Abstract
Background and purpose:
An inflammatory response in the central nervous system mediated by the activation of microglia is a key event in the early stages of the development of neurodegenerative diseases. LPS has been reported to cause marked microglia activation. It is very important to develop drugs that can inhibit microglia activation and neuroinflammation. Here, we investigated the inhibitory effect of YC-1, a known activator of soluble guanylyl cyclase, against LPS-induced inflammatory responses in microglia.
Experimental approach:
To understand the inhibitory effects of YC-1 on LPS-induced neuroinflammation, primary cultures of rat microglia and the microglia cell line BV-2 were used. To examine the mechanism of action of YC-1, LPS-induced nitric oxide (NO) and prostaglandin E2 (PGE2) production, iNOS, COX-2 and cytokine expression were analyzed by Griess reaction, ELISA, Western blotting and RT-PCR, respectively. The effect of YC-1 on LPS-induced activation of nuclear factor kappa B (NF-κB) was studied by NF-κB reporter assay and immunofluorocytochemistry.
Key results:
YC-1 inhibited LPS-induced production of NO and PGE2 in a concentration-dependent manner. The protein and mRNA expression of iNOS and COX-2 in response to LPS application were also decreased by YC-1. In addition, YC-1 effectively reduced LPS-induced expression of the mRNA for the proinflammatory cytokines, TNF-α and IL-1β. Furthermore, YC-1 inhibited LPS-induced NF-κB activation in microglia.
Conclusions and implications:
YC-1 was able to inhibit LPS-induced iNOS and COX-2 expression and NF-κB activation, indicating that YC-1 may be developed as an anti-inflammatory neuroprotective agent.
Keywords: COX-2, iNOS, LPS, microglia, NF-κB, YC-1
Introduction
Inflammation in the brain, characterized by the activation of microglia and astroglia, has been closely associated with the pathogenesis of Parkinsonism, as well as with several other degenerative neurological disorders, including Alzheimer's disease (McGeer et al., 1988). Inflammatory responses in the brain are now thought to be mainly associated with activity of glial cells. Microglia, the resident immune cells in the brain, serve the role of immune surveillance under normal conditions (Kreutzberg, 1996) and are the most responsive to environmental stress and immunological challenges. Under pathological conditions, microglia become activated and have been implicated as the predominant cell type governing inflammation-mediated neuronal damage (Liu and Hong, 2003). In particular, activated microglia exert cytotoxic effects by releasing inflammatory mediators, such as nitric oxide (NO), arachidonic acid metabolites, reactive oxygen species, tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) (McGeer et al., 1988; Minghetti and Levi, 1998; Le et al., 2001). Although these immunotoxic factors are necessary for normal function, the microglia response must be tightly regulated to avoid overactivation and disastrous neurotoxic consequences (Liu and Hong, 2003).
Microglia are cells of the monocyte/macrophage lineage that reside in the brain parenchyma. Microglia have been proposed to play a role in host defense and tissue repair in the central nervous system. Once activated chronically, microglia are capable of producing a variety of proinflammatory mediators and potentially neurotoxic compounds. The intracellular signaling mechanisms related to the effects of lipopolysaccharide (LPS) have been well studied in several types of cells including macrophage, microglia and astrocytes (Boulet et al., 1992; Bhat et al., 1998; Chen et al., 1998). LPS is known to activate mitogen-activated protein kinases, nuclear factor-kappa B (NF-κB), protein kinase C and tyrosine kinases, which have been implicated in the release of immune-related cytotoxic factors, such as NO and proinflammatory cytokines (Boulet et al., 1992; Bhat et al., 1998; Chen et al., 1998).
YC-1, 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole, has been reported to markedly increase the response of soluble guanylyl cyclase (sGC) and to raise the intracellular 3,5-cyclic monophosphorothioate (cGMP) concentration in platelets (Ko et al., 1994). It is well known that elevation of the cGMP levels can be achieved by YC-1 through the enhancement of sGC activity (Wu et al., 1995) and by inhibition of phosphodiesterase activity (Galle et al., 1999). Nevertheless, YC-1-mediated responses through a cGMP-independent pathway have also been reported (Ferrero and Torres, 2001; Hwang et al., 2003; Pan et al., 2005; Wang et al., 2005; Liu et al., 2006). In this study, we demonstrate that YC-1 significantly reduces the production of LPS-induced inflammatory mediators. In addition, sGC/protein kinase G (PKG) inhibitors do not antagonize the inhibitory effect of YC-1 on LPS-induced production of inducible nitric oxide synthase (iNOS) and COX-2 as well as NF-κB activation in microglia.
Materials and methods
Microglia culture
Rat primary microglia were cultured as described previously (Lu et al., 2006). Briefly, glial cells were cultured for 14 days in Dulbecco's modified Eagle's medium/F12 (DMEM/F12; Gibco, Grand Island, NY, USA) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT, USA). The separate microglial cells were plated into 24-well plates at a density of 2 × 105 cells/well. The purity of microglia cultures was assessed using CD-11b antibody and more than 90% of cells were stained positively. Cells were cultured for 2 days before drug treatment.
The murine BV-2 cell line developed by Dr V Bocchini (University of Perugia, Perugia, Italy) was a gift from Dr JS Hong (NIEHS, NIH, NC, USA). Immortalization of the BV-2 cell line by infecting primary microglial cell cultures with a v-raf/v-myc oncogene-carrying retrovirus (J2) has been described previously (Blasi et al., 1990). BV-2 cells were cultured in DMEM with 10% FCS at 37°C in a humidified incubator under 5% CO2 and 95% air. Confluent cultures were passaged by trypsinization.
Immunofluorocytochemistry
Cells were cultured in 12-mm coverslips. After treatment with LPS, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) at room temperature. Thirty minutes later, 4% nonfat milk in PBS containing 0.5% Triton X-100 was added to the cells. The cells were then incubated with rabbit anti-p65 (1:500; Santa Cruz Biotechnology, CA, USA) and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibody (1:500; Leinco Technology Inc., St Louis, MO, USA) for 1 h, respectively. The FITC was detected using a Zeiss fluorescence microscope.
