Abstract
Background and purpose:
The small and intermediate conductance, Ca2+-sensitive K+ channels (SKCa and IKCa, respectively) which are pivotal in the EDHF pathway may be differentially activated. The importance of caveolae in the functioning of IKCa and SKCa channels was investigated.
Experimental approach:
The effect of the caveolae-disrupting agent methyl-β-cyclodextrin (MβCD) on IKCa and SKCa localization and function was determined.
Key results:
EDHF-mediated, SKCa-dependent myocyte hyperpolarizations evoked by acetylcholine in rat mesenteric arteries (following blockade of IKCa with TRAM-34) were inhibited by MβCD. Hyperpolarizations evoked by direct SKCa channel activation (using NS309 in the presence of TRAM-34) were also inhibited by MβCD, an effect reversed by cholesterol. In contrast, IKCa-dependent hyperpolarizations (in the presence of apamin) were unaffected by MβCD. Similarly, in porcine coronary arteries, EDHF-mediated, SKCa-dependent (but not IKCa-dependent) endothelial cell hyperpolarizations evoked by substance P were inhibited by MβCD. In mesenteric artery homogenates subjected to sucrose-density centrifugation, caveolin-1 and SK3 (SKCa) proteins but not IK1 (IKCa) protein migrated to the buoyant, caveolin-rich fraction. MβCD pretreatment redistributed caveolin-1 and SK3 proteins into more dense fractions. In immunofluorescence images of porcine coronary artery endothelium, SK3 (but not IK1) and caveolin-1 were co-localized. Furthermore, caveolin-1 immunoprecipitates prepared from native porcine coronary artery endothelium contained SK3 but not IK1 protein.
Conclusions and Implications:
These data provide strong evidence that endothelial cell SKCa channels are located in caveolae while the IKCa channels reside in a different membrane compartment. These studies reveal cellular organisation as a further complexity in the EDHF pathway signalling cascade.
Keywords: caveolae, EDHF, calcium-activated potassium channels, NS309, apamin, TRAM-34
Introduction
The endothelium-dependent hyperpolarization and relaxation of vascular myocytes that is independent of the release of nitric oxide and prostacyclin from the vascular endothelium has been widely investigated. In both rat mesenteric and pig coronary arteries, a pivotal feature of this phenomenon, known widely as the endothelium-derived hyperpolarizing factor (EDHF) response, is the opening of endothelial small- and intermediate-conductance Ca2+-sensitive potassium channels (SKCa and IKCa respectively; Busse et al., 2002; Félétou and Vanhoutte, 2006). Complete inhibition of such endothelium-dependent hyperpolarization (and associated vasorelaxation) triggered by ligands like acetylcholine (ACh), bradykinin and substance P (Alexander et al., 2007) can often be achieved by blockade of both IKCa and SKCa (Busse et al., 2002). However, particularly in coronary and renal vascular beds, the sometimes additional involvement of endothelium-derived epoxyeicosatrienoic acids (EETs) necessitates the supplementary use of an EET-receptor antagonist (Gauthier et al., 2002; Weston et al., 2005b) or of iberiotoxin. The toxin blocks the myocyte large-conductance Ca2+-sensitive potassium channels (BKCa) that are opened by the EETs and which contribute to the observed vasorelaxation in these beds (Weston et al., 2005b).
In a study using pig coronary arteries (Graziani et al., 2004), it was shown that caveolae might play a role in the bradykinin-activated EDHF response that in these arteries involves not only the activation of SKCa and IKCa channels but also the release of EETs (Weston et al., 2005b). In these vessels, exposure to methyl β-cyclodextrin (MβCD), an agent known to disrupt caveolae and caveolae-dependent signalling, resulted in a substantial reduction of bradykinin-induced vasorelaxations. Graziani et al. (2004) concluded that cholesterol depletion by MβCD had disrupted the integrity of caveolin-rich domains in endothelial cells. They measured an associated reduction in the activity of phospholipase A2, a key enzyme in the generation of EETs from arachidonic acid.
The objective of the present study was to extend these observations in coronary vessels and to focus on the possible role of caveolin-rich domains in the SKCa- and IKCa-mediated components of the EDHF pathway in both porcine coronary and rat mesenteric artery. ACh was used to stimulate this pathway in the rat mesenteric artery, a vessel in which EETs are not involved in the EDHF response (Fukao et al., 1997; Vanheel and Van de Voorde, 1997). In parallel experiments, the EDHF pathway in the pig coronary artery was activated using substance P, which, unlike bradykinin (Fisslthaler et al., 1999), does not produce EETs in these vessels. Instead, substance P generates an EDHF response that only involves the opening of SKCa and IKCa channels (Weston et al., 2005b).
Methods
Animals
Experiments were performed on second- and third-order mesenteric artery branches dissected from male Wistar rats (body weight 280–360 g) previously killed by stunning and cervical dislocation in compliance with Schedule 1 of the UK Animals (Scientific Procedures) Act 1986. Pig hearts were obtained from a local abattoir and transported on ice in Krebs solution (mM; 118 NaCl, 3.4 KCl, 1.0 CaCl2, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 glucose, gassed with 5% CO2, 95% O2) before dissecting left anterior descending coronary arteries. An identical Krebs solution was used for the rat mesenteric artery studies.
