Abstract
Background and purpose:
NO/prostanoid independent, EDHF-mediated hyperpolarization and dilation in rat middle cerebral arteries is mediated solely by endothelial cell IKCa. However, when the NO-pathway is also active, both SKCa and IKCa contribute to EDHF responses. As the SKCa component can be inhibited by stimulation of thromboxane A2 (TxA2) TP receptors and NO has the potential ability to inhibit thromboxane synthesis, we investigated whether TxA2 might explain loss of functional input from SKCa during NOS inhibition in cerebral arteries.
Experimental approach:
Rat middle cerebral arteries were mounted in a wire myograph. Endothelium-dependent responses to the PAR2 agonist, SLIGRL were assessed as simultaneous changes in smooth muscle membrane potential and tension.
Key results:
Responses were obtained in the presence of L-NAME as appropriate. Inhibition of TP receptors with either ICI 192,605 or SQ 29,548, did not affect EDHF mediated hyperpolarization and relaxation, but in their presence neither TRAM-34 nor apamin (to block IKCa and SKCa respectively) individually affected the EDHF response. However, in combination they virtually abolished it. Similar effects were obtained in the presence of the thromboxane synthase inhibitor, furegrelate, which additionally revealed an iberiotoxin-sensitive residual EDHF hyperpolarization and relaxation in the combined presence of TRAM-34 and apamin.
Conclusions and implications:
In the rat middle cerebral artery, inhibition of NOS leads to a loss of the SKCa component of EDHF responses. Either antagonism of TP receptors or block of thromboxane synthase restores an input through SKCa. These data indicate that NO normally enables SKCa activity in rat middle cerebral arteries.
Keywords: thromboxane A2; small conductance calcium-activated potassium channel; intermediate conductance calcium-activated potassium channel; large conductance calcium-activated potassium channel; endothelium-derived hyperpolarizing factor, nitric oxide, cerebral artery
Introduction
In rat middle cerebral arteries treated with a nitric oxide synthase (NOS) inhibitor (L-NAME (NG-nitro-L-arginine methyl ester)), blockade of the intermediate conductance calcium-activated potassium channels (IKCa) in endothelial cells alone is sufficient to block smooth muscle hyperpolarization and relaxation due to endothelium-derived hyperpolarizing factor (EDHF; Marrelli et al., 2003; McNeish et al., 2005). This is in contrast to peripheral arteries, where EDHF-mediated responses are only abolished by the combined inhibition of both small conductance calcium-activated potassium channels (SKCa) and IKCa (Busse et al., 2002). A number of studies with peripheral arteries have provided functional, electrophysiological and immunohistochemical data showing that SKCa and IKCa channels are expressed only within the endothelium (Walker et al., 2001; Burnham et al., 2002; Bychkov et al., 2002). We recently investigated if the apparent solitary role of IKCa in the EDHF response of rat middle cerebral arteries reflected an absence of SKCa channels. Surprisingly in the light of the functional data, but in common with peripheral arteries, both IKCa and SKCa channels were demonstrated in the endothelium (McNeish et al., 2006).
The relative contribution from SKCa and IKCa channels to the EDHF response depends on the state of the arterial smooth muscle. In rat mesenteric arteries, during smooth muscle contraction evoked by phenylephrine, block of endothelium-dependent hyperpolarization and relaxation requires inhibition of both endothelial SKCa and IKCa, but in unstimulated vessels (where there is no contraction to reverse) inhibition of SKCa alone is sufficient to block EDHF-mediated hyperpolarization (Crane et al., 2003). In contrast to mesenteric arteries, arterial stimulation may not regulate KCa function in middle cerebral arteries, as increases in stretch-induced tension did not alter SKCa input (McNeish et al., 2006). However, basal release of NO suppresses myogenic tone, and an ability to elaborate NO is associated with maintained SKCa input to EDHF-evoked hyperpolarization in this artery. It is only after inhibition of NO synthase that the contribution of SKCa is lost, with EDHF responses becoming entirely reliant on IKCa (McNeish et al., 2005, 2006).
Although NO may suppress EDHF activity under some circumstances, these two separate dilator pathways do operate simultaneously in many arteries, including the rat middle cerebral artery (Zygmunt et al., 1998; Feletou and Vanhoutte, 2006; McNeish et al., 2006), but it is not clear how NO may alter SKCa activity. In non-vascular smooth muscle from the rat, there is evidence that NO can directly stimulate SKCa channel function (Geeson et al., 2002). Basal release of NO may also protect SKCa channel function indirectly. For example, NO may suppress an inhibitory mediator for the SKCa channel (and thus the EDHF response) such as the potent vasoconstrictor 20-HETE (20-hydroxy-(5Z,8Z,11Z,14Z)-eicosatetraenoic acid). 20-HETE does inhibit the EDHF response in small porcine coronary arteries (Randriamboavonjy et al., 2005) and NO can inhibit the synthesis of 20-HETE, by binding to the haem group of cytochrome P450 (CYP450) (Minamiyama et al., 1997). Another mediator that could potentially affect the EDHF response and in particular the SKCa channel is thromboxane A2 (TxA2). In rat mesenteric resistance arteries, stimulation of TxA2 receptors (TP receptors) with U46619 (9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α) attenuates SKCa function (Crane and Garland, 2004). NO has also been shown to inhibit the synthesis of TxA2, again by an interaction with a haem active site, this time in thromboxane synthase (Wade and Fitzpatrick, 1997). Indeed, increased TxA2 signalling has been reported to contribute to vasoconstriction and the development of vasomotion induced in middle cerebral arteries by NO synthase inhibitors (Benyo et al., 1998; Lacza et al., 2001). Finally, NO may also affect KCa function via indirect cGMP-mediated effects. So, for example, NO/cyclic 3′,5′-guanosine monophosphate (cGMP) causes desensitization of TP receptors via a protein kinase G (PKG)-dependent mechanism (Reid and Kinsella, 2003).