Assay of NO
Production of NO was assayed by measuring the levels of nitrite, the stable NO metabolite, in culture medium. Accumulation of nitrite in the medium was determined by colorimetric assay with Griess reagent. Cells (2 × 105 cells/well) in 24-well plates in 500 μl culture medium were stimulated with LPS (100 ng ml−1) for 18 h. 100 μl of culture supernatant reacted with an equal volume of Griess reagent (part 0.1% naphthylethylenediamine and part 1% sulfanilamide in 5% H3PO4) in 96-well culture plates for 10 min at room temperature in the dark. Nitrite concentrations were determined by using standard solutions of sodium nitrite prepared in cell-culture medium. The absorbance at 550 nm was determined using a microplate reader (Bio-Tek, Winooski, VT, USA). Each experiment was performed in triplicate.
Assay of PGE2
BV-2 cells and primary microglia cultured in 24-well plates were stimulated with the indicated agents for another 18 h. Production of prostaglandin E2 (PGE2) in culture supernatant was measured by a commercial kit (Cayman Chemicals, Ann Arbor, MI, USA) according to the manufacturer's instructions. Briefly, 50 μl PGE2–acetylcholinestrase conjugate (as tracer), 50 μl PGE2 monoclonal antibody and 50 μl culture supernatant per well, precoated with goat polyclonal anti-mouse immunoglobulin G, were incubated for 18 h at 4°C. The wells were emptied and rinsed five times with wash buffer. Ellman's reagent (200 μl) was added to each well to develop in the dark for 60–90 min. PGE2 concentrations were determined using PGE2 standard. The absorbance at 405 nm was determined using a microplate reader (Bio-Tek, Winooski, VT, USA).
Measurement of cell viability
Cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells cultured in 24-well plate were treated with different concentrations of YC-1. After incubation for 24 h, MTT (0.5 mg ml−1) was added for 60 min, the culture medium was then removed, and the cells were dissolved in dimethyl sulfoxide and shaken for 10 min. OD values at 550 nm were measured using a microplate reader. The absorbance indicates the enzymatic activity of mitochondria and provides information on cell viability.
Western blotting
Cells were incubated with LPS (100 ng ml−1) with or without drugs for 18 h (NF-κB p65 was detected after incubation with LPS for 30 min) and then washed in cold PBS, lysed for 30 min on ice with radioimmunoprecipitation assay buffer. Protein samples containing 30 μg protein were separated on 8% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were incubated for 1 h with 4% nonfat milk in PBS buffer to block nonspecific binding. The membranes were then incubated with rabbit anti-iNOS (1:1000), anti-COX-2 (1:1000) and mouse anti-α-tubulin (1:1000; Santa Cruz Biotechnology). Subsequently, the membranes were incubated with goat anti-rabbit or goat anti-mouse peroxidase-conjugated secondary antibody (1:1000; Santa Cruz Biotechnology) for 1 h. The blots were visualized by enhanced chemiluminescence (ECL; Santa Cruz Biotechnology) using Kodak X-OMAT LS film (Eastman Kodak, Rochester, NY, USA).
For the study of NF-κB p65 translocation, cells were rinsed with PBS and suspended in hypotonic buffer A (10 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES), pH 7.6, 10 mM KCl, 1 mM dithiothreitol (DTT), 0.1 mM ethylenediamine tetraacetic acid (EDTA), and 0.5 mM phenylmethylsulfonyl fluoride) for 10 min on ice and vortexed for 10 s. The lysates were separated into cytosolic and nuclear fractions by centrifugation at 12 000 g for 2 min. The supernatants containing cytosolic proteins were collected. The pellet containing nuclei was re-suspended in buffer C (20 mM HEPES, pH 7.6, 1 mM EDTA, 1 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride, 25% glycerol and 0.4 M NaCl) for 30 min on ice. The supernatants containing nuclei proteins were collected by centrifugation at 12 000 g for 20 min and stored at −70°C. All protein concentrations were determined by colorimetric assay using Bio-Rad assay kit (Bio-Rad, Hercules, CA, USA).
Equal protein amounts (50 μg) from cytosolic or nuclear fractions were separated by 10% SDS-PAGE and then electrotransferred to PVDF membranes. The blocked membranes were incubated overnight at room temperature with rabbit anti-p65 antibody (1:1000; Santa Cruz Biotechnology). After washing with PBS, the blots were incubated for 1 h at room temperature with goat anti-rabbit peroxidase-conjugated secondary antibody (1:1000; Santa Cruz Biotechnology). Quantitative data were obtained using a computing densitometer and ImageQuant software (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
mRNA analysis by semiquantitative RT-PCR
Total RNA was extracted from BV-2 microglia using a TRIzol kit (MDBio Inc., Taipei, Taiwan). Two micrograms of RNA from BV-2 cell were used for reverse transcriptase-polymerase chain reaction (RT-PCR) by using a commercial RT-PCR kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Polymerase chain reaction (PCR) was performed using an initial step of denaturation (5 min at 94°C), 28–32 cycles of amplification (94°C for 1 min, 56°C for 30 s and 68°C for 30 s), and an extension (68°C for 2 min). PCR products were analyzed on 2% agarose gels. The mRNA of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the internal control for sample loading and mRNA integrity. The band intensity was quantified using a densitometric scanner and ImageQuant software. The oligonucleotide primers were presented in Table 1.
Table 1.