Microelectrode recordings
Membrane potential recordings were performed on intact rat mesenteric artery myocytes and porcine coronary artery endothelial cells as described previously (Weston et al., 2005a, 2005b) in the absence of vasoconstrictor drugs. Tissues were constantly superfused with Krebs solution containing 10 μM indomethacin plus 300 μM Nω-nitro-L-arginine (L-NA) at 37°C and gassed with 95% O2, 5% CO2. To deplete tissues of cholesterol, 5 mM MβCD was added to the Krebs solution. To restore cholesterol following depletion, the water-soluble cholesterol-PEG 600 (5 mM) was included with the MβCD, as described elsewhere (Shaw et al., 2006).
Pressure myography
Second-order mesenteric arteries (approximately 300–350 μm diameter) of 1–2 mm length were cannulated and mounted in a pressure myograph (Living Systems Instrumentation, Burlington, VT, USA) as described previously (Izzard et al., 1996). Intraluminal pressure was held at 70 mm Hg and the superfusing Krebs solution contained 300 μM L-NA and 10 μM indomethacin. When appropriate, apamin or 1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole (TRAM-34) was added to both the superfusate and the luminal solutions. Arteries were preconstricted with an approximate EC50 concentration of phenylephrine (5–30 nM). Relaxations to ACh were assessed before and after 45 min incubation with 5 mM MβCD.
Co-localization analysis
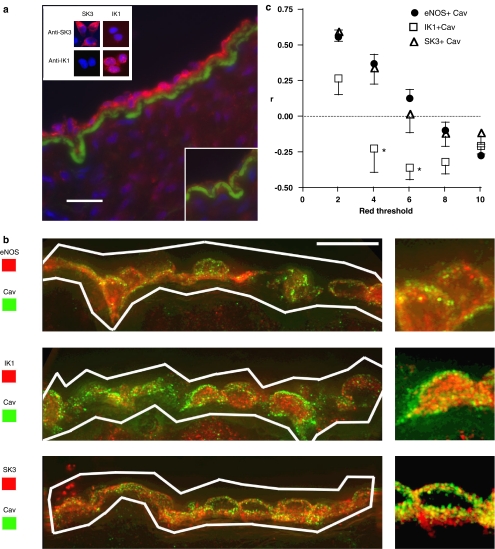
Dual labelling of porcine coronary artery sections was achieved using mouse anti-caveolin 1 monoclonal and rabbit anti-SK3, IK1 or eNOS polyclonal primary antibodies with subsequent incubation with species-specific, highly cross-absorbed secondary antibodies. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) and the internal elastic lamina was visualized using its intrinsic green autofluorescence. Sections were imaged by deconvolution microscopy (DeltaVision system, Issaquah, WA, USA; Applied Precision, USA) in the University of Manchester Imaging Facility. Co-localization analysis of proteins within the endothelium was performed using custom Matlab functions to calculate Pearson's correlation coefficient (r), a value between −1 and +1. A value of +1 indicated a direct and −1 an inverse relationship between the red and green intensity of a pixel (x and y), with ‘0' signifying a random distribution.
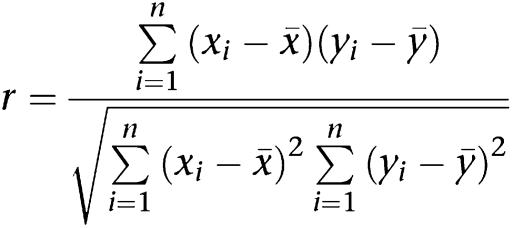 |
A region-of-interest covering the endothelium was defined manually in image stacks (25 slices). Background signal was removed by assigning separate red and green intensity thresholds and excluding pixels with below-threshold intensity in both colour channels. Green signal (caveolin labelling) was similar for all images and the threshold value was set at 2 times the maximum intensity present in unlabelled negative control images. A single threshold was inappropriate for the red signal, which represented anti-SK3, IK1 or eNOS immunoreactivity and so a range of thresholds (2, 4, 6, 8 and 10 times the maximum negative control intensity) was used for each analysis.
Sucrose density gradient ultracentrifugation and Western blotting
Rat mesenteric arteries were subjected to sucrose density gradient ultracentrifugation and Western blotting as described previously (Weston et al., 2005a). In initial experiments, the centrifuged sucrose gradient was divided into 13 equal-volume fractions and each fraction analysed by Western blot for the marker proteins caveolin-1 and β-adaptin. Subsequently, fractions were pooled as fraction 1 (F1), fractions 2–5 (caveolin-rich, C), fractions 6–9 (non-caveolin 1, NC1) and fractions 10–13 (non-caveolin 2, NC2), identical to the previous study (Weston et al., 2005a).
Immunoprecipitation
Immunoprecipitation using Dynabeads coupled to goat anti-mouse IgG antibodies was performed by the method of Oh and Schnitzer (1999), except that samples of porcine coronary artery endothelium were prepared in extraction buffer (20 mM Tris, pH 7.5, 250 mM sucrose, 5 mM EDTA, 10 mM EGTA, protease-inhibitor cocktail; P2714, Sigma-Aldrich, Gillingham, UK) and diluted with an equal volume of Tris-buffered saline before homogenization. Samples were analysed by Western blotting as described previously (Burnham et al., 2006b).
