Abstract
Background and purpose:
Activation of the pregnane X receptor (PXR) has been shown to protect against cholestatic hepatotoxicity. As PXR alters the expression of numerous hepatic bile acid transporters, we sought to delineate their potential role in hepatoprotection.
Experimental approach:
Wild-type (PXR+/+) and PXR-null (PXR-/-) mice were fed a 1% cholic acid (CA) diet with or without the PXR activator, PCN. Liver function was assessed along with the corresponding changes in hepatic gene expression.
Key results:
CA administration caused significant hepatotoxicity in PXR+/+ mice and was associated with induction of several FXR and PXR regulated genes, which encode for bile acid transport and metabolizing proteins. Compared to CA alone, co-administration of PCN to CA-fed PXR+/+ mice significantly decreased hepatotoxicity and was associated with induction of MRP3 mRNA as well as CYP3A11 mRNA and functional activity. Unexpectedly, PXR-/- mice, which expressed significantly higher basal and CA-induced levels of MRP2, MRP3, OSTα, OSTβ, OATP2 and CYP3A11, were dramatically less sensitive to CA hepatotoxicity than PXR+/+ mice.
Conclusions:
Protection of PXR+/+ mice against CA-induced hepatotoxicity by PCN is associated with the induction of MRP3 and CYP3A11 expression. Resistance against CA-induced hepatotoxicity in PXR-/- mice may result from higher basal and induced expression of bile acid transporters, particularly MRP3. These findings emphasize the importance of transport by MRP3 and metabolism as major protective pathways against cholestatic liver injury.
Keywords: PXR, transporters, cytochrome P450, cholestasis, bile acids, hepatotoxicity
Introduction
Bile acids (BAs) are endogenous cholesterol-derived compounds that play an important role in the digestion and absorption of lipids and hydrophobic compounds from the intestine. Despite their physiological importance, the surfactant properties of BAs render them toxic to membrane components of cells and elevated levels of BAs can result in damage to tissues such as the liver and bile duct. Under cholestatic conditions, BA levels are markedly increased in the liver and this is thought to contribute to further hepatocellular injury (Bremmelgaard and Alme, 1980; Fischer et al., 1996). Elevated intrahepatic BA levels cause changes in hepatic gene expression that lead to increased BA efflux as well as decreased biosynthesis and uptake (Fickert et al., 2001; Zollner et al., 2003; Ando et al., 2005). However, these intrinsic adaptive changes are not sufficient in protecting the liver against the high intrahepatic BA concentrations seen during cholestasis. Thus strategies to minimize toxic BA concentrations are of interest for the development of therapies to treat cholestatic liver diseases.
One potential approach that has recently emerged involves the use of activators of nuclear hormone receptors such as the pregnane X receptor (PXR), farnesoid X receptor (FXR) and constitutive androstane receptor (CAR). These ligand-activated transcription factors coordinately regulate genes involved in BA biosynthesis, metabolism and transport. In particular, PXR regulates the expression of cytochrome P450 (CYP)3A (Bertilsson et al., 1998) and CYP7A (Staudinger et al., 2001), which are respectively involved in BA hydroxylation and biosynthesis, as well as BA transporters including the multi-drug resistance associated proteins MRP2 (ABCC2) (Kast et al., 2002), MRP3 (ABCC3) (Teng et al., 2003) and the bile salt export pump (BSEP, ABCB11) (Teng and Piquette-Miller, 2005). One well known PXR activator, rifampicin, is used clinically for the treatment of complications associated with cholestatic diseases (Hoensch et al., 1985) and lithocholic acid-induced liver injury is attenuated by the rodent PXR activator, 5-pregnen-3β-ol-20-one-16α-carbonitrile (PCN) (Staudinger et al., 2001; Xie et al., 2001). However, the exact mechanism behind the effect of PXR activation against BA toxicity is still unclear. As transporters play a key role in BA homeostasis, it is likely that the mechanism involves the modulation of transporters resulting in decreased intrahepatic BA concentrations.
Therefore, the aim of this study was to compare the roles of individual BA transporters in the PXR activator-mediated protection against BA-induced hepatotoxicity. We compared the involvement of numerous BA uptake and efflux transporters, including the heterodimeric organic solute transporter (OSTα/OSTβ), which has only recently been found to function as a major BA transporter (Ballatori et al., 2005; Dawson et al., 2005) and which has not yet been examined for regulation by PXR. As cholic acid (CA), a major BA in both humans and rodents, is substantially elevated during cholestatic disease (Laatikainen and Ikonen, 1977; Fischer et al., 1996; Combes et al., 1999), we utilized an established CA model of cholestasis (Barone et al., 1996; Rost et al., 2003) to induce cholestatic hepatotoxicity. Overall, our novel findings point to altered expression of transporters, particularly MRP3, as being integral to hepatoprotection against BAs. Our study also demonstrates for the first time that the BA transporters MRP4 and OSTα/OSTβ are not regulated by PXR.
Methods
Animals
The animal studies were conducted in accordance with the guidelines of the Canadian Council on Animal Care. Wild-type (PXR+/+) eight- to 12-week-old male C57BL/6 mice were purchased from Charles River Canada (Montreal, PQ, Canada). A colony of PXR−/− mice was originally obtained from Dr Christopher Sinal (Dalhousie University, Halifax, NS, Canada) with permission from Dr Steven Kliewer (University of Texas Southwestern Medical Center, Dallas, TX, USA). Eight- to 12-week-old male PXR−/− mice derived from the originally-obtained colony were used in the experiments. Animals were kept in a temperature-controlled facility with 12-h light/dark cycles, and allowed to acclimatise for 1 week before treatment.