PCR primers used
| Gene target | Size (bp) | ||
|---|---|---|---|
| iNOS | Forward primer | CATGGCTTGCCCCTGGAAGTTTCTCTTCAAAG | 754 |
| reverse primer | GCAGCATCC CCTCTGATGGTGCCATCG | ||
| COX-2 | Forward primer | TTCAAAAGAAGTGCTGGAAAAGGT | 304 |
| Reverse primer | GATCATCTCTACCTGAGTGTCTTT | ||
| TNF-α | Forward primer | GACCCTCACACTCAGATCAT | 210 |
| Reverse primer | TTGAAGAGAACCTGGGAGTA | ||
| IL-1β | Forward primer | GCAACTGTTCCTGAACTC | 382 |
| Reverse primer | CTCGGAGCCTGTAGTGCA | ||
| GAPDH | Forward primer | CACCATGGAGAAGGCCGGGG | 418 |
| Reverse primer | GACGGACACATTGGGGGTAG | ||
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; iNOS, inducible nitric oxide synthase; IL-1β, interleukin-1β; TNF-α; tumor necrosis factor-alpha.
Transfection and reporter gene assay
κB-luciferase activity was measured after tran sfection with a plasmid vector containing the NF-κB binding site in the promoter region of the reporter luciferase gene. BV-2 microglia were cotransfected with 0.8 μg of κB-luciferase plasmid and 0.4 μg of β-galactosidase expression vector, which were premixed with lipofectamine (2.4 μg). After incubation for 20 min at room temperature, the mixture (1 ml/well) was applied to the cells, and 1 ml of DMEM containing 20% FCS was added 4 h later. After a 24 h transfection, the cell medium was replaced with a fresh serum-free DMEM medium and the cells were pretreated with indicated agents for 30 min, and LPS (100 ng ml−1) was then added for another 6 h. To prepare lysates, 100 μl of reporter lysis buffer (Promega, Madison, WI, USA) was added to each well, and cells were scraped from dishes. The supernatant was collected after centrifugation at 12 000 g for 2 min. Aliquots of cell lysates (10 μl) containing equal amounts of protein (20–30 μg) were plated into wells of an opaque black 96-well microplate. An equal volume of luciferase substrate was added to all samples, and luminescence was measured in a microplate luminometer. The luciferase activity value was normalized to transfection efficiency monitored by the cotransfected β-galactosidase expression vector.
Statistics
Statistical analysis was performed using software Graphpad Prism 4.01. (GraphPad Software Inc., San Diego, CA, USA). The values given are means±s.e.m. Statistical analysis between two samples was performed using Student's t-test. Statistical comparisons of more than two groups were performed using one-way analysis of variance (ANOVA) with Bonferroni's post hoc test. In all cases, P<0.05 was considered as significant.
Materials
LPS (0111:B4), KT5823, MTT, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), pyrrolidine dithiocarbamate (PDTC) and Rp-8-Br-PET-cGMPS were purchased from Sigma-Aldrich (St Louis, MO, USA). κB-luciferase plasmid was purchased from Stratagene (La Jolla, CA, USA). SV-β-galactosidase vector and luciferase assay kit were purchased from Promega (Madison, MA, USA). YC-1 was provided by Yung-Shin Pharmaceutical Industry Co., Ltd (Taichung, Taiwan).
Results
YC-1 inhibits the production of NO and PGE2 in LPS-stimulated primary microglia and BV-2 cell line
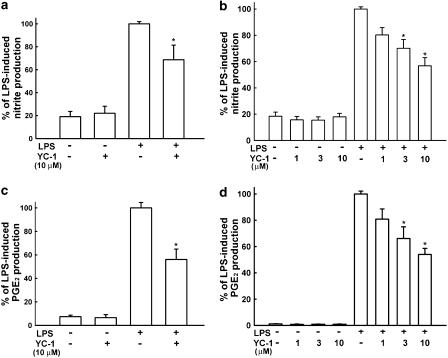
To investigate the effect of YC-1 on LPS-induced microglia activation, the primary microglia cells were pretreated with 10 μM YC-1 for 30 min and then stimulated with LPS (100 ng ml−1) for another 18 h. The cell-culture medium was then harvested. The content of nitrite and PGE2 in the medium was measured by Griess reaction and PGE2 enzyme-linked immunosorbent assay (ELISA) kit, respectively. The results show that LPS increased the production of NO (basal level: 6.1±2.5 μM, n=6) and PGE2 (basal level: 17.2±4.2 pg ml−1, n=5) by 5.5- and 13.4-fold, respectively. Pretreatment with YC-1 effectively decreased LPS-induced production of nitrite and PGE2 in primary microglia (Figure 1a and c). We also used BV-2 microglia cell line to study the action of YC-1. Exposure of BV-2 cells to LPS (100 ng ml−1) increased nitrite production approximately sevenfold over basal levels (basal level: 10.2±2.5 μM, n=6; Figure 1b). The nonselective NOS inhibitor Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME) (300 μM) completely inhibited LPS-induced nitrite production. As seen with nitrite production, LPS increased PGE2 synthesis from 7.3±1.9 to 648.5±14.5 pg ml−1 (n=5, Figure 1d). Cells were pretreated with various concentrations of YC-1 for 30 min and then stimulated with LPS for another 18 h. Preincubation of BV-2 cells with YC-1 concentration-dependently inhibited the production of nitrite and PGE2 (Figure 1b and d) induced by LPS. YC-1 at a concentration up to 10 μM by itself did not affect the release of NO and PGE2. YC-1 at concentrations ranging from 1 to 10 μM did not affect cell viability using MTT assay.
Figure 1.
Inhibition by YC-1 of LPS-induced NO and PGE2 release in rat primary microglia cultures and the BV-2 cell line. Cells were pretreated with YC-1 for 30 min and then stimulated with LPS (100 ng ml−1) for another 18 h, the culture medium of primary microglia culture (a and c) or BV-2 cell line (b and d) was then collected for the assay of nitrite (a and b) and PGE2 (c and d). The data represent the mean±s.e.m. of n=5–6. *Significantly different from LPS alone group (P<0.05, one-way ANOVA followed by Bonferroni's post hoc test).