Statistics
Data, expressed as mean±s.e.m., were analysed using analysis of variance (ANOVA) (GraphPad Prism software) followed by a Bonferroni post-test, where applicable. A value of P<0.05 was considered to be significant.
Drugs and solutions
Antibodies used were anti-SK3 (APC-025; Alomone Laboratories, Jerusalem, Israel), anti-IK1 (a kind gift of Dr M Chen, GlaxoSmithKline), anti-caveolin 1 mAb (clone 2234; BD Transduction Laboratories, San Diego, CA, USA), anti-caveolin 1 pAb (sc-894, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-eNOS (610298, BD Transduction Laboratories). Secondary antibodies were Cy3-conjugated anti-rabbit (Jackson ImmunoResearch, Cambridge, UK) and Alexa 488-conjugated anti-mouse (Molecular Probes, Eugene, OR, USA). Apamin was supplied by Latoxan. TRAM-34 (1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole) and TRAM-39 (2-(2-chlorophenyl)-2,2-diphenylacetonitrile) were kind gifts of Dr H Wulff (UC Davis School of Medicine, CA, USA). NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) was kindly donated by Dr P Christophersen, NeuroSearch A/S. Cholesterol-PEG 600 (C1145) and all other chemicals were from Sigma-Aldrich (UK).
Results
Rat mesenteric artery microelectrode studies
The smooth-muscle membrane potential in segments of rat mesenteric artery with an intact endothelium was −54.1±0.2 mV (n=8) and was unchanged in the presence of 100 nM apamin (−54.3±0.2 mV; n=4) or 10 μM TRAM-34 (−54.0±0.2 mV; n=4). In the presence of 100 nM apamin (to block endothelial SKCa channels and reveal responses mediated by IKCa channels), the peak hyperpolarizations to 1 and 3 μM ACh were similar before (8.7±0.6 and 15.0±0.5 mV, respectively, n=4) and after 30 min exposure to 5 mM MβCD (10.1±0.6 and 14.9±0.3 mV, respectively, n=4, Figure 1a). The residual ACh-induced hyperpolarizations in the continued presence of apamin and MβCD were abolished by 10 μM TRAM-34, indicating that they were due to the opening of IKCa channels (n=4; data not shown).
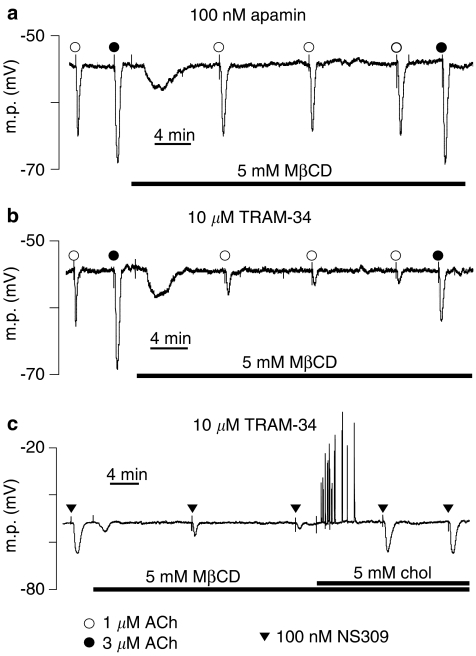
Figure 1.
Typical traces showing the effect of MβCD on the SKCa and IKCa-induced myocyte hyperpolarizations in rat mesenteric artery segments. MβCD had no effect on (a, presence of apamin) the IKCa component of the response to ACh (b, presence of TRAM-34) but markedly reduced the SKCa component. (c, presence of TRAM-34) Similarly, the SKCa component of the hyperpolarization to NS 309 was also reduced by MβCD, an effect that was reversed by simultaneous exposure to cholesterol (chol).
In the presence of 10 μM TRAM-34 (to block endothelial IKCa channels and reveal responses mediated by SKCa channels), peak hyperpolarizations to both 1 and 3 μM ACh (8.2±0.4 and 14.6±0.5 mV, respectively, n=4) were similar to those in the presence of 100 nM apamin. In contrast, however, both were significantly reduced by 30 min MβCD treatment (2.3±0.2 and 6.1±0.5 mV, respectively; P<0.001, two-way ANOVA n=4, Figure 1b).
To determine whether the inhibitory effects of MβCD on ACh-induced, SKCa-mediated hyperpolarizations (Figure 1b) somehow involved an action on the endothelial muscarinic receptors, vessels were exposed to 100 nM NS309 (a direct opener of both IKCa and SKCa channels; Strøbæk et al., 2004) in the presence of the IKCa blocker TRAM-34 (10 μM). Under these conditions, NS309 hyperpolarized mesenteric artery myocytes by 13.2±0.3 mV (n=4). MβCD (5 mM) time dependently reduced this hyperpolarization (to 7.4±0.9 mV after 15 min and to 3.9±0.5 mV after 30 min, n=4), effects that were reversed by subsequent inclusion of 5 mM cholesterol-PEG 600 (hyperpolarizations to NS309 were 11.2±0.5 mV after 10 min and 13.9±0.1 mV after 20 min exposure to cholesterol-PEG 600 in the presence of MβCD, n=4, Figure 1c).