The mice were fed either a powdered standard diet or a 1% (w/w) CA-supplemented diet for 5 days, and injected daily with PCN (50 mg kg−1 intraperitoneal) or corn oil vehicle control, with n=4–6 for each group. The CA-supplemented diet was prepared in-house by adding powdered CA to the same standard diet fed to control animals. Total body weight was measured each day throughout the treatment period. Mice were placed in metabolic cages for the final 24 h of the study period for urine collection. Mice were fasted during the final 4 h to ensure that elevations in serum BA concentrations were not owing to their release from the gall bladder and subsequent circulation following a meal. The mice were killed 24 h after the final injection. Blood was collected, and the liver was removed; a section of the liver was put into 4% paraformaldehyde for histological analysis and the remainder was snap-frozen in liquid nitrogen and kept frozen at −80°C until analysed.
Determination of mRNA expression
Total RNA was isolated from mouse liver using the QuickPrep RNA extraction kit (Amersham Biosciences Inc., Piscataway, NJ, USA) according to manufacturer's protocol. cDNA was synthesized from 0.5 μg of RNA using the First Strand cDNA synthesis kit (MBI Fermentas, Flamborough, ON, Canada). The mRNA levels of various genes were determined by real-time quantitative PCR using LightCycler technology with SYBR Green I fluorescence detection (Roche Diagnostics, Mannhei, Germany). PCR primer sequences have been previously published (Kitada et al., 2003; Teng and Piquette-Miller, 2005; Zollner et al., 2006). All mRNA levels were normalized to 18S rRNA.
Measurement of hepatic enzymes, bilirubin and bile acids in serum and urine
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and bilirubin (total and direct) levels were measured using reagent kits from Thermo Electron (Louisville, CO, USA) according to manufacturer's instructions. Total BAs in serum and urine were measured using a kit from Trinity Biotech (St Louis, MO, USA). BAs in serum were analysed directly, whereas urine was first extracted with methanol.
Histopathological analysis
Paraformaldehyde-fixed liver samples were re-sectioned in paraffin (thickness of 4 μm), stained with haematoxylin-eosin at the University Health Network Clinical Research Programme Histology Lab (Toronto, ON, Canada) and assessed by pathologist Dr Eugene Hsieh at Sunnybrook and Women's College Health Sciences Centre (Toronto, ON, Canada).
Isolation of liver microsomes and measurement of CYP3A activity
Liver samples were homogenized in a buffer (pH 7.4) containing 0.25 M sucrose, 10 mM Tris-HCl and protease inhibitor cocktail, centrifuged at 10 000 g for 20 min and the resulting supernatant was centrifuged at 100 000 g for 60 min. The pellet containing the microsomal fraction was resuspended in the same buffer, and protein concentrations were measured using the Bradford method (Bio-Rad, Hercules, CA, USA).
Microsomal CYP3A activity was measured using 7-benzyloxyquinoline (7-BQ) as a substrate. 7-BQ is demethylated by CYP3A to the fluorescent metabolite 7-hydroxyquinoline. A NADPH-generating system (5 mM MgCl2, 7.5 mM glucose-6-phosphate, 0.3 mM NADP+ and 1 unit ml−1 glucose-6-phosphate dehydrogenase) was added to samples containing 0.5 mg ml−1 of microsomal protein and 25 μM 7-BQ. Reactions were incubated at 37°C for 30 min and terminated by the addition of 0.1 ml 30% trichloroacetic acid. Samples were vortexed, centrifuged (2300 g for 5 min) and supernatant aliquots were measured at excitation and emission wavelengths of 410 and 520 nm, respectively, using a Spectra Max Gemini XS microplate fluorimeter (Molecular Devices, Sunnyvale, CA, USA). Because 7-BQ is also metabolized to a small extent by other CYP isoforms (Renwick et al., 2001), reactions in the presence of the CYP3A inhibitor ketoconazole (30 μM) were also performed, and values were subtracted from the inhibitor-free measurements to obtain metabolic activity due to CYP3A only.
Statistical analysis
All studies were performed using n=4 or more. Differences between the treatment groups were analysed using ANOVA and significance was determined by the Tukey test. In all cases, P<0.05 was considered to be statistically significant. Analysis was performed using SigmaStat 2.03 (SPSS Inc., Chicago, IL, USA).
Drugs and chemicals
Primers for PCR were synthesized by the DNA Synthesis Centre, Hospital for Sick Children (Toronto, ON, Canada). 7-BQ was purchased from BD Gentest (Woburn, MA, USA). Bradford reagent for protein quantification was from Bio-Rad Laboratories (Hercules, CA, USA). All other reagents and chemicals including CA and PCN were purchased from Sigma-Aldrich Canada (Oakville, ON, Canada).
Results
Effect of CA feeding on liver function and hepatoprotection by PXR activation
CA feeding caused significant hepatotoxicity in PXR+/+ mice as indicated by an increase in serum ALT, AST and ALP levels (Table 1). Total and direct bilirubin levels in serum were also significantly increased by CA in PXR+/+ mice. Co-administration of PCN significantly attenuated hepatotoxicity in CA-fed PXR+/+ mice as demonstrated by a 70–78% reduction in ALT, AST and ALP levels as compared to CA alone. Likewise, total and direct bilirubin levels also decreased (by 70 and 83%, respectively). Of particular interest was the finding that PXR−/− mice demonstrated dramatically lower susceptibility to CA-induced hepatotoxicity. Although CA feeding did cause a small but significant degree of hepatotoxicity in PXR−/− mice, ALT, AST and ALP levels were 17-, 15- and 6.5-fold lower, respectively, in PXR−/− as compared to PXR+/+ mice following CA feeding. This indicates that PXR−/− mice are considerably protected from BA-induced hepatotoxicity. In addition, total and direct bilirubin levels in serum were not increased by CA in PXR−/− mice. CA-mediated changes in serum ALT and AST levels were not altered by the addition of PCN treatment in PXR−/− mice, confirming a PXR-dependent mechanism of hepatoprotection by PCN. Treatment of either PXR+/+ or PXR−/− mice with PCN alone did not significantly impact bilirubin levels or the other markers of hepatotoxicity.
Table 1.