YC-1 inhibits the expression of iNOS and COX-2
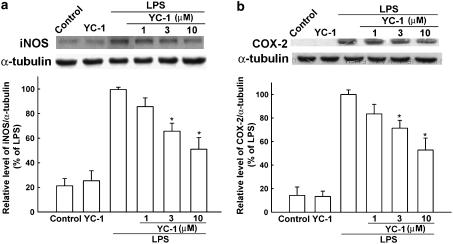
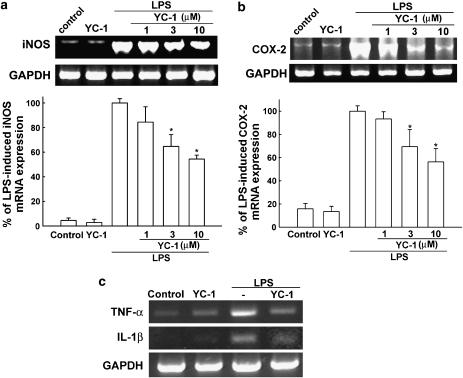
To determine the effect of YC-1 on the cytosolic protein levels of iNOS and COX-2, BV-2 cells were treated with LPS or LPS plus various concentrations of YC-1 for 18 h and the protein levels of iNOS and COX-2 were detected by Western blotting. Pretreatment with YC-1 led to a significant decrease in LPS-induced production of iNOS (Figure 2a) and COX-2 (Figure 2b) in a concentration-dependent manner. We further examined the effect of YC-1 on LPS-induced mRNA expression of iNOS (Figure 3a), COX-2 (Figure 3b), TNF-α and IL-1β (Figure 3c) using RT-PCR analysis. Total RNA were extracted from BV-2 microglia after LPS stimulation for 5 h. mRNA of iNOS and COX-2 increased by 14.2- and 8.3-fold after 5 h treatment by LPS, respectively. Expression of mRNA for iNOS, COX-2, TNF-α and IL-1β was also decreased by YC-1 cotreatment.
Figure 2.
Inhibition by YC-1 of the protein levels of iNOS and COX-2 in LPS-stimulated BV-2 microglia. BV-2 cells were pretreated with vehicle or various concentrations of YC-1 for 30 min and then stimulated with LPS (100 ng ml−1) for another 18 h. Cell lysates were prepared for the determination of protein levels of iNOS (a), COX-2 (b) and α-tubulin. The data represent the mean±s.e.m. from five independent experiments. *Significantly different from LPS alone group (P<0.05, one-way ANOVA followed by Bonferroni's post hoc test).
Figure 3.
Inhibition by YC-1 of expression of the mRNA for iNOS and COX-2 in LPS-stimulated BV-2 microglia. BV-2 cells were pretreated with vehicle or various concentrations of YC-1 for 30 min and then stimulated with LPS (100 ng ml−1) for another 5 h. Cell lysates were prepared for the determination of mRNA levels for iNOS (a), COX-2 (b) and TNF-α and IL-1β (c) and GAPDH using RT-PCR. The band intensity was quantifed with a densitometric scanner and is presented as relative to the level of GAPDH. The data represent the mean±s.e.m. of n=5. *Significantly different from LPS alone group (P<0.05, one-way ANOVA followed by Bonferroni's post hoc test).
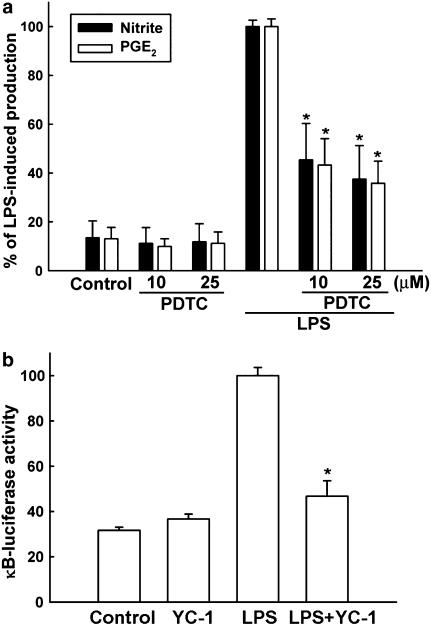
Inhibition of LPS-induced NF-κB activation by YC-1
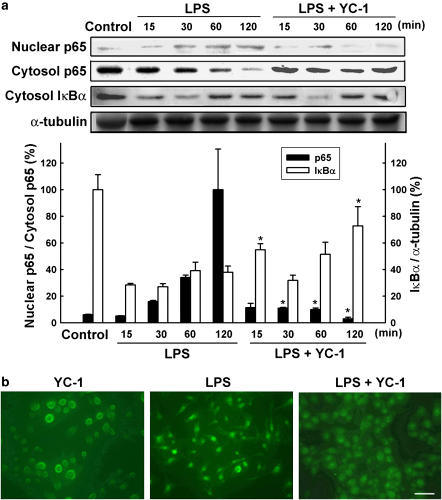
NF-κB has been demonstrated to play an essential role in the LPS-induced expression of both iNOS and COX-2 genes. PDTC, which is a NF-κB inhibitor, antagonized LPS-induced production of nitrite and PGE2 in microglia (Figure 4a). We then examined the effects of YC-1 on the transcription factor, NF-κB. NF-κB activity was evaluated by the luciferase assay in BV-2 cells transfected with the κB-luciferase plasmid. As shown in Figure 4b, LPS elicited a significant activation of NF-κB, which was inhibited by YC-1 (10 μM). We then further examined the effects of YC-1 on nuclear translocation of p65 in LPS-treated BV-2 cells. The p65 subunit of NF-κB translocates into the nucleus to exert its transcription activity. As shown in Figure 5a, LPS enhanced the nuclear translocation of p65 subunit, an effect preceded by the cytosolic decrease in IκBα. Both effects were inhibited by YC-1 treatment. In addition, immunocytochemical examination of p65 localization showed that the p65 protein was primarily located in the cytosol during the resting state. In response to LPS, the p65 protein appeared in nuclei within 1 h and BV-2 cells changed their morphology from a round shape to a spindle shape (Figure 5b). YC-1 did not affect per se the subcellular distribution of p65, but reduced the p65 nuclear immunoreactivity, as well as the morphological change elicited by LPS.