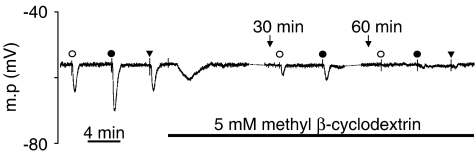
A further series of experiments was undertaken to determine whether the small residual SKCa-dependent hyperpolarizations recorded after 30 min MβCD treatment represented a genuine MβCD-resistant component of the response or were the result of the relatively short exposure to MβCD. Responses to 1 and 3 μM ACh in the presence of 10 μM TRAM-34 were recorded before MβCD treatment and again in the same arteries after 30 and 60 min incubation with 5 mM MβCD (n=4; Figure 2). Before MβCD treatment, ACh (1 and 3 μM) produced hyperpolarizations of 8.3±1.0 and 16.3±1.4 mV, respectively. These responses were reduced to 2.3±0.3 and 7.2±1.4 mV after 30 min treatment and essentially abolished after 60 min exposure to MβCD (0.0±0.0 and 1.5±0.6 mV). In two of these experiments, responses to 100 nM NS309 were also determined. The mean hyperpolarization of 9.3 mV recorded before MβCD treatment was reduced to 0.7 mV after 60 min.
Figure 2.
Typical trace showing the effect of prolonged MβCD treatment on SKCa-dependent responses in rat mesenteric artery. Myocyte membrane potential was continuously recorded in the presence of TRAM-34. Responses to ACh were reduced after 30 min treatment with MβCD and these residual responses were essentially abolished by a further 30 min incubation with MβCD, as were responses to NS 309. Symbols used are identical to those in Figure 1. The broken lines in the membrane potential record indicate where a portion has been omitted for clarity; the cell remained impaled by the electrode.
Porcine coronary artery microelectrode studies
To extend the data to more than one species and, additionally, to exclude the possibility that MβCD exerted its effects on the coupling between endothelium and smooth muscle, membrane potential recordings were obtained directly from the endothelium of porcine coronary arteries. The mean basal membrane potential of porcine coronary artery endothelial cells before the start of experimentation was −51.2±4.1 mV (n=8). In the presence of 100 nM apamin to block endothelial SKCa channels, their resting membrane potential was −50.6±0.3 mV (n=4). Control hyperpolarizations were first recorded to 100 nM substance P (21.3±0.6 mV); on superfusion with 1 mM MβCD, responses to substance P were not significantly different from controls after 10 min (20.4±1.0 mV), 20 min (19.9±1.0 mV) or 30 min (20.2±0.9 mV), whereas additional application of 10 μM TRAM-39 abolished substance P responses (0.3±0.1 mV; n=3).
The above protocol was repeated in fresh tissue segments (n=4) with 10 μM TRAM-39 (in place of apamin) to block IKCa channels leaving only SKCa channels available for activation by substance P. From a basal membrane potential of −50.4±0.1 mV, mean control hyperpolarizations of endothelial cells to substance P were 20.0±1.9 mV. On superfusion with MβCD, substance P responses were significantly reduced after 10 min (13.2±3.6 mV) and further reduced at 20 (7.3±1.7 mV) and 30 min (6.3±2.0 mV). Subsequent application of 100 nM apamin in the continuing presence of MβCD effectively abolished the residual substance P hyperpolarizations (0.2±0.0 mV; n=3).
Pressure myography
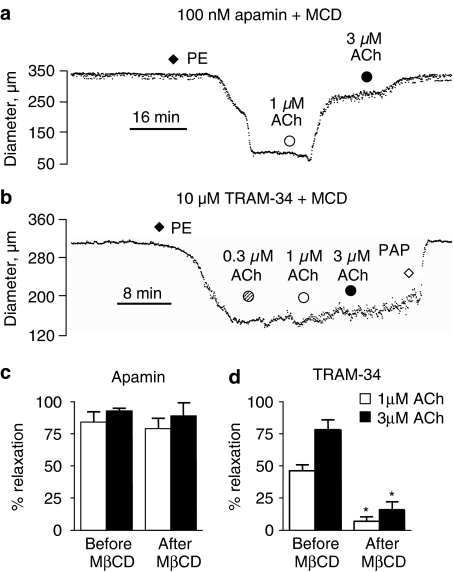
The mean diameter of rat mesenteric arteries used for pressure myography was 326±15 μm (n=16). In the presence of 100 nM apamin to block SKCa channels, 1 and 3 μM ACh relaxed a phenylephrine-induced contraction by 84±8 and 93±2%, respectively (n=4). Following 5 mM MβCD treatment for 40 min, relaxations to 1 and 3 μM ACh were unaffected (79±8 and 89±10%, respectively, n=4, Figure 3a). In separate artery segments in the presence of 10 μM TRAM-34 to block IKCa channels, 1 and 3 μM ACh relaxed phenylephrine contractions by 46±5 and 78±8%, respectively (n=4). After MβCD treatment for 40 min, relaxations to both 1 and 3 μM ACh were significantly reduced (to 7±3 and 16±6%, respectively; two-way ANOVA, P<0.001, n=4, Figure 3b).
Figure 3.
Typical traces showing the effect of MβCD on IKCa- and SKCa-dependent vasorelaxation in rat mesenteric arteries. Arteries were mounted on a pressure myograph and pressurized to 70 mm Hg. Arteries were pre-incubated for 45 min with 5 mM MβCD and either (a) 100 nM apamin or (b) 10 μM TRAM-34. Arteries were constricted with 5–30 nM phenylephrine (PE) and 0.3–3 μM ACh applied. Full relaxation was induced with 100 μM papaverine. Mean data showing relaxations to ACh in the presence of (c) apamin and (d) TRAM-34 before and after incubation with MβCD (n=4).