Effect of CA feeding with or without co-treatment with PXR activators on liver function and total body weight
| ALT (U/l) | AST (U/l) | ALP (U/l) | Total bilirubin (μM) | Direct bilirubin (μM) | Change in total body weight (%) | |
|---|---|---|---|---|---|---|
| PXR+/+ | ||||||
| Control (4) | 23±4 | 45±6 | 42±6 | 10.4±1.28 | 4.27±0.44 | +2 |
| PCN (4) | 28±6 | 64±14 | 38±2 | 15.5±3.08 | 8.60±2.05 | NC |
| CA (6) | 1618±485a | 1350±356a | 202±32a | 39.3±7.52a | 33.8±10.4a | −23 |
| CA+PCN (5) | 358±77b | 380±108b | 62±12b | 12.0±1.20b | 7.52±0.68b | −12 |
| PXR−/− | ||||||
| Control (4) | 19±3 | 42±4 | 19±7 | 12.3±1.74 | 6.50±2.74 | +2 |
| PCN (4) | 13±5 | 38±12 | 11±6 | 15.8±4.10 | 11.2±4.27 | −10 |
| CA (4) | 95±8a | 91±3a | 31±7 | 10.7±1.54 | 6.87±1.03 | −15 |
| CA+PCN (4) | 85±6a | 80±13a | 23±5 | 15.3±1.37 | 7.58±0.68 | −18 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine amino transferase; AST, aspartate aminotransferase; CA, cholic acid; PXR, pregnane X receptor; PCN, 5-pregnen-3β-ol-20-one-16α-carbonitrile; NC, no change.
PXR+/+ and PXR−/− mice were fed standard chow or a 1% CA-supplemented diet for 5 days with or without injection of the PXR activator PCN. Numbers in brackets indicate the number of mice in each treatment group. Blood samples were analysed for ALT, AST, ALP and bilirubin levels as indicators of liver function. Mice were weighed throughout the treatment period; the change in weight represents the difference in weight between the start and end of treatment. Results are presented as mean enzyme or bilirubin concentrations±s.e.m.
Significant from control P<0.05.
Significant from CA P<0.05.
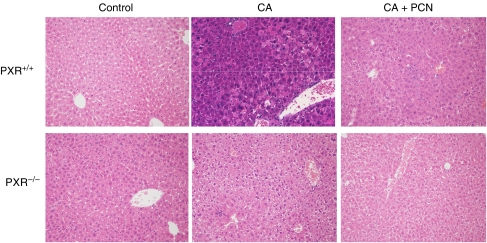
Total body weight was also measured throughout the treatment period because hepatotoxicity is often associated with decreased appetite and weight loss. Consistent with the serum biochemistry findings, CA feeding caused severe weight loss in PXR+/+ mice and this was prevented by nearly 50% in mice given PCN along with CA (Table 1). Weight loss also occurred in CA-fed PXR−/− mice, although this was significantly less pronounced compared to PXR+/+ mice. Also in agreement with the serum biochemistry results, H&E-stained liver samples from CA-fed PXR+/+ mice showed tissue damage characterized by large zones of dysplastic and degenerative hepatocytes (Figure 1). This was absent in the liver from PXR+/+ mice co-treated with PCN. On the other hand, no major histological abnormalities were observed in the livers of CA-fed PXR−/− mice other than some areas of mild inflammation.
Figure 1.
Histological changes in mouse liver following CA and CA+PCN treatment. PXR+/+ and PXR−/− mice were fed a 1% CA-supplemented diet for 5 days with or without cotreatment with the PXR activator PCN (50 mg kg−1). The liver was removed and a section was fixed and stained with hematoxylin-eosin. Images are shown at × 20 magnification. Large areas with degenerative hepatocytes are evident in the sections from CA-fed PXR+/+ mice, whereas only a few areas with increased macrophage infiltration in the sinusoids are notable in those from CA+PCN treated mice. Likewise in the CA-fed PXR−/− mice, some inflammation represented by clustered macrophages is present.
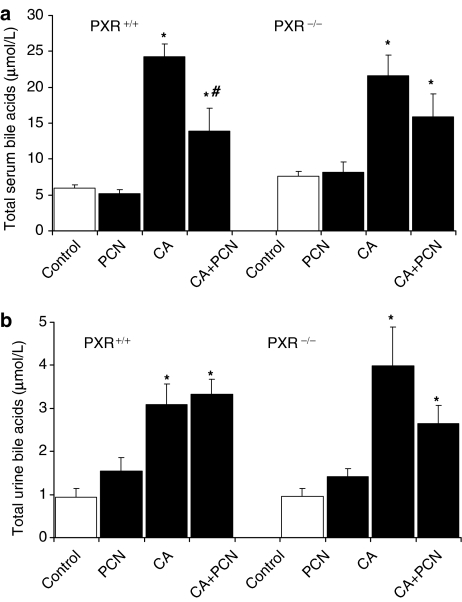
Total bile acid levels in serum and urine following CA feeding
Total BA levels in serum and urine were also measured to assess BA distribution (Figure 2). Significant 3- to 4-fold increases in serum BAs were seen in the CA-fed PXR+/+ and PXR−/− mice as compared to controls. Serum BA levels were similar between CA-fed PXR+/+ and PXR−/− mice. Co-administration of PCN imposed a significant (42%) reduction in serum BAs in the PXR+/+ mice. CA feeding also caused a 3- and 4-fold elevation of urinary total BAs versus control levels in PXR+/+ and PXR−/− mice, respectively. Interestingly, in contrast to the changes seen in serum, urinary BAs were not further altered by administration of PCN to the CA-fed PXR+/+ mice. PCN administration alone had no significant effect on BA levels in either the serum or urine of all mice fed standard diets. Renal clearance of BAs was significantly higher (P<0.05) in CA- (42.6±6.6 μl h−1), PCN- (33.3±4.1 μl h−1) and CA+PCN- (39.6±9 μl h−1) treated mice compared to controls (17.4±3 μl h−1) but significant differences were not seen between CA and CA-PCN treated mice.
Figure 2.