Therefore, we hypothesized that constriction following inhibition of NOS and the associated loss of the SKCa component of agonist-induced hyperpolarization may be underpinned by an increase in the synthesis and/or function of TxA2 or 20-HETE in the rat middle cerebral artery. The aim of the present study was therefore to assess the contribution of KCa channel subtypes to hyperpolarization and relaxation in rat middle cerebral artery smooth muscle cells by stimulating the endothelium with the protease activated receptor 2 (PAR2) receptor agonist, SLIGRL; SLIGRL was the only agent used to stimulate endothelium-dependent responses as other mediators such as acetylcholine (ACh), adenosine diphosphate (ADP) and bradykinin elicit a much less reliable EDHF response in middle cerebral arteries (unpublished data). Subsequently, we investigated whether inhibiting TP receptors, thromboxane synthesis or synthesis of 20-HETE can restore the SKCa component of the EDHF response that is lost after inhibition of NOS. The ability of these treatments to reverse the L-NAME induced depolarization and constriction was also evaluated.
Methods
Male Wistar rats (200–300 g) were killed by cervical dislocation and the brains removed and immediately placed in ice-cold Krebs solution. Segments of the middle cerebral artery (∼2 mm long) were dissected and stored in ice-cold Krebs solution for use within 30 min, with similar size vessels used in all experimental groups
Experimental protocols
Segments of middle cerebral artery (internal diameter ∼150 μm) were mounted in a Mulvany-Halpern myograph (model 400A, Danish Myotechnology) in Krebs solution containing (mM): NaCl, 118.0, NaCO3, 24; KCl, 3.6; MgSO4·7H2O, 1.2; glucose, 11.0; CaCl2, 2.5; gassed with 95% O2 and 5% CO2 and maintained at 37°C. After equilibration for 20 min, vessels were tensioned to 1–1.5 mN (approximates wall tension at 60 mm Hg). Smooth muscle tension was recorded with an isometric pressure transducer and Powerlab software (ADI, Australia). Vessel viability was assessed by adding exogenous K+ (15–55 mM, total K+ concentration); only vessels developing tension of ⩾3 mN were used. Endothelial cell viability was assessed by the ability of SLIGRL (20 μM) to relax myogenic tone and to hyperpolarize the smooth muscle cell membrane by >15 mV.
All EDHF responses to SLIGRL (20 μM) were obtained in the presence of L-NAME (100 μM) to block NOS, unless otherwise stated. EDHF-mediated responses were assessed in the presence of the KCa channel blockers, apamin (SKCa, 50 nM), 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34) (IKCa, 1 μM) and iberiotoxin (large conductance calcium-activated potassium channel (BKCa), 100 nM). The effect of KCa blockers on EDHF-mediated responses was also assessed after addition of the TP receptor antagonists SQ 29,548 (10 μM) and ICI 192,605 (100 μM); the TxA2 synthase inhibitor, furegrelate (10 μM); the cyclo-oxygenase inhibitor, indomethacin (10 μM) and the phospholipase A2 inhibitor, AACOCF3 (10 μM). In some experiments, endothelium-dependent hyperpolarization was assessed in vessels without L-NAME and still able to synthesize NO. In these experiments, the effect of the KCa channel blockers was assessed on endothelium-dependent hyperpolarization induced by SLIGRL (20 μM) in the presence of the TP receptor agonist U46619 (5 nM). Papaverine (150 μM) was added at the end of each experiment to assess overall tone. All drugs were allowed to equilibrate for at least 20 min before vasodilator responses were stimulated. In most experiments, smooth muscle membrane potential (Em) and tension were measured simultaneously as previously described, using glass microelectrodes (filled with 2 M KCl; tip resistance, 80–120 MΩ) to measure Em (Garland and McPherson, 1992).
Data analysis and statistical procedures
Results are expressed as the mean±s.e.m. of n animals. Tension values are given in mN (always per 2 mm segment) and Em as mV. Vasodilatation is expressed as percentage reduction of the total vascular tone (myogenic tone plus vasoconstrictor response), quantified by relaxation with papaverine (150 μM). Graphs were drawn and comparisons made using one-way analysis of variance with Tukeys' post-test (Prism, Graphpad). P⩽0.05 was considered significant.