Figure 4.
Involvement of NF-κB in LPS-induced NO and PGE2 production in microglia. (a) Cells were pretreated with the inhibitor of NF-κB (PDTC: 10 or 25 μM) for 30 min and then stimulated with LPS (100 ng ml−1) for another 18 h. The culture medium was collected for the assay of nitrite and PGE2. The data represent the mean±s.e.m. of n=5. *Significantly different from LPS alone group (P<0.05, one-way ANOVA followed by Bonferroni's post hoc test). Note that PDTC inhibited both NO and PGE2 production in response to LPS. (b) Cells cotransfected with κB-luciferase reporter gene and SV-β-galactosidase vector were treated with LPS (100 ng ml−1) in the presence or absence of YC-1 (10 μM). Luciferase activity was normalized to the transfection efficiency with β-galactosidase expression vector. Note that LPS increased κB expression, which was antagonized by YC-1. The data represent the mean±s.e.m. of n=5. *Significantly different from LPS alone group (P<0.05, one-way ANOVA followed by Bonferroni's post hoc test).
Figure 5.
Inhibition of LPS-induced NF-κB activation by YC-1. (a) Both nuclear and cytosolic cell lysates were prepared from BV-2 cells treated with LPS (100 ng ml−1) in the presence or absence of YC-1 (10 μM) for the indicated times. Lower panel shows the quantitative data of p65 translocation and IκBα degradation. The data represent the mean±s.e.m. from five independent experiments. Both p65 tranlocation and IκBα degradation are significantly different between LPS and LPS+YC-1 groups (one-way ANOVA followed by Bonferroni's post hoc test). *Significantly different from LPS alone group. (b) The nuclear translocation of p65 after 1 h treatment of LPS was shown by immunofluorescence, which was inhibited by YC-1. Scale bar=10 μm.
Inhibition of LPS-induced iNOS and COX-2 expression by YC-1 is not antagonized by sGC/PKG inhibitors
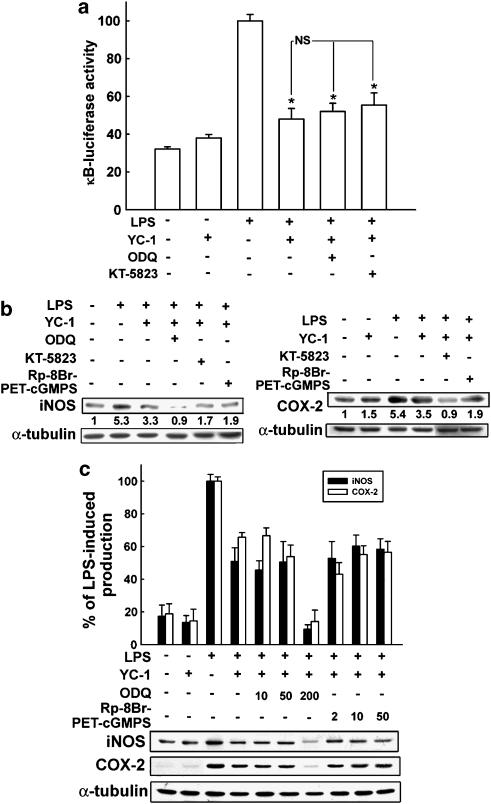
It is well known that elevation of the cGMP levels can be achieved by YC-1 through the enhancement of sGC–NO interaction (Wu et al., 1995). We then examined whether cGMP/PKG pathway is involved in the inhibition of LPS-induced iNOS and COX-2 expression in microglia. The sGC inhibitor, ODQ, and PKG inhibitors, KT5823 and Rp-8-Br-PET-cGMPS, did not antagonize the inhibitory action of YC-1 on LPS-induced NF-κB activation (Figure 6a), as well as iNOS and COX-2 (Figure 6b) induction in BV-2 cells. Treatment with higher concentrations of cGMP inhibitor, ODQ (up to 200 μM), or PKG inhibitor, Rp-8-Br-PET-cGMPS (up to 50 μM), did not affect the inhibitory action of YC-1. Therefore, the anti-inflammatory action of YC-1 may not act through a cGMP/PKG-dependent pathway.
Figure 6.
cGMP/PKG inhibitors were not able to antagonize the inhibitory response of YC-1 in LPS-stimulated microglia. BV-2 microglia were pretreated with ODQ (10 μM), KT5823 (2 μM) or Rp-8-Br-PET-cGMPS (2 μM) for 15 min, followed by vehicle or YC-1 (10 μM) for 20 min, and then stimulated with LPS (100 ng ml−1) for different time intervals (6 h: κB-luciferase (a); 18 h: iNOS and COX-2 (b) protein expression). The data represent the mean±s.e.m. from at least five independent experiments. *P<0.05, significantly different from LPS alone. NS, not significant. (c) Pretreatment of various concentrations of the guanylate cyclase inhibitor, ODQ (10, 50 or 200 μM) or PKG inhibitor, Rp-8-Br-PET-cGMPS (2, 10 or 50 μM) and the cell lysates were prepared for the determination of protein levels of iNOS and COX-2. The data represent the mean±s.e.m. (n=6). Note that the inhibitory effect of YC-1 on LPS-induced NF-κB luciferase activity and iNOS and COX-2 protein expression was not antagonized by sGC or PKG inhibitors.