Total bile acid levels in serum and urine. Serum (a) was collected from mice (n=4–6) at the time of killed, after 4 h of fasting. (b) Urine was collected from mice for 24 h before killed. Total BA levels were analysed as described in Methods. Results are presented as mean BA levels±s.e.m. *P<0.05 versus control; #P<0.05 versus CA.
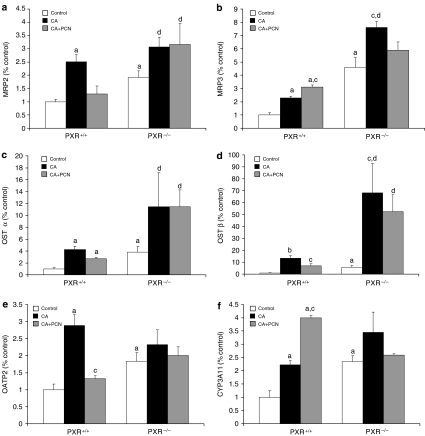
Basal expression of efflux transporters and CYP3A11 in PXR+/+ versus PXR−/− mice
To investigate the reason for the relative insensitivity of PXR−/− mice to CA toxicity, we compared PXR−/− and PXR+/+ mice for differences in the basal levels of genes involved in BA homeostasis. Figure 3 depicts expression levels of genes that were significantly different between PXR+/+ and PXR−/− mice. The basal levels of MRP2, MRP3 and CYP3A11 were 2- to 4-fold higher in PXR−/− mice than in PXR+/+ mice. In addition, basal OATP2 levels in PXR−/− mice were nearly double that in PXR+/+ mice, and that OSTα and OSTβ were nearly 4- and 6-fold higher, respectively. Several genes were not altered between PXR+/+ and PXR−/− mice, and these are depicted in Figure 4. Basal levels of FXR and CAR mRNA, which also regulate many of the genes being examined, did not differ between PXR+/+ and PXR−/− mice (Table 2).
Figure 3.
Effect of CA and CA+PCN treatment on the expression of hepatic genes with higher basal levels in PXR−/− mice: (a) MRP2; (b) MRP3; (c) OSTα; (d) OSTβ; (e) OATP2 and (f) CYP3A11. PXR+/+ and PXR−/− mice (n=4–6) were fed a control diet, a 1% CA-supplemented diet or CA diet with PCN. Levels of mRNA were measured by RT-PCR as described in Methods. Values are presented as a ratio versus PXR+/+ controls±s.e.m. aP<0.05 versus PXR+/+ control; bP<0.001 versus PXR+/+ control; cP<0.05 versus PXR+/+ fed CA; dP<0.05 versus PXR−/− control.
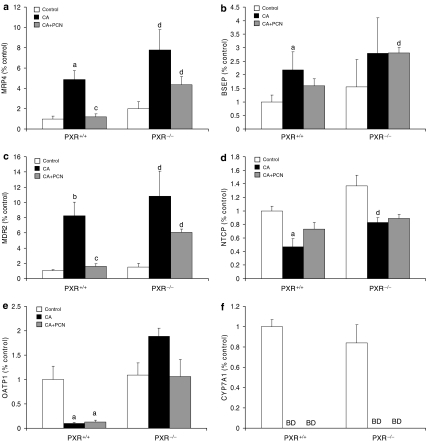
Figure 4.
Effect of CA and CA+PCN treatment on the expression of hepatic genes with comparable basal levels between PXR+/+ and PXR−/− mice: (a) MRP4; (b) BSEP; (c) MDR2; (d) NTCP; (e) OATP1 and (f) CYP7A1. PXR+/+ and PXR−/− mice (n=4–6) were fed a control diet, a 1% CA-supplemented diet or CA diet with PCN and levels of hepatic mRNA were measured by RT-PCR. Values are presented as a ratio versus PXR+/+ controls±s.e.m. aP<0.05 versus PXR+/+ control; bP<0.001 versus PXR+/+ control; cP<0.05 versus PXR+/+ fed CA; dP<0.05 versus PXR−/− control; BD, below detection limit.
Table 2.
Effect of CA feeding with or without co-treatment with PCN on hepatic nuclear receptor expression
|
Relative levels of PXR, FXR and CAR mRNA |
||||||
|---|---|---|---|---|---|---|
|
PXR+/+ |
PXR−/− |
|||||
| Control | CA | CA+PCN | Control | CA | CA+PCN | |
| PXR | 100±26 | 346±42a | 300±21a | — | — | — |
| FXR | 100±7 | 359±42a | 158±19 | 156±39 | 423±98c | 279±69 |
| CAR | 100±27 | 58±27 | 77±25 | 156±56 | 335±53b,c | 223±57 |
Abbreviations: CA, cholic acid; CAR, constitutive androstane receptor; FXR, farnesoid X receptor; PCN, 5-pregnen-3β-ol-20-one-16α-carbonitrile; PXR, pregnane X receptor.
PXR+/+ and PXR−/− mice (n=4–6) were fed a 1% CA-supplemented diet and treated with the PXR activator PCN or corn oil control. Levels of hepatic nuclear receptor mRNA were measured by RT-PCR. Values are presented as a percentage of the mRNA levels in PXR+/+ control mice (±s.e.m.).
P<0.05 vs PXR+/+ control.
P<0.05 vs PXR+/+ fed CA.
P<0.05 vs PXR−/− control.
We also considered whether differences in CA-induced hepatotoxicity between PXR+/+ and PXR−/− mice might be related to differences in the relative expression of genes following CA feeding. Indeed, we observed that CA-fed PXR−/− mice expressed more than 3-fold higher levels of MRP3 than their PXR+/+ counterparts (P<0.05). Moreover, CA-induced hepatotoxicity, as determined by plasma ALT levels, was found to be significantly correlated to MRP3 mRNA expression (r=−0.501, P<0.05) in all animals. The basolateral transporter OSTβ was nearly 5-fold higher in PXR−/− mice than PXR+/+ mice following CA feeding but no correlations in its expression were detected with indices of hepatotoxicity. No further significant differences were detected between CA-fed PXR−/− versus PXR+/+ mice nor did the expression of other hepatic genes significantly correlate to hepatotoxicity (data not shown).