Drugs, chemicals, reagents and other materials
Exogenous K+ was added as an isotonic physiological salt solution in which all the NaCl was replaced with an equivalent amount of KCl. Concentrations of K+ used are expressed as final bath concentration. AACOCF3 (1,1,1-Trifluoromethyl-6,9,12,15-heieicosatetraen-2-one), L-NAME, papaverine HCl and U46619 were all obtained from Sigma (Poole, UK); apamin and iberotoxin, from Latoxan (Valence, France); ICI 192,605 (4(Z)-6-(2-o-chlorophenyl-4-o-hydroxyphenyl-1,3-dioxan-cis-5-yl)hexenoic acid) from Tocris (Nottingham, UK); SLIGRL (serine, leucine, isoleucine, glycine, arginine, leucine) from Auspep (Parkville, Australia); furegrelate and SQ 29,548 ([1S-[1α,2α(Z),3α,4α]]-7-[3-[[2-[(phenylamino)carnonyl]hydrazine]methyl]-7-oxabicyclo[2.2.1]hept-2-yl]-5-heptenoic acid) from Cayman-Europe (Tallinn, Estonia). TRAM-34 was a generous gift from Dr H Wulff (University of California, Davis, USA). All stock solutions (100 mM) were prepared in dimethyl sulphoxide (DMSO) except L-NAME, apamin, iberiotoxin, papaverine and SLIGRL, which were dissolved in 0.9% NaCl, and indomethacin, which was dissolved in Na2CO3 (1 M); vehicle controls were performed when necessary.
Results
We have previously reported myogenic tone equivalent to about 14% of the maximal vasoconstriction induced in rat middle cerebral arteries by raising [K+]o to 55 mM, and that the NOS inhibitor, L-NAME (100 μM) further contracts the artery to Circa 70% of this maximum associated with smooth muscle depolarization of 12.8±0.7 mV (McNeish et al., 2005). In the current study, in the presence of L-NAME-induced vasoconstriction, SLIGRL induced EDHF-mediated hyperpolarization and relaxation to 19.6±3.1 mV and 80.9±6.7% (n=4, respectively). In agreement with our previous studies, this hyperpolarization and relaxation was abolished by the selective IKCa channel inhibitor TRAM-34 (to 1.2±0.8 mV and 6.0±1.8%, respectively, n=4).
Effect of inhibiting TP receptors or CYP 450 on L-NAME constriction and EDHF-mediated hyperpolarization and relaxation
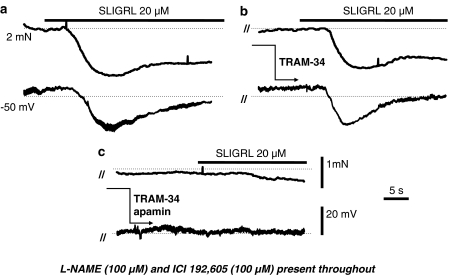
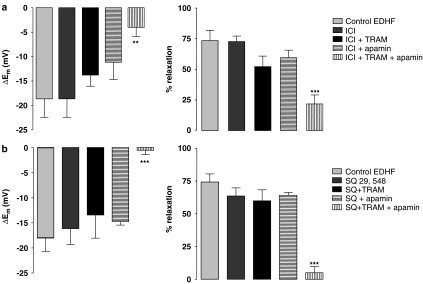
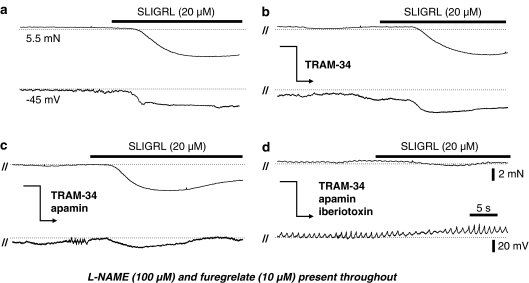
The TP receptor antagonist ICI 192,605 (100 μM) reversed depolarization and contraction to L-NAME (by 12.9±2.9 mV, n=3 and 67.9±6.3%, n=7, respectively), but did not alter the EDHF-mediated hyperpolarization (n=7) and relaxation (n=9) to SLIGRL (20 μM; Figure 2a). In the presence of ICI 192,605, EDHF responses were now resistant to blockade of IKCa with 1 μM TRAM-34 (Figures 1b and 2a; n=4) and remained insensitive to blockade of SKCa with apamin (50 nM, Figure 2a; n=5). However, in combination, these blockers markedly attenuated the EDHF response (Figures 1c and 2a; n=10).
Figure 1.
Original recordings of EDHF-mediated relaxation (upper trace) and hyperpolarization (lower trace) from a rat middle cerebral artery preconstricted with the NOS inhibitor L-NAME (100 μM) and in the presence of the of the TP inhibitor ICI 192,605 (100 μM; a) also shown is the subsequent effects either of the IKCa channel inhibitor TRAM-34 (1 μM) alone (b) or the combined blockade of IKCa and SKCa channels with TRAM-34 and apamin (50 nM; c) on the EDHF response. Dotted lines represent the control tension and resting membrane potential, respectively. In the presence of ICI 192,605, EDHF responses have a functional input from SKCa, as they were only blocked by the combination of TRAM-34 and apamin. Parallel lines (//) indicate a time break between separate recordings from a single vessel.
Figure 2.
Histograms showing the effect of the structurally distinct TP antagonists ICI 193,605 (100 μM; a and SQ 29,548 (10 μM; b) on SLIGRL (20 μM)-induced, EDHF-mediated hyperpolarization (left panels) and relaxation (right panels) in rat middle cerebral artery preconstricted with the NOS inhibitor L-NAME (100 μM). Also shown is the effect of the IKCa blocker, TRAM-34 (1 μM) and the SKCa blocker apamin (50 nM), both alone and in combination. When TPs were inhibited, the EDHF response was only blocked by combined incubation of both TRAM-34 and apamin, indicating that there is a functional input from SKCa in this response. **P<0.01 and ***P<0.001 indicate a statistically significant difference from control.