Sucrose-density gradient fractionation
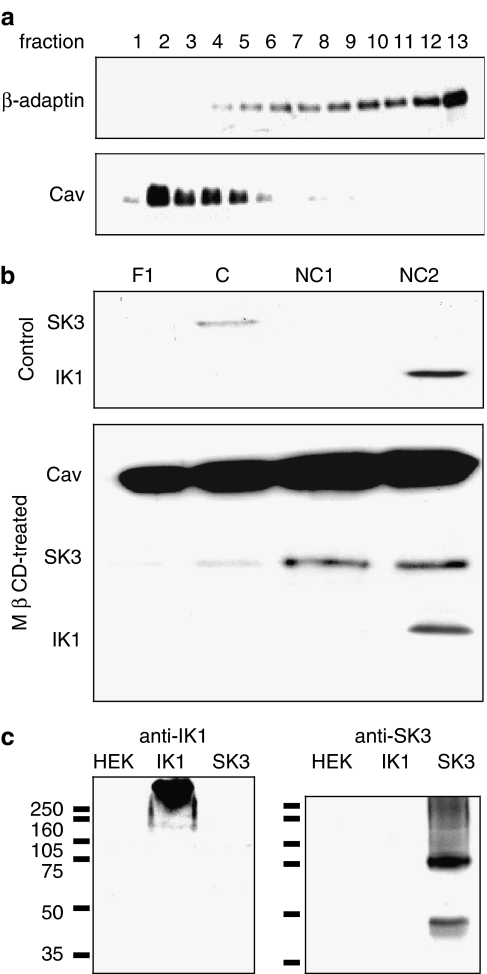
In the present study, the distribution of the proteins caveolin-1 (caveolae-associated marker) and β-adaptin (non-caveolae marker; Sampson et al., 2004) were determined in the unpooled sucrose gradient fractions and a clear differentiation was observed (Figure 4a). When the fractions, pooled as described in the Methods section, were analysed for SK3 and IK1 protein, a distinct pattern was observed under control conditions (Figure 4b), as previously reported (Weston et al., 2005a). The SK3 protein migrated with caveolin-1 into the lipid-rich fractions, whereas IK1 was retained in the dense, non-caveolin fractions. However, when rat mesenteric arteries were pretreated with 5 mM MβCD for 30 min, significant amounts of both caveolin and SK3 protein were present in all fractions, but predominantly in the higher-density fractions rather than the caveolin-rich (C) fraction only. In contrast, MβCD treatment failed to affect the distribution of IK1 protein, which remained predominantly in the high-density fractions (Figure 4b). To demonstrate antibody specificity, Western blotting experiments were performed with lysates of transfected human embryonic kidney (HEK) cells and these experiments confirmed that anti-SK3 and -IK1 immunoreactivity was limited to samples of SK3- and IK1-transfected cells, respectively (Figure 4c). Aggregation of subunit proteins may underlie the high molecular weight smearing observed (Soulie et al., 1996).
Figure 4.
(a) Sucrose density gradient fractions analysed by Western blot for the presence of caveolin-1 (Cav) and β-adaptin protein (a non-caveolae marker). Subsequently, fractions were pooled as fraction 1 (F1), fractions 2–5 (caveolin-rich, C), fractions 6–9 (non-caveolin 1, NC1) and fractions 10–13 (non-caveolin 2, NC2). (b) Effect of MβCD on SK3, IK1 and caveolin-1 protein distribution. Arteries were incubated with 5 mM MβCD for 30 min (lower panel) or left untreated (upper panel) before subjecting the resultant sucrose density gradient fractions to Western blotting. Note the migration of the SK3 and caveolin proteins into the NC fractions on MβCD treatment. (c) Western blot analysis showing the specificity of the anti-IK1 and anti-SK3 antibodies used in the sucrose density gradient experiments shown in (b) above. Note that each antibody recognizes only protein in HEK cells transfected with the appropriate subunit and does not react with any proteins in either untransfected HEK cells or those transfected with the alternative subunit. Molecular weight markers (kDa) are indicated. Images representative of three separate experiments are shown.
Deconvolution microscopy and image analysis
Antibody specificity control experiments with HEK cells expressing SK3 or IK1 proteins indicated that both anti-SK3 and -IK1 antibodies labelled only those cells containing the appropriate subunit and that the antibodies did not crossreact with the other subunit or with other HEK cell proteins (Figure 5a). Sections of porcine coronary artery labelled with anti-IK1 antibodies displayed strong endothelial immunoreactivity that was prevented by pre-incubation of the antibody with immunogenic peptide (Figure 5a). Weaker labelling that was not blocked by immunogenic peptide pre-incubation was visible in the smooth muscle. Previous experiments have shown that anti-SK3 immunoreactivity is restricted to the endothelium in this tissue (Burnham et al., 2002).
Figure 5.