Hepatic gene expression in CA-fed and PCN-treated mice
Administration of the CA-supplemented diet to PXR+/+ mice caused significant changes in the expression of hepatic CYPs and BA transporters, and these changes are depicted in Figures 3 and 4. As compared to standard diet controls, the CA-supplemented diet induced mRNA levels of MRP2, MRP3, MRP4, BSEP, multi-drug resistance protein 2 (MDR2), OSTα, OSTβ, OATP2 and CYP3A11, whereas CYP7A1, OATP1 and NTCP were downregulated. The nuclear receptors PXR and FXR, but not CAR, were also induced (Table 2).
To determine potential mechanisms of PCN-mediated hepatoprotection, we examined the pattern of hepatic gene expression in CA-fed PXR+/+ mice co-administered PCN. We found that the mRNA expression of MRP3 and CYP3A11 was significantly higher in mice treated with CA+PCN as compared to mice fed CA alone (Figure 3b and f). Interestingly, the expression of several FXR regulated genes including MDR2, OSTα and OSTβ showed a pronounced downregulatory trend in CA+PCN treated PXR+/+ mice as compared to CA feeding alone, suggesting possible negative regulatory feedback mechanisms. Indeed, mRNA levels of FXR were approximately 50% lower in PCN treated mice, although this did not reach statistical significance.
In order to delineate the contribution of PXR to these changes, we also examined the effect of CA and CA+PCN treatment on gene expression in PXR−/− mice. Administration of the CA-supplemented diet to PXR−/− mice caused significant changes in the expression of several FXR-regulated genes including MDR2, MRP2, MRP4, NTCP, OSTα, OSTβ and CYP7A1 (Figures 3 and 4). MRP2 and MRP3 induction still occurred, but to a significantly lesser extent (P<0.05) than that seen in PXR+/+ mice (Figure 3a and b). This suggests both PXR-dependent and independent pathways of induction by CA. Induction of CYP3A11 and OATP2 as observed in CA-fed PXR+/+ mice was not seen in CA-fed PXR−/− mice (Figure 3e and f), indicating that induction by CA occurred through PXR. Clearly, changes in gene expression in response to BA feeding involve multiple regulatory mechanisms. On the other hand, as compared to CA-feeding alone, co-administration of PCN to PXR−/− mice did not impose any significant changes in gene expression. As the effect of PXR activation on the expression of MRP4 and OSTα/OSTβ is unknown, we also examined the impact of PCN treatment alone in PXR+/+ versus PXR−/− mice. PCN administration did not significantly alter the expression of MRP4 (1.5±0.22 fold), OSTα (1.3±0.1 fold) or OSTβ (1.4±0.1 fold) in PXR+/+ mice. Likewise PCN treatment did not significantly affect these genes in PXR−/− mice.
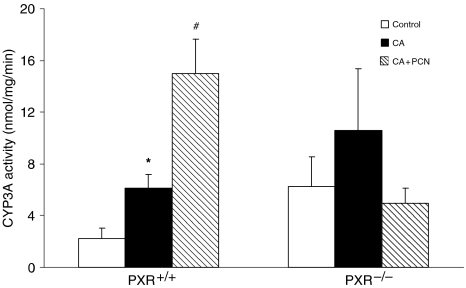
The metabolic activity of CYP3A in isolated microsomes also confirmed a significant increase in CYP3A-mediated metabolism of 7-BQ in CA-fed PXR+/+ mice as compared to controls. Metabolism was further induced in mice administered CA+PCN than in mice fed CA only (Figure 5). On the other hand, neither CA feeding nor CA+PCN treatment had a significant impact on CYP3A activity in PXR−/− mice.
Figure 5.
Effect of CA feeding with or without cotreatment with PCN on hepatic CYP3A activity in microsomes. Microsomes were isolated from the liver of PXR+/+ and PXR−/− mice (n=4–6) fed a 1% CA-supplemented diet and treated with the PXR activator PCN or corn oil control. CYP3A metabolic activity was determined by measuring the fluorescence generated by the demethylation of 7-BQ to a fluorescent metabolite at 410 nm and 520 nm excitation and emission wavelengths, respectively. Results are presented as mean activity±s.e.m. *P<0.05 versus control; #P<0.05 versus CA.
Discussion
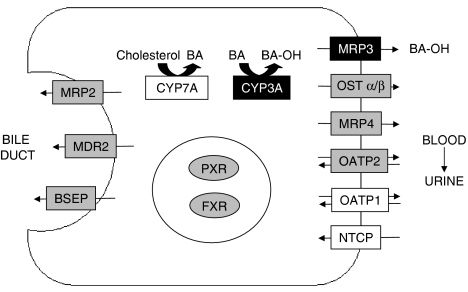
Transporters in the liver play an integral role in BA homeostasis (Figure 6). To understand the relationship between transporter expression and BA-induced liver injury and also the impact of PXR activator-mediated modulation of transporter expression on liver function, we treated PXR+/+ and PXR−/− mice with a CA-supplemented diet that results in hepatotoxicity along with plasma total BA levels that are similar to those seen in patients with cholestatic disease (Bremmelgaard and Alme, 1980) and other animal models of cholestasis (Bossard et al., 1993; Wang et al., 2001). Consistent with recent reports, the increased expression of many FXR-regulated BA transporters in CA-fed PXR+/+ and PXR−/− mice suggest CA-mediated activation of FXR. We also observed a lesser or abolished CA-mediated induction of PXR target genes (OATP2, CYP3A11, MRP2, MRP3) in PXR−/− mice, which indicates that CA can also activate PXR. As CA and its conjugate taurocholate are relatively weak PXR activators (Staudinger et al., 2001; Moore et al., 2002), this may occur through a secondary pathway. Recently, Jung et al. (2006) demonstrated that CA-mediated induction of PXR occurs through the activation of FXR; hence FXR is believed to play the primary role in gene regulation in response to CA. However, despite these intrinsic adaptive changes in gene expression, hepatotoxicity caused by CA feeding persisted. Administration of the potent PXR activator, PCN, to PXR+/+ mice substantially attenuated CA-induced hepatotoxicity as indicated by decreased serum liver enzymes, bilirubin and BA levels, histological abnormalities and changes in total body weight. Thus it is highly plausible that activation of PXR by PCN mediates the observed hepatoprotective effects.