The structurally distinct TP receptor antagonist SQ 29,546 (10 μM) did not modify L-NAME-induced tone, but did have similar effects to ICI 192,605 against the EDHF response. In the presence of SQ 29,546 (Figure 2b), EDHF-mediated hyperpolarization (n=10) and relaxation (n=10) was not significantly altered (n=7). Neither apamin (50 nM; n=3) nor TRAM-34 (n=4) had a significant effect on the EDHF hyperpolarization and relaxation (Figure 2), but in combination abolished the response (Figure 2b; n=6).
The non-selective CYP450 inhibitor, 17-octadecynoic acid (17-ODYA) (10 μM), did not alter L-NAME-induced constriction (total tone 4.2±0.4 and 4.0±0.4 mN in the absence and presence of 17-ODYA, respectively, n=6), or EDHF-mediated hyperpolarization and relaxation (19.8±4.3 mV and 74.9±5.8%, versus 19.0±2.3 mV and 74.4±5.5%, respectively, n=4). Furthermore, in the presence of 17-ODYA, TRAM-34 alone still effectively abolished EDHF responses (residual, 2.2±2.4 mV and 13.1±5.1%, n=4).
Effect of TP receptor stimulation on endothelium-dependent hyperpolarization in the absence of L-NAME
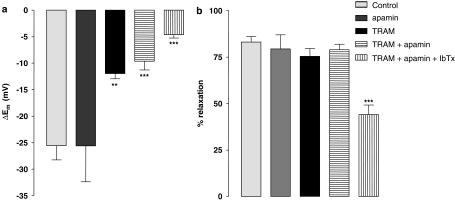
With NO (basal) synthesis extant, endothelium-dependent hyperpolarization to SLIGRL (20 μM) in cerebral arteries reflects activation of both SKCa and IKCa channels (McNeish et al., 2006). In the present study, under similar conditions, the TP receptor agonist U46619 (5 nM) depolarized and contracted the cerebral arteries (by 7.2±2.8 mV and 3.5±0.3 mN; n=5 and n=13, respectively). During constriction with U46619, SLIGRL-induced hyperpolarization (Figure 3a; n=13) was resistant to apamin (n=4) but partially inhibited by TRAM-34 (P<0.01). The inhibitory action of TRAM-34 was not increased by the additional presence of apamin. The remaining, residual hyperpolarization was, however, attenuated by the inhibitor of BKCa, iberiotoxin (Figure 3, P<0.001; n=5). In Figure 3b, apamin and TRAM-34 alone or in combination did not affect relaxation to SLIGRL, reflecting the direct smooth muscle vasodilator action of NO in these vessels. However, a combination of apamin, TRAM-34 and iberiotoxin did significantly inhibit SLIGRL-induced relaxation (Figure 3b). This probably reflects block of NO action, as hyperpolarization (McNeish et al., 2006) and relaxation (unpublished observation) to the NO donor DEA-NONOate is inhibited by iberiotoxin in this artery.
Figure 3.
Histograms showing the effect of the TxA2 mimetic U46619 (5 nM) on hyperpolarization (a) and relaxation (b) produced by SLIGRL (20 μM) in rat middle cerebral arteries that had not been treated with an NOS inhibitor. Also shown are the effects of the IKCa inhibitor TRAM-34 (1 μM), the SKCa channel inhibitor, apamin (50 nM) and the BKCa inhibitor, iberiotoxin (IbTx; 100 nM) on these responses. TRAM-34 alone inhibited the SLIGRL-induced hyperpolarization, whereas apamin had no effect. Combination of TRAM-34 and apamin had no additional effect when compared to TRAM-34 alone. The combination of apamin, TRAM-34 and iberiotoxin did further attenuate hyperpolarization and relaxation. Relaxations were unaffected by apamin and TRAM-34 alone or in combination with apamin as these vessels are able to synthesize NO. The NO-dependent relaxation was affected by iberiotoxin. **P<0.01 and ***P<0.001 indicate a statistically significant difference from control.
Effect of inhibiting TxA2 synthase, cyclo-oxygenase or phospholipaseA2 on L-NAME-induced tone and EDHF responses
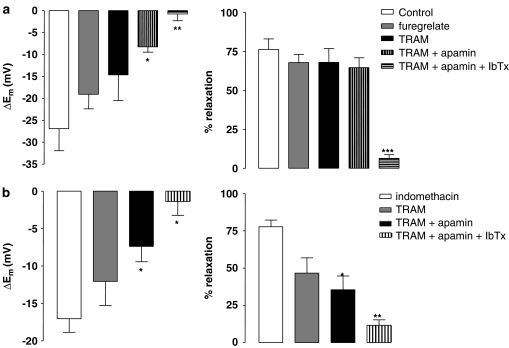
The thromboxane synthase inhibitor, furegrelate (10 μM), did not affect L-NAME-induced constriction (5.0±0.4 and 5.0±0.4 mN before and after furegrelate treatment, respectively, n=6). EDHF-mediated hyperpolarization (n=5) and relaxation (n=5) evoked by SLIGRL (20 μM) was also not significantly modified by furegrelate (Figure 5a; n=6) and TRAM-34 did not have any additional effect (Figures 4b and 5a; n=4). However, in combination, apamin and TRAM-34 did attenuate EDHF-mediated hyperpolarization (Figures 4c and 5a; P<0.05; n=5), but without significantly altering relaxation (n=5). In the additional presence of the BKCa channel inhibitor iberiotoxin (100 nM), EDHF responses were blocked (Figures 4d and 5a, P<0.001; n=4).