(a) Detection and localization of IK1 in sections of porcine coronary artery. Anti-IK1 immunoreactivity (red), nuclei (blue) and internal elastic lamina (green) are shown (representative of n=4 animals). All scale bars are 12.5 μm. Negative control experiment with anti-IK1 antibody pre-incubated with antigenic peptide (inset bottom right). Antibody specificity control data showing HEK cells expressing SK3 and IK1 immunolabelled with anti-SK3 and anti-IK1 antibodies (inset top left; representative of three separate experiments). (b) Deconvolution microscopy images of porcine coronary artery sections co-labelled with anti-caveolin-1 (Cav) and anti-eNOS, anti-IK1 or anti-SK3 antibodies. Low and high power magnification images shown are histogram-equalized z-projections of 25-image stacks. White lines enclose endothelial areas included in the co-localization analysis. (c) Graph of co-localization analysis mean data (eNOS, n=100 images, four animals; SK3, IK1, n=75 images, three animals) showing Pearson's correlation coefficient (r) versus red threshold for anti-eNOS, SK3 or IK1 with anti-caveolin-1 immunoreactivity (r=+1 indicates perfect co-localization, r=0 indicates no correlation and r=−1 indicates perfect negative correlation). (For colour figure see online version.)
Co-localization of caveolin-1 with IK1, SK3 or eNOS proteins within the endothelium was determined by co-labelling of porcine coronary sections followed by deconvolution microscopy and image analysis (Figures 5b and c). Vertical spacing within a 25-image stack was 0.2 μm, indicating that an individual caveola <100 nm in diameter (Gratton et al., 2004) would not appear on consecutive images. Co-localization (Pearson's correlation coefficient r, determined across a range of background thresholds) was positive for caveolin and SK3 and this parameter was not significantly different when calculated for caveolin and eNOS, a protein known to be associated with caveolae (Garcia-Cardena et al., 1996; Shaul et al., 1996). However, caveolin/IK1 co-localization was negative and significantly different from caveolin/eNOS and caveolin/SK3, strongly suggesting that IK1 was not spatially associated with caveolin in the manner of eNOS or SK3.
Immunoprecipitation
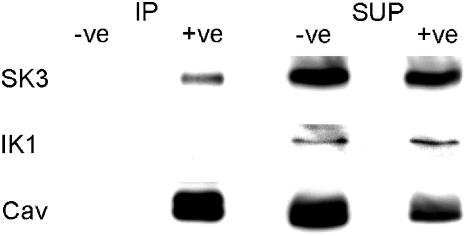
Anti-caveolin-1 immunoprecipitates prepared from homogenates of porcine coronary artery endothelium were analysed by anti-SK3, -IK1 and -caveolin-1 Western blotting (Figure 6). Caveolin-1 protein was robustly detected in immunoprecipitates and this was accompanied by a reduction in caveolin protein in unbound supernatants. Anti-caveolin immunoprecipitates also contained SK3 protein (n=5), whereas IK1 protein was only detected in unbound supernatants (n=3). As a control for non-specific binding, the immunoprecipitation procedures were performed without anti-caveolin-1 antibody and no signal was detected upon Western blotting.
Figure 6.
Anti-SK3, IK1 and caveolin-1 Western blot analysis of anti-caveolin-1 immunoprecipitates prepared from porcine coronary artery endothelium. Immunoprecipitation (IP) was performed with and without anti-caveolin-1 antibody (+ve and −ve, respectively). Supernatants remaining after IP (SUP) were also subjected to Western blot analysis. Note that the SK3 protein co-precipitates with the caveolin-1 protein, whereas the IK1 protein does not. Images are representative of five (SK3) and three (IK1) experiments.
Discussion
The aim of the present study was to investigate the importance of caveolin-rich domains in EDHF-mediated responses involving KCa channels in both rat and porcine blood vessels with the primary objective of learning more about the intracellular organization of the EDHF-signalling cascade. The results show that in both vascular beds, there is a fascinating difference between the location of the endothelial SKCa and IKCa channels that play a pivotal role in the EDHF response. Although the functional evidence is critically dependent on the results obtained with the cholesterol-depleting agent MβCD, these data, when combined with the results from imaging and other biochemical approaches obtained independently of MβCD, all indicate that SKCa channels reside within caveolin-rich lipid domains (almost certainly caveolae) rather than in lipid rafts and that this position within the cell is one that they do not share with IKCa.
Rat mesenteric artery
The general strategy adopted was first to isolate the SKCa and IKCa components of agonist-induced EDHF responses using TRAM-34 or apamin, respectively, and then to employ MβCD as the cholesterol-depleting agent. Thus, in the presence of TRAM-34 and following treatment with MβCD over a 30 min period, the SKCa component of the endothelium-dependent hyperpolarization to ACh became markedly reduced. In contrast, after isolating the ACh-induced, IKCa-mediated hyperpolarization using apamin, exposure to MβCD over a 30 min period had no significant effect on responses to ACh. This finding was reproduced in the pressure myograph experiments in which treatment with MβCD reduced only the SKCa-mediated EDHF-dependent dilation of rat mesenteric arteries.