Figure 6.
Metabolism and transport of BA, and the proposed mechanism for PCN-mediated protection against CA-induced hepatotoxicity. CA feeding results in the feed-forward induction of bile acid efflux transporters and hydroxylation by CYP3A. Hepatic uptake of BA by NTCP and OATP1 and synthesis by CYP7A are downregulated. CYP3A and MRP3 are further induced by the co-administration of PCN. Thus BAs are cleared from the liver by PXR activation-dependent hydroxylation (CYP3A) and basolateral efflux (MRP3), leading to renal elimination. Genes in boxes with grey shading were induced by CA feeding, whereas those in boxes with dark shading were induced further by co-administration of PCN. Genes in boxes without shading were downregulated by CA feeding.
Of particular significance was the finding that PXR−/− mice were quite tolerant to the toxic effects of CA in that CA-induced hepatotoxicity was dramatically attenuated in PXR−/− mice. In agreement with previous findings (Teng and Piquette-Miller, 2005), we detected significant differences in the basal expression of several genes involved in BA homeostasis in the PXR−/− mice; thus it is conceivable that reduced CA sensitivity in PXR−/− mice stems from these differences. However, we also cannot exclude the potential for other yet-to-be-identified BA detoxification and/or transport pathways which could have elevated activity in PXR−/− mice. Our results suggest that expression of the basolateral anion transporter, MRP3, is an important determinant of CA toxicity. Basal and CA-induced MRP3 expression were significantly higher in PXR−/− mice. Furthermore, we observed a significant correlation between MRP3 expression and serum ALT among all CA and CA+PCN-treated PXR+/+ and PXR−/− mice. PCN-mediated hepatoprotection in PXR+/+ mice was also associated with a significant induction of MRP3 mRNA expression, further suggesting that sinusoidal efflux is a major route by which PXR activation mediates BA homeostasis. As MRP3 is involved in the efflux of CA and its main conjugate, taurocholic acid (Hirohashi et al., 2000; Akita et al., 2002), it is likely that CA efflux by this transporter is essential during BA overload. Elevated MRP3 expression is often observed in patients with cholestasis or Dubin–Johnson syndrome, and has been linked to protection against hepatocellular necrosis during obstructive cholestasis in mice (Bohan et al., 2003). Our findings provide evidence that upregulation of MRP3 is indeed a hepatoprotective event during cholestasis, as part of both the inherent feedforward response to elevated BA levels as well as, reported here for the first time, through the exogenous PXR activator-mediated mechanism.
Hepatoprotection by PCN was also associated with a significant induction of CYP3A11 mRNA and activity. CYP3A catalyzes the hydroxylation of BAs including CA, rendering them more hydrophilic and therefore less toxic (Stedman et al., 2004; Zollner et al., 2006). Indeed, CYP3A11 induction in CA-fed mice is associated with higher proportions of hydroxylated CA metabolites in serum (Zollner et al., 2006) and increased proportions of hydroxylated urinary BAs have been reported in PCN-treated bile duct ligated mice (Wagner et al., 2005). It is important to note that CYP3A11 levels in CA-fed PXR−/− mice were similar to levels in CA-fed PXR+/+ mice despite the lesser toxicity in PXR−/− mice. This suggests that the role of CYP3A11 in the overall feedforward response to CA feeding in PXR+/+ mice and in the inherent resistance of PXR−/− mice against CA is modest. Rather, the contribution of CYP3A11 in hepatoprotection appears to be associated exclusively with PXR activation by PCN in PXR+/+ mice. On the other hand, our results do not preclude the possibility that other metabolic pathways such as sulphation could contribute to BA detoxification. However, sulphation has little relevance in mice, where it has only been demonstrated to occur with lithocholic acid (Kitada et al., 2003).
It is generally hypothesized that the hydroxylation of CA enables the hydrophilic metabolites to be more readily secreted into urine. Indeed we collected high levels of BAs in the urine of CA-fed PXR+/+ and PXR−/− mice. Surprisingly, though, while we expected the PCN-treated mice to have further increased hydroxylated metabolites owing to CYP3A11 induction, there was no difference in urinary total BA levels between the CA-fed and CA+PCN-treated PXR+/+ mice. These findings could reflect saturation of renal BA transporters. Indeed, the renal clearance of BAs was significantly higher in CA-treated mice as compared to controls, however addition of PCN to CA treatments did not further increase renal clearance. Of note, CA+PCN treatment resulted in a pronounced decrease in the expression of MRP2 and MRP4 in liver. If these genes are similarly downregulated by PCN in the kidney, this could lead to a reduction in renal tubular secretion of BAs. However, regulation of these transporters in the kidney by PXR activation is still unknown. Nevertheless, despite the similar total BA levels, we cannot discard the possibility that the proportion of hydroxylated BAs could have increased in the PCN-treated mice.
Unexpectedly, PCN administration decreased expression of the apical transporters MRP2 and BSEP to near basal levels in CA fed PXR+/+ mice. This was surprising because hepatobiliary excretion is a major route of BA elimination. Consistent with other reports, expression of these transporters was induced by the CA-supplemented diet, suggesting a role for MRP2 and BSEP in BA homeostasis (Fickert et al., 2001). The absence of further induction by PCN may be because of a negative feedback whereby high biliary concentrations of toxic BAs may inhibit further induction of apical transporters such as MRP2 and BSEP in order to prevent damage to the bile duct. Indeed, it is thought that elevated expression of BSEP in bile duct ligated mice is detrimental owing to over-exposure of the already vulnerable bile duct to toxic BAs (Stedman et al., 2006).