Figure 5.
Histograms showing the EDHF-mediated hyperpolarization (left panels) and relaxation (right panels) in the presence of the NOS inhibitor L-NAME (100 μM) and either: (a) the thromboxane synthase inhibitor, furegrelate (10 μM), or (b) the cyclo-oxygenase inhibitor indomethacin (10 μM). Also shown are the effects of the IKCa inhibitor, TRAM-34 (1 μM), the SKCa inhibitor apamin (50 nM) and the BKCa inhibitor iberiotoxin (IbTx; 100 nM). Only combined application of TRAM-34, apamin and iberiotoxin fully blocked the EDHF response in the presence of either furegrelate or indomethacin. *P<0.05, **P<0.01 and ***P<0.001 indicate a statistically significant difference from control.
Figure 4.
Original traces showing SLIGRL (20 μM)-induced, EDHF-mediated relaxation (upper panels) and hyperpolarization (lower panels) in a rat middle cerebral artery treated with the NOS inhibitor L-NAME (100 μM) and the thromboxane synthase inhibitor, furegrelate (10 μM; a). Also shown is the additional effect of: (b) the IKCa inhibitor, TRAM-34 (1 μM); (c) the combination of TRAM-34 and the SKCa inhibitor, apamin (50 nM) and (d) the combination of TRAM-34, apamin and the BKCa inhibitor, iberiotoxin (100 nM). Dotted lines represent the control tension and membrane potential. Only the combination of apamin, TRAM-34 and iberiotoxin fully blocked the EDHF response, indicating that functional inputs from SKCa, IKCa and BKCa contribute to the EDHF response under these conditions. Parallel lines (//) indicate a time break between separate recordings from a single vessel.
Control EDHF responses obtained in the presence of L-NAME alone (hyperpolarization of 26.0±6.0 mV and relaxation of 84.6±4.7%, n=5) were unaffected by the addition of iberiotoxin (hyperpolarization of 21.2±6.3 mV and relaxation of 75.8±4.2%, n=5, respectively).
Similar results were obtained after inhibition of cyclo-oxygenase with 10 μM indomethacin (Figure 5b). Indomethacin did not affect L-NAME-induced vasoconstriction or the EDHF response (tone of 5.0±0.6 and 4.7±0.4 mN, n=6 and 11, before and after indomethacin treatment, respectively). TRAM-34 alone did not significantly depress EDHF-mediated hyperpolarization or relaxation (n=10), but, in combination with apamin, TRAM-34 did significantly attenuate these responses (P<0.05; n=6). The residual response was blocked by the addition of iberiotoxin (Figure 5b; P<0.05; n=3).
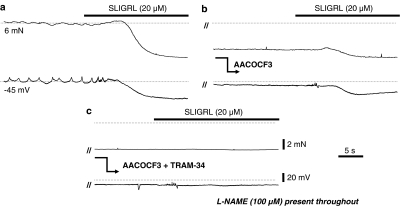
The PLA2 inhibitor AACOCF3 (10 μM; Figure 6) reversed L-NAME-induced tone by 85.6±3.8% and inhibited EDHF hyperpolarization and relaxation (27.7±6.0 mV and 72.0±7.4%, n=7, versus 9.4±3.5 mV and 32.7±15.0%, n=6, respectively; Figure 6b). The residual EDHF response was blocked by TRAM-34 alone (5.6±2.8 mV and 4.5±6.5%, respectively, n=4, P<0.05; Figure 6c).
Figure 6.
Original traces showing SLIGRL (20 μM)-induced EDHF-mediated relaxation (upper traces) and hyperpolarization (lower traces) in a rat middle cerebral artery treated with the NOS inhibitor L-NAME (100 μM; a). Also shown is the effect of the PLA2 inhibitor AACOCF3 (10 μM) on L-NAME-induced tone and the EDHF response (b) and the subsequent effect of IKCa blocker, TRAM-34 (1 μM) on the residual EDHF response (c). Dotted lines represent the control membrane potential and tension before addition of AACOCF3. AACOCF3 relaxed the L-NAME-induced tone as well as attenuating the EDHF response, the residual EDHF response was completely blocked by TRAM-34 alone. Parallel lines (//) indicate a time break between separate recordings from a single vessel.
Discussion
These data demonstrate that activation of TxA2 receptors in the rat middle cerebral artery can explain the absence of SKCa input to endothelium-dependent hyperpolarization when NO synthesis is inhibited. Furthermore, inhibition of NOS may result in an increase in TxA2 synthesis, as inhibiting TxA2 synthesis restores SKCa input as well as uncovering a previously unrecognized role for BKCa in the EDHF response. These results help to explain our previous observation that NO protects a functional input from SKCa in the rat middle cerebral artery (McNeish et al., 2006) and provide a link to our demonstration that the stimulation of TP receptors inhibits SKCa function (Plane and Garland, 1996; Crane and Garland, 2004).