Such selective impairment by MβCD of the SKCa component of the EDHF response could have resulted if the coupling between muscarinic-receptor activation and SKCa opening involved a mechanism not shared by the muscarinic receptor – IKCa interaction. To clarify this, we employed NS309, an opener of both IKCa and SKCa channels by a mechanism independent of muscarinic receptor activation (Strøbæk et al., 2004). In these experiments, the selective NS309-induced opening of SKCa channels seen in the presence of TRAM-34 was also markedly reduced by MβCD. Thus, the ability of MβCD to modify the SKCa component of both ACh- and NS309-induced KCa channel opening shows that the action of MβCD is not exerted on the muscarinic receptor but rather on the SKCa channel itself. Significantly, the reduction in the SKCa-mediated component was rapidly reversed by the inclusion of cholesterol in the bathing medium. Collectively, these data strongly suggest that cholesterol depletion by MβCD in rat mesenteric vessels selectively impairs the activation of SKCa channels. From this, it can be inferred that these are probably located in caveolae or lipid rafts in marked contrast to the IKCa channels that are not associated with these structures.
Porcine coronary artery
The EDHF response has been widely studied in the porcine coronary artery, often using the local hormone bradykinin to activate the system (Fisslthaler et al., 1999). In this vascular bed, the bradykinin-induced EDHF response is more complex than in some other vessels as this agent not only activates IKCa and SKCa channels but also stimulates the production of 11,12- and 14,15-EET. These then subsequently activate endothelial IKCa and SKCa channels and also BKCa channels on the vascular myocytes (Weston et al., 2005b). To avoid these complexities, substance P acting via NK1 receptors (Alexander et al., 2007) was used to activate the pathway. In porcine coronary arteries, substance P produces an EDHF response which results solely from the opening of IKCa and SKCa channels and which is independent of the production of EETs (Weston et al., 2005b).
Consistent with the findings in the rat mesenteric artery, porcine coronary artery endothelial cell hyperpolarizations to substance P which were elicited through SKCa channels alone (i.e., in the presence of TRAM-39) were attenuated following exposure to MβCD, whereas IKCa-mediated hyperpolarizations (in the presence of apamin) were unaffected. The sustained IKCa-mediated response in the presence of MβCD is a strong indication that substance P receptors were neither desensitized nor uncoupled from downstream effectors during MβCD treatment. In addition, the fact that microelectrode recordings were made from the endothelial cells of these arteries (as opposed to the measurements from myocytes in the rat mesenteric artery study) means that the reduction in SKCa-mediated hyperpolarizations did not reflect an inhibitory action on endothelium–myocyte coupling processes.
Molecular studies
To consolidate the electrophysiological findings, the subcellular distributions of the SKCa and IKCa channel-forming proteins SK3 and IK1, respectively, were investigated. In rat mesenteric arteries treated with MβCD, sucrose-density gradient experiments showed that both caveolin-1 and SK3 immunoreactivities were redistributed across a range of density fractions, whereas in untreated arteries both caveolin-1 and SK3 proteins were restricted to the buoyant caveolin-rich fraction. Thus, it appears that the presence of SK3 in the buoyant fraction may be dependent on intact caveolae and/or lipid rafts, whereas the occurrence of IK1 in denser fractions is cholesterol-independent.
From these studies with MβCD, it can be concluded that membranes rich in cholesterol (e.g., lipid rafts or caveolae) harboured the SK3 protein and that MβCD treatment disrupted these membranes and hence SK3 localization through a cholesterol-depleting mechanism. To delineate more clearly the cellular location of SK3, further studies were conducted using freshly-isolated native endothelial cells from the porcine coronary artery. In deconvolved immunofluorescence images, the co-localization of SK3 and caveolin-1 immunoreactivities occurred to a degree similar to that observed between caveolin-1 and eNOS (a known caveolae-associated protein; Garcia-Cardena et al., 1996; Shaul et al., 1996). Additionally, a physical interaction between SK3 and caveolin-1 was demonstrated by the presence of SK3 in caveolin immunoprecipitates. This close association between SK3 and caveolin is consistent with the localization of SKCa channels within caveolae rather than with lipid rafts. In contrast, IK1 was not physically associated with caveolin-1 and these two proteins apparently occupy distinct membrane compartments. Collectively, these findings are entirely consistent with the electrophysiological data. They strengthen the view that the endothelial SKCa channels so important in the EDHF response are located in caveolae, a location not shared by the IKCa channels.
Conclusions
The findings of the present study have shown that in two dissimilar vascular beds, the endothelial SKCa channels are located in caveolin-rich domains, whereas IKCa is found in a different cellular fraction not associated with either caveolin or MβCD-sensitive lipid rafts. The present results in porcine coronary vessels show the importance of caveolae in generating not only the SKCa component of the EDHF response but, when combined with the results of earlier investigators (Haasemann et al., 1998; Graziani et al., 2004), also in the production of EETs. In the rat mesenteric bed, the different locations of the endothelial SKCa and IKCa channels appear identical to those in the porcine coronary vessels. These differences may go some way to explain the recently-reported selective impairment of the SKCa-mediated component of EDHF responses in the Zucker diabetic fatty rat (Burnham et al., 2006a).
The list of membrane proteins that are associated with caveolin/caveolae is growing rapidly (Frank et al., 2003) and it is thus remarkable that the IK1 protein does not share the same location as the SK3 protein with which it is mechanistically linked in the EDHF cascade. However, it is not alone in this respect as a recent study (Weston et al., 2005a; also in rat and porcine vessels) has shown that the extracellular Ca2+-sensing receptor is also not associated with the caveolin-rich domains. Further studies are in progress to clarify how the IKCa channel, in contrast to SKCa, can function outside a microdomain that is so important in facilitating the interactions between receptors, channels and signalling mechanisms.
