and F. Biochemistry. 2003;42:5065–5073. doi: 10.1021/bi020482m. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Schaible HG. Prostaglandins and cyclooxygenases in the spinal cord. Prog Neurobiol. 2001;64:327–363. doi: 10.1016/s0301-0082(00)00063-0. [DOI] [PubMed] [Google Scholar]
- Vlahov V, Badian M, Verho M, Bacracheva N. Pharmacokinetics of metamizol metabolites in healthy subjects after a single oral dose of metamizol sodium. Eur J Clin Pharmacol. 1990;38:61–65. doi: 10.1007/BF00314805. [DOI] [PubMed] [Google Scholar]
- Voegtle HL, Sono M, Adak S, Pond AE, Tomita T, Perera R, et al. Spectroscopic characterization of five- and six-coordinate ferrous-NO heme complexes. Evidence for heme Fe-proximal cysteinate bond cleavage in the ferrous-NO adducts of the Trp-409Tyr/Phe proximal environment mutants of neuronal nitric oxide synthase. Biochemistry. 2003;42:2475–2484. doi: 10.1021/bi0271502. [DOI] [PubMed] [Google Scholar]
- Wang MX, Wei A, Yuan J, Trickett A, Knoops B, Murrell GA. Expression and regulation of peroxiredoxin 5 in human osteoarthritis. FEBS Lett. 2002;531:359–362. doi: 10.1016/s0014-5793(02)03511-1. [DOI] [PubMed] [Google Scholar]
- Warner TD, Mitchell JA. Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum. Proc Natl Acad Sci USA. 2002;99:13371–13373. doi: 10.1073/pnas.222543099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren I. Pain research. Enzyme might relieve research headache. Science. 2002;297:1976. doi: 10.1126/science.297.5589.1976a. [DOI] [PubMed] [Google Scholar]
- Wong BS, Brown DR, Pan T, Whiteman M, Liu T, Bu X, et al. Oxidative impairment in scrapie-infected mice is associated with brain metals perturbations and altered antioxidant activities. J Neurochem. 2001;79:689–698. doi: 10.1046/j.1471-4159.2001.00625.x. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci USA. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.