EDHF responses in the rat middle cerebral artery are unusual, in being dependent only on activation of IKCa. In most vessels that exhibit an EDHF response, inhibition of both SKCa and IKCa channels is necessary to block the EDHF response (Busse et al., 2002). Despite this difference, the rat middle cerebral artery does exhibit morphological features similar to other vessels, that is, both IKCa and SKCa channels are expressed within the endothelium (McNeish et al., 2006) and the endothelium is coupled to the smooth muscle layer by myo-endothelial gap junctions (McNeish et al., 2006; Sokoya et al., 2006). Furthermore, SKCa channels can contribute to endothelium-dependent hyperpolarization in the middle cerebral artery, but only when the vessels are still able to synthesize NO (McNeish et al., 2006). The NO-dependent contribution of SKCa to endothelium-dependent hyperpolarization did not appear to involve a concomitant inhibition of IKCa, because in the presence of apamin, a normal, maximum hyperpolarization and relaxation was still evoked (McNeish et al., 2006). The mechanism responsible for the apparent ability of NO to protect SKCa function is, however, unclear and may involve both a direct effect of NO and downstream signalling mediators such as cGMP-linked effects. For example, NO may directly interact with SKCa channels, as it does in smooth muscle of the rat fundus (Geeson et al., 2002). Alternatively or additionally, as NO readily interacts with other signalling pathways, particularly those involving haem-containing enzymes and the metabolism of arachidonic acid, a protective role may reflect an interaction of the NO/cGMP pathway with factors elaborated within the artery wall.
One possibility is an alteration in the synthesis of 20-HETE, a potent vasoconstrictor derived from arachidonic acid by CYP450-dependent enzymes. 20-HETE is involved in myogenic constriction/autoregulation in cerebral vessels (Harder et al., 1994; Gebremedhin et al., 2000) and is also known to inhibit EDHF-mediated responses by reducing KCa function in small coronary arteries (Randriamboavonjy et al., 2005). Furthermore, synthesis of 20-HETE can be inhibited by NO binding to the haem active site of its synthetic enzyme (Minamiyama et al., 1997). However, despite the fact that 20-HETE has a role in myogenic tone, we failed to demonstrate any input to cerebral constriction after inhibition of NOS. The non-selective CYP450 inhibitor 17-octadecynoic acid (17-ODYA) did not alter the L-NAME-induced constriction in the middle cerebral artery. Furthermore, 17-ODYA also failed to reveal any functional role for the SKCa channel in the EDHF response, as TRAM-34 alone was still able to abolish this response. This suggests that NO does not normally protect SKCa channel function by inhibiting the synthesis/function of 20-HETE or a related metabolite generated by CYP450-dependent enzymes.
Another autacoid that could affect KCa channel function is the potent vasoconstrictor and platelet activator TxA2. As well as being involved in NOS-mediated vasoconstriction in rat middle cerebral arteries (Benyo et al., 1998; Lacza et al., 2001; Gonzales et al., 2005), we have previously shown that stimulation of TPs results in a fairly rapid loss of the SKCa component of EDHF hyperpolarization and associated relaxation in peripheral arteries of the rat (Crane and Garland, 2004). NO does inhibit the formation of TxA2, by binding to the haem active site of TxA2 synthase (Wade and Fitzpatrick, 1997). It may also inhibit cyclo-oxygenase (Kanner et al., 1992), responsible for synthesizing the precursor of TxA2 (and other prostaglandins), prostaglandin H2 (PGH2). In addition to inhibiting synthesis, NO is also known to desensitize the TP receptor through a PKG-dependent mechanism (Reid and Kinsella, 2003). In the present study, L-NAME-induced constriction was significantly reduced by the TP receptor antagonist, ICI 192,605, indicating that receptor activation might account for at least some of the constriction following inhibition of NOS. In contrast, a structurally unrelated TP antagonist SQ 29,548 failed to alter L-NAME-induced constriction, suggesting that ICI 192,605 may have been acting non-selectively. Indeed, the concentration of ICI 192,605 used in this study (100 μM) is known to have effects on prostaglandin E2 (PGE2) (EP) receptors, which may provide an explanation (Brewster et al., 1988). However, in vessels pretreated with L-NAME, both of the TP receptor antagonists uncovered a functional role for the SKCa channel in the EDHF response. Simultaneous block of both SKCa and IKCa channels with apamin and TRAM-34 was necessary to abolish the response as the functional ability of either channel appeared sufficient to elicit adequate hyperpolarization for full EDHF-mediated relaxation. Therefore, stimulation of TP receptors could explain the loss of the SKCa-dependent component of endothelium-dependent hyperpolarization after inhibition of NOS. This suggestion is supported by the observation that activation of TP with U46619 abolishes the SKCa component of endothelium-dependent hyperpolarization, in arteries still able to synthesize NO. NO-dependent inhibition of TPs appears to depend on activation of PKG (Reid and Kinsella, 2003), which may explain our previous observation that ODQ (1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one), an inhibitor of soluble guanylate cyclase, revealed endothelium-dependent hyperpolarization mainly dependent upon IKCa (McNeish et al., 2006). The possible involvement of cGMP-mediated effects in the regulation of SKCa channel function is a subject of ongoing investigation.