It is also conceivable that hepatoprotection against CA could be attributed to changes in the expression of other basolateral BA efflux transporters such as MRP4 or OSTα/OSTβ. FXR and CAR regulate these novel BA transporters (Assem et al., 2004; Landrier et al., 2006; Zollner et al., 2006) and our results in CA-fed mice imply that MRP4 and OSTα/OSTβ are involved in innate BA feedback mechanisms. However, we found that despite a 4- and 13-fold induction of OSTα and OSTβ, respectively, by CA feeding in PXR+/+ mice, extensive toxicity still ensued. Furthermore, PCN treatment in CA-fed PXR+/+ mice was associated with a decreased rather than increased expression of MRP4, OSTα and OSTβ, and changes in their expression among CA-fed PXR+/+ or PXR−/− mice did not significantly correlate with any indices of hepatotoxicity. On the other hand, OSTβ levels in PXR−/− mice following CA feeding were seven-fold higher compared to their PXR+/+ counterparts, possibly indicating that the expression of this gene must reach extremely high levels in order to contribute to hepatoprotection. Thus changes in the expression of MRP4, OSTα and OSTβ are not entirely consistent with our toxicity data but, nevertheless indicate that unlike MRP3, these basolateral transporters do not appear to play a role in the PCN-mediated hepatoprotection. Our results showing that the impact of CA, PCN or CA+PCN treatments on gene expression is the same between PXR+/+ and PXR−/− mice demonstrate for the first time that MRP4, OSTα and OSTβ are not regulated by PXR.
Our finding of significant differences in basal gene expression between PXR+/+ and PXR−/− mice was not entirely unexpected. Similar trends have been reported in other nuclear receptor knockout models (Guo et al., 2003; Saini et al., 2005; Marschall et al., 2006). For example, hepatic CYP3A11, MRP3 and MRP4 expression are elevated in FXR knockout mice, and this has been linked to protection against cholestatic liver injury through increased BA metabolism and excretion (Schuetz et al., 2001; Wagner et al., 2003; Marschall et al., 2006; Zollner et al., 2006). Saini et al. (2005) also detected higher bilirubin clearance in PXR−/− than PXR+/+ mice, which they associated with elevated basal levels of a number of genes including MRP2. Although the phenomenon of increased gene expression in knockout mice is often reported, the mechanism behind this effect remains unclear. The differences could be interpreted as being indicative of PXR as a negative regulator of transporter expression and that the presence of PXR promotes CA-induced hepatotoxicity. It is possible that in an unliganded state, PXR may suppress gene expression; this might explain the higher basal gene expression and lesser hepatotoxicity in PXR−/− mice. On the other hand, ligand-activated PXR acts as a positive regulator of gene expression. Alternatively, the differences in transporter expression and sensitivity to CA between PXR+/+ and PXR−/− mice could stem not from the presence or absence of PXR itself, but because of a compensatory induction of other pathways that regulate transporter genes in PXR−/− mice. This is an area that is certainly worthy of further investigation.
In conclusion, our results are suggestive of an important role for efflux transporters, particularly MRP3, in protecting the liver from cholestatic injury. We have elucidated, at least in part, the mechanism of PCN-mediated protection against CA-induced hepatotoxicity; this involves the induction of MRP3 as well as CYP3A11. Our findings also demonstrate that unlike MRP3, other basolateral efflux pathways including MRP4 and OSTα/OSTβ are not regulated by PXR and do not contribute to this PCN-mediated effect. Our study significantly adds to the current knowledge of changes in gene expression that occur during cholestasis and the mechanisms by which the liver attempts to minimize injury. Our study brings to the forefront the hepatoprotective role of MRP3 in BA transport under cholestatic conditions and our findings provide further evidence for the potential of BA transporters and PXR activators as foci for the development of novel therapies to treat cholestasis.
Acknowledgments
We would like to thank Dr Eugene Hsieh of Sunnybrook and Women's College Health Sciences Centre (Toronto, ON, Canada) for evaluating the histopathology specimens. Funding for this study was provided by a grant from the Canadian Institutes of Health Research (CIHR).
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BA
bile acid
- BSEP
bile salt export pump
- 7-BQ
7-benzyloxyquinoline
- CA
cholic acid
- CAR
constitutive androstane receptor
- CYP
cytochrome P450
- FXR
farnesoid X receptor
- 7-HQ
7-hydroxyquinoline
- MDR
multidrug resistance
- MRP
multidrug resistance-associated protein
- NTCP
sodium-taurocholate transporting polypeptide
- OATP
organic anion transporting polypeptide
- OST
organic solute transporter
- PCN
5-pregnen-3β-ol-20-one-16α-carbonitrile
- PXR
pregnane X receptor
Conflict of interest
The authors state no conflict of interest.