Acknowledgments
We acknowledge the support of the University of Aleppo, Syria (MA), the British Heart Foundation (Grants PG/05/058, PG/05/010; MB, GE, AHW) and the Royal Society (GE) for supporting this work. We thank Dr H Wulff (UC Davis School of Medicine, CA) and Dr P Christophersen (NeuroSearch A/S) for TRAM and NS309 compounds, respectively, and the staff of the abattoir (Steve, Alan and Howard).
Abbreviations
- ACh
acetylcholine
- BKCa
large-conductance Ca2+-sensitive potassium channel
- EDHF
endothelium-derived hyperpolarizing factor
- EET
epoxyeicosatrienoic acid
- IKCa
intermediate-conductance Ca2+-sensitive potassium channel
- L-NA
Nω-nitro-L-arginine
- MβCD
methyl β-cyclodextrin
- NS309
6,7-dichloro-1H-indole-2,3-dione 3-oxime
- SKCa
small-conductance Ca2+-sensitive potassium channel
- TRAM-34
1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole
- TRAM-39
2-(2-chlorophenyl)-2,2-diphenylacetonitrile
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA.Guide to receptors and channels (GRAC) Br J Pharmacol 2007150Suppl 1S1–S168.2nd edition (2007 revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham MP, Bychkov R, Félétou M, Richards GR, Vanhoutte PM, Weston AH, et al. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham MP, Johnson IT, Weston AH. Impaired small-conductance Ca2+-activated K+ channel-dependent EDHF responses in Type II diabetic ZDF rats. Br J Pharmacol. 2006a;148:434–441. doi: 10.1038/sj.bjp.0706748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham MP, Johnson IT, Weston AH. Reduced Ca2+-dependent activation of large-conductance Ca2+-activated K+ channels from arteries of Type 2 diabetic Zucker diabetic fatty rats. Am J Physiol. 2006b;290:H1520–H1527. doi: 10.1152/ajpheart.00827.2005. [DOI] [PubMed] [Google Scholar]
- Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor. Where are we now. Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, et al. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Evidence against a role of cytochrome P450-derived arachidonic acid metabolites in endothelium-dependent hyperpolarization by acetylcholine in rat isolated mesenteric artery. Br J Pharmacol. 1997;120:439–446. doi: 10.1038/sj.bjp.0700932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem. 1996;271:27237–27240. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, et al. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004;94:1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- Graziani A, Bricko V, Carmignani M, Graier WF, Groschner K. Cholesterol- and caveolin-rich membrane domains are essential for phospholipase A2-dependent EDHF formation. Cardiovasc Res. 2004;64:234–242. doi: 10.1016/j.cardiores.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Haasemann M, Cartaud J, Muller-Esterl W, Dunia I. Agonist-induced redistribution of bradykinin B2 receptor in caveolae. J Cell Sci. 1998;111:917–928. doi: 10.1242/jcs.111.7.917. [DOI] [PubMed] [Google Scholar]
- Izzard AS, Bund SJ, Heagerty AM. Myogenic tone in mesenteric arteries from spontaneously hypertensive rats. Am J Physiol. 1996;270:H1–H6. doi: 10.1152/ajpheart.1996.270.1.H1. [DOI] [PubMed] [Google Scholar]
- Oh P, Schnitzer JE. Immunoisolation of caveolae with high affinity antibody binding to the oligomeric caveolin cage. Toward understanding the basis of purification. J Biol Chem. 1999;274:23144–23154. doi: 10.1074/jbc.274.33.23144. [DOI] [PubMed] [Google Scholar]
- Sampson LJ, Hayabuchi Y, Standen NB, Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ Res. 2004;95:1012–1018. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, et al. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- Shaw L, Sweeney MA, O'Neill SC, Jones CJ, Austin C, Taggart MJ. Caveolae and sarcoplasmic reticular coupling in smooth muscle cells of pressurised arteries: the relevance for Ca2+ oscillations and tone. Cardiovasc Res. 2006;69:825–835. doi: 10.1016/j.cardiores.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Soulie S, Moller JV, Falson P, le Maire M. Urea reduces the aggregation of membrane proteins on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1996;236:363–364. doi: 10.1006/abio.1996.0183. [DOI] [PubMed] [Google Scholar]
- Strøbæk D, Teuber L, Jorgensen TD, Ahring PK, Kjaer K, Hansen RS, et al. Activation of human IK and SK Ca2+ -activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) Biochim Biophys Acta. 2004;1665:1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Vanheel B, Van de Voorde J. Evidence against the involvement of cytochrome P450 metabolites in endothelium-dependent hyperpolarization of the rat main mesenteric artery. J Physiol. 1997;501:331–341. doi: 10.1111/j.1469-7793.1997.331bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston AH, Absi M, Ward DT, Ohanian J, Dodd RH, Dauban P, et al. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: studies with calindol and Calhex 231. Circ Res. 2005a;97:391–398. doi: 10.1161/01.RES.0000178787.59594.a0. [DOI] [PubMed] [Google Scholar]
- Weston AH, Félétou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. Br J Pharmacol. 2005b;145:775–784. doi: 10.1038/sj.bjp.0706256. [DOI] [PMC free article] [PubMed] [Google Scholar]