As stimulation of TPs appeared to account for the loss of SKCa function in arteries treated with an NOS inhibitor, we investigated if receptor stimulation reflected an increased synthesis of TxA2 (or its precursor PGH2, which can also stimulate these receptors) as opposed to an inhibitory action of NO/cGMP-dependent signalling on TPs. The former appeared to be the case, as inhibition of TxA2 synthase with furegrelate or inhibition of cyclo-oxygenase (to inhibit synthesis of PGH2 and thus TxA2) with indomethacin each revealed a role for SKCa channels in the EDHF response. Neither treatment had any effect on the L-NAME-induced constriction, again indicating that stimulation of TP receptors does not form a major component of this response. Interestingly, pretreatment with either indomethacin or furegrelate also revealed a role for BKCa in the EDHF response. The explanation for this unexpected observation is the subject of ongoing investigation. One possibility is that altering the prostanoid profile in the vessel wall uncovers an eicosanoid pathway able to directly activate BKCa on the smooth muscle cells in this artery (McNeish et al., 2006).
The observation that stimulation of either TP receptors or 20-HETE did not appear to contribute to the contraction following inhibition of NOS was surprising, as both signalling pathways have previously been implicated in this response (Harder et al., 1994; Benyo et al., 1998; Lacza et al., 2001). However, our data do indicate that constriction involves a metabolite of arachidonic acid, as the phopholipase A2 (PLA2) inhibitor AACOCF3 fully reversed L-NAME-induced constriction. Interestingly, the EDHF response was also attenuated by inhibition of PLA2, which is consistent with previous observations in the rat middle cerebral artery (You et al., 2002). However, a significant EDHF response remained after treatment with AACOCF3 and was abolished by TRAM-34. Overall, these observations suggest there are at least two components of the EDHF response in the rat middle cerebral artery. One component appears to involve a metabolite of arachidonic acid. A recent study by You et al. (2005) appears to rule out the involvement of metabolites of either the lipoxygenase (epoxyeicosatrienoic acids, EETs) or epoxgenase pathways (hydroxyeicosatetraenoic acids, HETEs) in rat middle cerebral arteries (You et al., 2005), so the identity of such an active metabolite remains uncertain. Other components of the EDHF response appear to rely solely upon the activation of endothelial KCa channels, and may lead to smooth muscle hyperpolarization/relaxation through an increase in extracellular [K+] (McNeish et al., 2005) and/or a direct transfer of hyperpolarization via myoendothelial gap junctions (McNeish et al., 2006; Sokoya et al., 2006).
In summary, inhibition of NOS leads to pronounced constriction in cerebral arteries that appears to involve an unidentified metabolite of arachidonic acid. This metabolite does not appear to be either of the potent endogenous vasoconstrictors 20-HETE or TxA2. However, block of the SKCa-mediated component of endothelium-dependent (EDHF) hyperpolarization, which follows inhibition of NOS, can be reversed by inhibiting TPs or by reducing the synthesis of TxA2. Therefore, increased thromboxane signalling after inhibition of NOS may underlie blockade of a fundamental part of the EDHF response in these arteries. The fact that loss of NO signalling can disrupt the EDHF pathway and associated vasodilatation, through increased activity of the potent vasoconstrictor/platelet activator TxA2, is likely to be of fundamental relevance in disease states where NO release is known to be compromised.
Acknowledgments
This work was supported by the British Heart Foundation.
Abbreviations
- 20-HETE
20-hydroxy-(5Z,8Z,11Z,14Z)-eicosatetraenoic acid
- 17-ODYA
17-octadecynoic acid
- BKCa
large conductance calcium-activated potassium channel
- CYP450
cytochrome P450
- EDHF
endothelium-derived hyperpolarizing factor
- EETs
epoxyeicosatrienoic acids
- HETEs
hydroxyeicosatetraenoic acids
- IKCa
intermediate conductance calcium-activated potassium channel
- NOS
nitric oxide synthase
- ODQ
1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one
- PAR2
protease activated receptor 2
- PKG
protein kinase G
- SKCa
small conductance calcium-activated potassium channel
- TRAM-34
1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole
- TxA2
thromboxane A2
- TP
thromboxane A2 receptor
Conflict of interest
The authors state no conflict of interest.