Discussion
Microglia cells are the resident immune cells of the brain. In response to injury or infection, microglia cells readily become activated and consequently release proinflammatory cytokines, free radicals and eicosanoids. These factors are believed to contribute to microglia-mediated neurodegeneration (McGeer et al., 1988; Minghetti and Levi, 1998; Le et al., 2001). It has been reported that iNOS and COX-2 were induced in various types of central nervous injuries and diseases (Hunot et al., 1996; Teismann et al., 2003). These two enzymes are often coexpressed in disease states associated with gliosis. It was found that iNOS and COX-2 expressed in glial cells of substantia nigra of post-mortem Parkinson's patients (Knott et al., 2000). In addition, the expression of iNOS and COX-2 has been identified in microglia cells in rodent brain after LPS treatment (Boje and Arora, 1992; Minghetti et al., 1999). Microglia-derived NO and PGE2 have been presumed to be neurotoxic. Previous studies have demonstrated that iNOS and COX-2 inhibitors (Araki et al., 2001; Arimoto and Bing, 2003; Teismann et al., 2003) provided neuroprotective effects against LPS-induced neurotoxicity. Therefore, antagonism of the induction of iNOS, COX-2 and proinflammatory cytokines from microglia could inhibit neuroinflammation after LPS treatment.
Previous studies have reported that YC-1 inhibits the production of proinflammatory cytokines in several cell types. Hsiao et al. (2004) showed that YC-1 significantly attenuated lipoteichoic acid-induced iNOS expression and IκB-α degradation in macrophages. Pan et al. (2005) showed that YC-1 inhibited proinflammatory cytokine production in human leukocytes. Our results show that YC-1 effectively inhibits LPS-induced iNOS, COX-2 and proinflammatory cytokine expression in primary microglia culture and BV-2 cells. Recently, it has been reported that YC-1 inhibited HepG2 tumor cell growth under hypoxic condition but did not induce cell apoptosis (Lau et al., 2006). Huang et al. (2005) also showed that YC-1 induced apoptosis of PC-3 cells at concentrations upto 50∼100 μM. On the other hand, YC-1 alone did not affect cell viability in smooth muscle cell but inhibited NO-induced cell death (Pan et al., 2004). Therefore, the effect of YC-1 on cell viability is dependent on cell types, concentration used and experimental condition.
NF-κB is an essential and ubiquitous transcription factor for the expression of many inflammation-related genes, including iNOS, COX-2, TNF-α, IL-1β and IL-6. LPS has also been reported to activate NF-κB in microglia (Wang et al., 2002; Lee et al., 2004). Here, we confirm that LPS significantly activated NF-κB in BV-2 microglia. It is well established that the nuclear accumulation of NF-κB relies in large part upon Ikappa B kinase-dependent phosphorylation and subsequent degradation of the cytosolic inhibitor, IκBα. LPS treatment in BV-2 microglia led to a rapid degradation of IκBα and nuclear translocation of p65. Previous reports have shown that YC-1 inhibits NF-κB activation in several cell types. High concentrations of YC-1 inhibited NF-κB activation and induced apoptosis in human prostate cancer cells (Huang et al., 2005). YC-1 inhibited cytokine release and NF-κB activation in endotoxemic mouse models (Pan et al., 2005). Our results show that the signaling pathways of NF-κB activated by LPS were also inhibited by YC-1. Immunocytochemistry consistently shows that YC-1 alone did not affect the translocation of p65 but inhibited the nuclear translocation of NF-κB induced by LPS. In parallel to the rapid movement of p65 into the nuclear compartment, the decrease in cytosolic p65 was also observed after LPS application. The BV-2 cell line, as shown previously, exhibits morphological and functional properties of microglial cells (Blasi et al., 1990). BV-2 cells have a round shape in resting state, but display morphological changes after stimulation with LPS or granulocyte–macrophage colony stimulating factor (Suzumura et al., 1990; Laurenzi et al., 2001). As shown in Figure 5b, cells began to have spindle shape and a few thin cytoplasmatic processes after LPS treatment. YC-1 pretreatment effectively decreased morphological change in response to LPS. Therefore, inhibition of NF-κB translocation might be the downstream target by which YC-1 exerts an anti-inflammatory effect in microglia.
It is well known that a marked elevation of the cGMP levels can be achieved by YC-1 through the potentiation of sGC (Wu et al., 1995; Friebe and Koesling, 1998) or by the inhibition of phosphodiesterase activity (Galle et al., 1999). However, many studies have shown that several effects of YC-1 may result from acting through a cGMP/PKG-independent pathway. Liu et al. (2006) showed that YC-1 inhibited neointima formation in balloon-injured carotid artery not through a cGMP-elevating pathway. It has also been reported that inhibition of proinflammatory cytokine production by YC-1 in human leukocytes or neutrophil was not through a cGMP-elevating pathway (Hwang et al., 2003; Pan et al., 2005). Wang et al. (2005) showed that YC-1 induced antiproliferative effects in HA22T cells also via a cGMP-independent pathway. In addition, Garthwaite et al. (2002) reported that the powerful axonoprotective action of YC-1 is unrelated to its activity on sGC but is explained by a novel action on voltage-dependent Na+ channels. We thus examined the role of cGMP/PKG in the inhibitory action of YC-1 on BV-2 microglia. As shown in Figure 6, sGC inhibitor ODQ and PKG inhibitors of KT5823 and Rp-8-Br-PET-cGMPS did not antagonize the inhibitory action of YC-1 on LPS-induced NF-κB activation as well as the induction of iNOS and COX-2. Even if the higher concentrations of ODQ (up to 200 μM) and Rp-8-Br-PET-cGMPS (up to 50 μM) were used, they still did not antagonize the inhibitory effect of YC-1. The PKG inhibitor KT5823 alone was reported to inhibit LPS-induced iNOS expression in primary glial cells (Choi et al., 1999). In addition, LPS-induced production of TNF-α, IL-1β, and NO were also inhibited by ODQ and KT5823 in glial cells (Choi et al., 2002). Therefore, YC-1 may not act through a cGMP/PKG-dependent pathway to reduce inflammation in microglia.