References
- Akita H, Suzuki H, Hirohashi T, Takikawa H, Sugiyama Y. Transport activity of human MRP3 expressed in Sf9 cells: comparative studies with rat MRP3. Pharm Res. 2002;19:34–41. doi: 10.1023/a:1013699130991. [DOI] [PubMed] [Google Scholar]
- Ando H, Tsuruoka S, Yamamoto H, Takamura T, Kaneko S, Fujimura A. Regulation of cholesterol 7alpha-hydroxylase mRNA expression in C57BL/6 mice fed an atherogenic diet. Atherosclerosis. 2005;178:265–269. doi: 10.1016/j.atherosclerosis.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, et al. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem. 2004;279:22250–22257. doi: 10.1074/jbc.M314111200. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, et al. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42:1270–1279. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- Barone M, Francavilla A, Polimeno L, Ierardi E, Romanelli D, Berloco P, et al. Modulation of rat hepatocyte proliferation by bile salts: in vitro and in vivo studies. Hepatology. 1996;23:1159–1166. doi: 10.1053/jhep.1996.v23.pm0008621149. [DOI] [PubMed] [Google Scholar]
- Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, et al. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci USA. 1998;95:12208–12213. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohan A, Chen WS, Denson LA, Held MA, Boyer JL. Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3(Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem. 2003;278:36688–36698. doi: 10.1074/jbc.M304011200. [DOI] [PubMed] [Google Scholar]
- Bossard R, Stieger B, O'Neill B, Fricker G, Meier PJ. Ethinylestradiol treatment induces multiple canalicular membrane transport alterations in rat liver. J Clin Invest. 1993;91:2714–2720. doi: 10.1172/JCI116511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmelgaard A, Alme B. Analysis of plasma bile acid profiles in patients with liver diseases associated with cholestasis. Scand J Gastroenterol. 1980;15:593–600. doi: 10.3109/00365528009182221. [DOI] [PubMed] [Google Scholar]
- Combes B, Carithers RL, Jr, Maddrey WC, Munoz S, Garcia-Tsao G, Bonner GF, et al. Biliary bile acids in primary biliary cirrhosis: effect of ursodeoxycholic acid. Hepatology. 1999;29:1649–1654. doi: 10.1002/hep.510290618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, et al. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Pojer C, Zenz R, et al. Effects of ursodeoxycholic and cholic acid feeding on hepatocellular transporter expression in mouse liver. Gastroenterology. 2001;121:170–183. doi: 10.1053/gast.2001.25542. [DOI] [PubMed] [Google Scholar]
- Fischer S, Beuers U, Spengler U, Zwiebel FM, Koebe HG. Hepatic levels of bile acids in end-stage chronic cholestatic liver disease. Clin Chim Acta. 1996;251:173–186. doi: 10.1016/0009-8981(96)06305-x. [DOI] [PubMed] [Google Scholar]
- Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB, Jr, Kliewer SA, et al. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem. 2003;278:45062–45071. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3) J Biol Chem. 2000;275:2905–2910. doi: 10.1074/jbc.275.4.2905. [DOI] [PubMed] [Google Scholar]
- Hoensch HP, Balzer K, Dylewizc P, Kirch W, Goebell H, Ohnhaus EE. Effect of rifampicin treatment on hepatic drug metabolism and serum bile acids in patients with primary biliary cirrhosis. Eur J Clin Pharmacol. 1985;28:475–477. doi: 10.1007/BF00544371. [DOI] [PubMed] [Google Scholar]
- Jung D, Mangelsdorf DJ, Meyer UA. Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem. 2006;281:19081–19091. doi: 10.1074/jbc.M600116200. [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Kitada H, Miyata M, Nakamura T, Tozawa A, Honma W, Shimada M, et al. Protective role of hydroxysteroid sulfotransferase in lithocholic acid-induced liver toxicity. J Biol Chem. 2003;278:17838–17844. doi: 10.1074/jbc.M210634200. [DOI] [PubMed] [Google Scholar]
- Laatikainen T, Ikonen E. Serum bile acids in cholestasis of pregnancy. Obstet Gynecol. 1977;50:313–318. [PubMed] [Google Scholar]
- Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol. 2006;290:G476–G485. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- Marschall HU, Wagner M, Bodin K, Zollner G, Fickert P, Gumhold J, et al. Fxr(−/−) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J Lipid Res. 2006;47:582–592. doi: 10.1194/jlr.M500427-JLR200. [DOI] [PubMed] [Google Scholar]
- Moore LB, Maglich JM, McKee DD, Wisely B, Willson TM, Kliewer SA, et al. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol Endocrinol. 2002;16:977–986. doi: 10.1210/mend.16.5.0828. [DOI] [PubMed] [Google Scholar]
- Renwick AB, Lewis DF, Fulford S, Surry D, Williams B, Worboys PD, et al. Metabolism of 2,5-bis(trifluoromethyl)-7-benzyloxy-4-trifluoromethylcoumarin by human hepatic CYP isoforms: evidence for selectivity towards CYP3A4. Xenobiotica. 2001;31:187–204. doi: 10.1080/00498250110043526. [DOI] [PubMed] [Google Scholar]
- Rost D, Herrmann T, Sauer P, Schmidts HL, Stieger B, Meier PJ, et al. Regulation of rat organic anion transporters in bile salt-induced cholestatic hepatitis: effect of ursodeoxycholate. Hepatology. 2003;38:187–195. doi: 10.1053/jhep.2003.50256. [DOI] [PubMed] [Google Scholar]
- Saini SP, Mu Y, Gong H, Toma D, Uppal H, Ren S, et al. Dual role of orphan nuclear receptor pregnane X receptor in bilirubin detoxification in mice. Hepatology. 2005;41:497–505. doi: 10.1002/hep.20570. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Strom S, Yasuda K, Lecureur V, Assem M, Brimer C, et al. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem. 2001;276:39411–39418. doi: 10.1074/jbc.M106340200. [DOI] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez JG, Evans RM, et al. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci USA. 2006;103:11323–11328. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman C, Robertson G, Coulter S, Liddle C. Feed-forward regulation of bile acid detoxification by CYP3A4: studies in humanized transgenic mice. J Biol Chem. 2004;279:11336–11343. doi: 10.1074/jbc.M310258200. [DOI] [PubMed] [Google Scholar]
- Teng S, Jekerle V, Piquette-Miller M. Induction of ABCC3 (MRP3) by pregnane X receptor activators. Drug Metab Dispos. 2003;31:1296–1299. doi: 10.1124/dmd.31.11.1296. [DOI] [PubMed] [Google Scholar]
- Teng S, Piquette-Miller M. The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther. 2005;312:841–848. doi: 10.1124/jpet.104.076141. [DOI] [PubMed] [Google Scholar]
- Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–838. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42:420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, et al. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci USA. 2001;98:2011–2016. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, et al. Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J Hepatol. 2003;39:480–488. doi: 10.1016/s0168-8278(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, et al. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G923–G932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]