References
- Benyo Z, Gorlach C, Wahl M. Involvement of thromboxane A2 in the mediation of the contractile effect induced by inhibition of nitric oxide synthesis in isolated rat middle cerebral arteries. J Cereb Blood Flow Metab. 1998;18:616–618. doi: 10.1097/00004647-199806000-00003. [DOI] [PubMed] [Google Scholar]
- Brewster AG, Brown GR, Foubister AJ, Jessup R, Smithers MJ. The synthesis of a novel thromboxane receptor antagonist 4(Z)-6-(2-o-chlorophenyl-4-o-hydroxyphenyl-1,3-dioxan-cis-5-yl) hexenoic acid ICI 192605. Prostaglandins. 1988;36:173–178. doi: 10.1016/0090-6980(88)90304-8. [DOI] [PubMed] [Google Scholar]
- Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, et al. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Feletou M, et al. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol. 2002;137:1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol (Lond) 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane GJ, Garland CJ. Thromboxane receptor stimulation associated with loss of SKCa activity and reduced EDHF responses in the rat isolated mesenteric artery. Br J Pharmacol. 2004;142:43–50. doi: 10.1038/sj.bjp.0705756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now. Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Garland CJ, McPherson GA. Evidence that nitric oxide does not mediate the hyperpolarisation and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, et al. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- Geeson J, Larsson K, Hourani SM, Toms NJ. Sodium nitroprusside-induced rat fundus relaxation is ryanodine-sensitive and involves L-type Ca2+ channel and small conductance Ca(2+)-sensitive K+ channel components. Auton Autacoid Pharmacol. 2002;22:297–301. doi: 10.1046/j.1474-8673.2002.00271.x. [DOI] [PubMed] [Google Scholar]
- Gonzales RJ, Ghaffari AA, Duckles SP, Krause DN. Testosterone treatment increases thromboxane function in rat cerebral arteries. Am J Physiol. 2005;289:H578–H585. doi: 10.1152/ajpheart.00958.2004. [DOI] [PubMed] [Google Scholar]
- Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB, et al. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266:H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- Kanner J, Harel S, Granit R. Nitric oxide, an inhibitor of lipid oxidation by lipoxygenase, cyclooxygenase and hemoglobin. Lipids. 1992;27:46–49. doi: 10.1007/BF02537058. [DOI] [PubMed] [Google Scholar]
- Lacza Z, Herman P, Gorlach C, Hortobagyi T, Sandor P, Wahl M, et al. NO synthase blockade induces chaotic cerebral vasomotion via activation of thromboxane receptors. Stroke. 2001;32:2609–2614. doi: 10.1161/hs1101.098526. [DOI] [PubMed] [Google Scholar]
- Marrelli SP, Eckmann MS, Hunte MS. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am J Physiol. 2003;285:H1590–H1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- McNeish AJ, Dora KA, Garland CJ. Possible role for K+ in endothelium-derived hyperpolarizing factor-linked dilatation in rat middle cerebral artery. Stroke. 2005;36:1526–1532. doi: 10.1161/01.STR.0000169929.66497.73. [DOI] [PubMed] [Google Scholar]
- McNeish AJ, Sandow SL, Neylon CB, Chen MX, Dora KA, Garland CJ. Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke. 2006;37:1277–1282. doi: 10.1161/01.STR.0000217307.71231.43. [DOI] [PubMed] [Google Scholar]
- Minamiyama Y, Takemura S, Imaoka S, Funae Y, Tanimoto Y, Inoue M. Irreversible inhibition of cytochrome P450 by nitric oxide. J Pharmacol Exp Ther. 1997;283:1479–1485. [PubMed] [Google Scholar]
- Plane F, Garland CJ. Influence of contractile agonists on the mechanism of endothelium-dependent relaxation in rat isolated mesenteric artery. Br J Pharmacol. 1996;119:191–193. doi: 10.1111/j.1476-5381.1996.tb15970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamboavonjy V, Kiss L, Falck JR, Busse R, Fleming I. The synthesis of 20-HETE in small porcine coronary arteries antagonizes EDHF-mediated relaxation. Cardiovas Res. 2005;65:487–494. doi: 10.1016/j.cardiores.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Reid HM, Kinsella BT. The {alpha}, but not the {beta}, isoform of the human thromboxane A2 receptor is a target for nitric oxide-mediated desensitization: independent modulation of TP{alpha} signaling by nitric oxide and prostacyclin. J Biol Chem. 2003;278:51190–51202. doi: 10.1074/jbc.M309314200. [DOI] [PubMed] [Google Scholar]
- Sokoya EM, Burns AR, Setiawan CT, Coleman HA, Parkington HC, Tare M. Evidence for the involvement of myoendothelial gap junctions in EDHF-mediated relaxation in the rat middle cerebral artery. Am J Physiol. 2006;291:H385–H393. doi: 10.1152/ajpheart.01047.2005. [DOI] [PubMed] [Google Scholar]
- Wade ML, Fitzpatrick FA. Nitric oxide modulates the activity of the hemoproteins prostaglandin I2 synthase and thromboxane A2 synthase. Arch Biochem Biophys. 1997;347:174–180. doi: 10.1006/abbi.1997.0348. [DOI] [PubMed] [Google Scholar]
- Walker SD, Dora KA, Ings NT, Crane GJ, Garland CJ. Activation of endothelial cell IKCa with 1-ethyl-2-benzimidazolinone evokes smooth muscle hyperpolarization in rat isolated mesenteric artery. Br J Pharmacol. 2001;134:1548–1554. doi: 10.1038/sj.bjp.0704415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Golding EM, Bryan RM., Jr Arachidonic acid metabolites, hydrogen peroxide, and EDHF in cerebral arteries. Am J Physiol. 2005;289:H1077–H1083. doi: 10.1152/ajpheart.01046.2004. [DOI] [PubMed] [Google Scholar]
- You J, Marrelli SP, Bryan RM., Jr Role of cytoplasmic phospholipase A2 in endothelium-derived hyperpolarizing factor dilations of rat middle cerebral arteries. J Cereb Blood Flow Metab. 2002;22:1239–1247. doi: 10.1097/01.WCB.0000037996.34930.2E. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Plane F, Paulsson M, Garland CJ, Hogestatt ED. Interactions between endothelium-derived relaxing factors in the rat hepatic artery: focus on regulation of EDHF. Br J Pharmacol. 1998;124:992–1000. doi: 10.1038/sj.bjp.0701893. [DOI] [PMC free article] [PubMed] [Google Scholar]