YC-1 is lipid soluble and able to reach the central nervous system. We previously found that YC-1 can enhance learning and memory in water-maze task (Chien et al., 2005). It has been previously reported that intravenous injection of YC-1 (5 mg kg−1 or 1.2 μmol kg−1) induces a hypotensive response in male Wistar Kyoto rats and Lewis rats (Rothermund et al., 2000; Foresti et al., 2004). We used the noninvasive computerized tail-cuff system to measure blood pressure in rats and found that the intraperitoneal injection of YC-1, up to 10 mg kg−1, did not exert significant effects on blood pressure and heart rate. Therefore, the hemodynamic effect of YC-1 is dependent on the route of administration. Here, we further found that YC-1 can inhibit LPS-induced production of iNOS and COX-2 as well as NF-κB activation in microglia. Our preliminary results also show that intranigral or systemic application of YC-1 can inhibit LPS-induced dopaminergic neuronal death (data not shown). The direct protective effect on blood–brain barrier and neurons also cannot be excluded. These results indicate that YC-1 may be developed as a neuroprotective agent.
Acknowledgments
This work was supported by research grants form the National Science Council of Taiwan.
Abbreviations
- DMSO
dimethyl sulfoxide
- L-NAME
Nω-nitro-L-arginine methyl ester hydrochloride
- LPS
lipopolysaccharide
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF-κB
nuclear factor-kappa B
- NO
nitric oxide
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PD
Parkinson's disease
- PDTC
pyrrolidine dithiocarbamate
- PGE2
prostaglandin E2
- PVDF
polyvinylidene difluoride
- ROS
reactive oxygen species
- Rp-8-Br-PET-cGMPS
Rp-β-phenyl-1,N2-etheno-8-bromoguanosine 3,5-cyclic monophosphorothioate
- YC-1
3-(5-hydroxymethyl-2-furyl)-1-benzyl indazole
Conflict of interest
The authors state no conflict of interest.
References
- Araki E, Forster C, Dubinsky JM, Ross ME, Iadecola C. Cyclooxygenase-2 inhibitor ns-398 protects neuronal cultures from lipopolysaccharide-induced neurotoxicity. Stroke. 2001;32:2370–2375. doi: 10.1161/hs1001.096057. [DOI] [PubMed] [Google Scholar]
- Arimoto T, Bing G. Up-regulation of inducible nitric oxide synthase in the substantia nigra by lipopolysaccharide causes microglial activation and neurodegeneration. Neurobiol Dis. 2003;12:35–45. doi: 10.1016/s0969-9961(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- Boulet I, Ralph S, Stanley E, Lock P, Dunn AR, Green SP, et al. Lipopolysaccharide- and interferon-gamma-induced expression of hck and lyn tyrosine kinases in murine bone marrow-derived macrophages. Oncogene. 1992;7:703–710. [PubMed] [Google Scholar]
- Chen CC, Wang JK, Chen WC, Lin SB. Protein kinase C beta mediates lipopolysaccharide-induced nitric-oxide synthase expression in primary astrocytes. J Biol Chem. 1998;273:19424–19430. doi: 10.1074/jbc.273.31.19424. [DOI] [PubMed] [Google Scholar]
- Chien WL, Liang KC, Teng CM, Kuo SC, Lee FY, Fu WM. Enhancement of learning behaviour by a potent nitric oxide-guanylate cyclase activator YC-1. Eur J Neurosci. 2005;21:1679–1688. doi: 10.1111/j.1460-9568.2005.03993.x. [DOI] [PubMed] [Google Scholar]
- Choi SH, Choi DH, Song KS, Shin KH, Chun BG. Zaprinast, an inhibitor of cGMP-selective phosphodiesterases, enhances the secretion of TNF-alpha and IL-1 beta and the expression of iNOS and MHC class II molecules in rat microglial cells. J Neurosci Res. 2002;67:411–421. doi: 10.1002/jnr.10102. [DOI] [PubMed] [Google Scholar]
- Choi SH, Shin KH, Kang SW, Chun YS, Chun BG. Guanosine 5′,3′-cyclic monophosphate enhances lipopolysaccharide-induced nitric oxide synthase expression in mixed glial cell cultures of rat. Neurosci Lett. 1999;276:29–32. doi: 10.1016/s0304-3940(99)00783-1. [DOI] [PubMed] [Google Scholar]
- Ferrero R, Torres M. Prolonged exposure to YC-1 induces apoptosis in adrenomedullary endothelial and chromaffin cells through a cGMP-independent mechanism. Neuropharmacology. 2001;41:895–906. doi: 10.1016/s0028-3908(01)00131-9. [DOI] [PubMed] [Google Scholar]
- Foresti R, Hammad J, Clark JE, Johnson TR, Mann BE, Friebe A, et al. Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Br J Pharmacol. 2004;142:453–460. doi: 10.1038/sj.bjp.0705825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe A, Koesling D. Mechanism of YC-1-induced activation of soluble guanylyl cyclase. Mol Pharmacol. 1998;53:123–127. doi: 10.1124/mol.53.1.123. [DOI] [PubMed] [Google Scholar]
- Galle J, Zabel U, Hubner U, Hatzelmann A, Wagner B, Wanner C, et al. Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br J Pharmacol. 1999;127:195–203. doi: 10.1038/sj.bjp.0702495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite G, Goodwin DA, Neale S, Riddall D, Garthwaite J. Soluble guanylyl cyclase activator YC-1 protects white matter axons from nitric oxide toxicity and metabolic stress, probably through Na(+) channel inhibition. Mol Pharmacol. 2002;61:97–104. doi: 10.1124/mol.61.1.97. [DOI] [PubMed] [Google Scholar]
- Hsiao G, Huang HY, Fong TH, Shen MY, Lin CH, Teng CM, et al. Inhibitory mechanisms of YC-1 and PMC in the induction of iNOS expression by lipoteichoic acid in RAW 264.7 macrophages. Biochem Pharmacol. 2004;67:1411–1419. doi: 10.1016/j.bcp.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Huang YT, Pan SL, Guh JH, Chang YL, Lee FY, Kuo SC, et al. YC-1 suppresses constitutive nuclear factor-kappaB activation and induces apoptosis in human prostate cancer cells. Mol Cancer Ther. 2005;4:1628–1635. doi: 10.1158/1535-7163.MCT-05-0090. [DOI] [PubMed] [Google Scholar]
- Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y, et al. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience. 1996;72:355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- Hwang TL, Hung HW, Kao SH, Teng CM, Wu CC, Cheng SJ. Soluble guanylyl cyclase activator YC-1 inhibits human neutrophil functions through a cGMP-independent but cAMP-dependent pathway. Mol Pharmacol. 2003;64:1419–1427. doi: 10.1124/mol.64.6.1419. [DOI] [PubMed] [Google Scholar]
- Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson's disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16:724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994;84:4226–4233. [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lau CK, Yang ZF, Lam CT, Tam KH, Poon RT, Fan ST. Suppression of hypoxia inducible factor-1alpha (HIF-1alpha) by YC-1 is dependent on murine double minute 2 (Mdm2) Biochem Biophys Res Commun. 2006;348:1443–1448. doi: 10.1016/j.bbrc.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Laurenzi MA, Arcuri C, Rossi R, Marconi P, Bocchini V. Effects of microenvironment on morphology and function of the microglial cell line BV-2. Neurochem Res. 2001;26:1209–1216. doi: 10.1023/a:1013911205494. [DOI] [PubMed] [Google Scholar]
- Le W, Rowe D, Xie W, Ortiz I, He Y, Appel SH. Microglial activation and dopaminergic cell injury: an in vitro model relevant to Parkinson's disease. J Neurosci. 2001;21:8447–8455. doi: 10.1523/JNEUROSCI.21-21-08447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Yune TY, Kim SJ, Kim YC, Oh YJ, Markelonis GJ, et al. Minocycline inhibits apoptotic cell death via attenuation of TNF-alpha expression following iNOS/NO induction by lipopolysaccharide in neuron/glia co-cultures. J Neurochem. 2004;91:568–578. doi: 10.1111/j.1471-4159.2004.02780.x. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Liu YN, Pan SL, Peng CY, Guh JH, Huang DM, Chang YL, et al. YC-1 [3-(5′-hydroxymethyl-2′-furyl)-1-benzyl indazole] inhibits neointima formation in balloon-injured rat carotid through suppression of expressions and activities of matrix metalloproteinases 2 and 9. J Pharmacol Exp Ther. 2006;316:35–41. doi: 10.1124/jpet.105.090563. [DOI] [PubMed] [Google Scholar]
- Lu DY, Liou HC, Tang CH, Fu WM. Hypoxia-induced iNOS expression in microglia is regulated by the PI3-kinase/Akt/mTOR signaling pathway and activation of hypoxia inducible factor-1alpha. Biochem Pharmacol. 2006;72:992–1000. doi: 10.1016/j.bcp.2006.06.038. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Levi G. Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog Neurobiol. 1998;54:99–125. doi: 10.1016/s0301-0082(97)00052-x. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Walsh DT, Levi G, Perry VH. In vivo expression of cyclooxygenase-2 in rat brain following intraparenchymal injection of bacterial endotoxin and inflammatory cytokines. J Neuropathol Exp Neurol. 1999;58:1184–1191. doi: 10.1097/00005072-199911000-00008. [DOI] [PubMed] [Google Scholar]
- Pan SL, Guh JH, Chang YL, Kuo SC, Lee FY, Teng CM. YC-1 prevents sodium nitroprusside-mediated apoptosis in vascular smooth muscle cells. Cardiovasc Res. 2004;61:152–158. doi: 10.1016/j.cardiores.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Pan SL, Guh JH, Peng CY, Chang YL, Cheng FC, Chang JH, et al. A potential role of YC-1 on the inhibition of cytokine release in peripheral blood mononuclear leukocytes and endotoxemic mouse models. Thromb Haemost. 2005;93:940–948. doi: 10.1160/TH04-03-0195. [DOI] [PubMed] [Google Scholar]
- Rothermund L, Friebe A, Paul M, Koesling D, Kreutz R. Acute blood pressure effects of YC-1-induced activation of soluble guanylyl cyclase in normotensive and hypertensive rats. Br J Pharmacol. 2000;130:205–208. doi: 10.1038/sj.bjp.0703320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A, Sawada M, Yamamoto H, Marunouchi T. Effects of colony stimulating factors on isolated microglia in vitro. J Neuroimmunol. 1990;30:111–120. doi: 10.1016/0165-5728(90)90094-4. [DOI] [PubMed] [Google Scholar]
- Teismann P, Vila M, Choi DK, Tieu K, Wu DC, Jackson-Lewis V, et al. COX-2 and neurodegeneration in Parkinson's disease. Ann NY Acad Sci. 2003;991:272–277. doi: 10.1111/j.1749-6632.2003.tb07482.x. [DOI] [PubMed] [Google Scholar]
- Wang MJ, Lin WW, Chen HL, Chang YH, Ou HC, Kuo JS, et al. Silymarin protects dopaminergic neurons against lipopolysaccharide-induced neurotoxicity by inhibiting microglia activation. Eur J Neurosci. 2002;16:2103–2112. doi: 10.1046/j.1460-9568.2002.02290.x. [DOI] [PubMed] [Google Scholar]
- Wang SW, Pan SL, Guh JH, Chen HL, Huang DM, Chang YL, et al. YC-1 [3-(5′-hydroxymethyl-2′-furyl)-1-benzyl indazole] exhibits a novel antiproliferative effect and arrests the cell cycle in G0-G1 in human hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2005;312:917–925. doi: 10.1124/jpet.104.077230. [DOI] [PubMed] [Google Scholar]
- Wu CC, Ko FN, Kuo SC, Lee FY, Teng CM. YC-1 inhibited human platelet aggregation through NO-independent activation of soluble guanylate cyclase. Br J Pharmacol. 1995;116:1973–1978. doi: 10.1111/j.1476-5381.1995.tb16400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]