Abstract
Background and purpose:
Endothelial NO synthase (eNOS) is a dynamic enzyme tightly controlled by co- and post-translational lipid modifications, phosphorylation and regulated by protein-protein interactions. Here we have pharmacologically modulated the activation of eNOS, at different post-translational levels, to assess the role of eNOS-derived NO and of these regulatory mechanisms in intestinal injury associated with splanchnic artery occlusion (SAO) shock.
Experimental approach:
SAO shock was induced by clamping both the superior mesenteric artery and the celiac trunk for 45 min followed by 30 min of reperfusion. During ischemia, 15 min prior to reperfusion, mice were given geldanamycin, an inhibitor of hsp90 recruitment to eNOS, or LY-294002 an inhibitor of phosphatidylinositol 3-kinase (PI3K), an enzyme that initiates Akt–catalysed phosphorylation of eNOS on Ser1179. After 30 min of reperfusion, samples of ileum were taken for histological examination or for biochemical studies.
Key results:
Either LY-294002 or geldanamycin reversed the increased activation of eNOS and Akt observed following SAO shock. These molecular effects were mirrored in vivo by an exacerbation of the intestinal damage. Histological damage also correlated with neutrophil infiltration, assessed as myeloperoxidase activity, and with an increased expression of the adhesion proteins: ICAM-I, VCAM, P-selectin and E-selectin.
Conclusions and implications:
Overall these results suggest that activation of the Akt pathway in ischemic regions of reperfused ileum is a protective event, triggered in order to protect the intestinal tissue from damage induced by ischaemia/reperfusion through a fine tuning of the endothelial NO pathway.
Keywords: splanchnic artery occlusion, ischemia-reperfusion, eNOS, PI3K, Akt, adhesion proteins
Introduction
Until recently, endothelial nitric oxide synthase (eNOS) had been regarded as a static enzyme that produced a constant low amount of NO both in physiological and pathological conditions. Now, it is clear that eNOS is a dynamic enzyme tightly controlled by co- and post-translational lipid modifications, phosphorylation and regulated by protein–protein interactions (Sessa, 2004). Generally, in the basal state, eNOS is localized in the caveolae, where it interacts with caveolin-1 (CAV-1), thus maintaining the enzyme in an inactive state. Following endothelial cell activation by increased shear stress or local autacoids, the increase in cytoplasmic calcium levels activates calmodulin, which weakens the eNOS-CAV-1 interaction thereby promoting hsp90 recruitment to eNOS and eNOS activation. Recently, many investigators have shown that protein phosphorylation of eNOS by several serine/threonine kinases is a critical control step for NO production by endothelial cells (Fulton et al., 1999). In particular, phosphorylation by Akt on Ser1179 of eNOS leads to enhanced activity of the enzyme and, thus, augments production of NO. Indeed mutation of Ser1179 to an alanine residue prevents Akt-dependent phosphorylation and NO production, proving that this residue is indispensable for the activation of the enzyme by this kinase (Dimmeler et al., 1999; Fulton et al., 1999). The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is an important survival pathway involved in protection against various stressors by modulation of various downstream elements involved in apoptosis (Scheid and Woodgett, 2001; Toth et al., 2003). Furthermore, the activation of the PI3K-Akt-eNOS signalling pathway seems to play a key role in the protection afforded by preconditioning, decreasing organ damage. Recent findings demonstrate that the acute activation of the serine-threonine kinase Akt is cardioprotective and reduces dysfunction following ischemia/reperfusion (I/R) injury (Ackah et al., 2005; O'Neill and Abel, 2005). PI3K/Akt pathway activation has similar protective effects on hepatic I/R injury (Izuishi et al., 2006).
Here, we have used pharmacological modulation of eNOS activation at post-translational level with LY-294002, an inhibitor of PI3K, which thereby prevents activation of Akt and consequently inhibits eNOS phosphorylation on Ser1179 (Fulton et al., 1999). We also sought to modify Akt-induced phosphorylation of eNOS with geldanamycin, a compound that binds specifically to the ATP pocket of hsp90 and prevents it acting as an adaptor for Akt (Fontana et al., 2002). These procedures were designed to assess the role of eNOS activation via Akt in intestinal injury associated with splanchnic artery occlusion (SAO) shock (Cuzzocrea et al., 2002).
Materials and methods
Animals
The study was carried out in 6–8-weeks-old (20–25 g) male mice CD1 (Harlan Nossan, Italy). The animals were housed in a controlled environment and provided with standard rodent chow and water. Animal care was in compliance with Italian regulations on protection of animals used for experimental and other scientific purposes (DM 116192) as well as with the EEC regulations (OJ of ECL 358/1, 18 December 1986).
Surgical procedures
Mice were allowed access to food and water ad libitum. On the day of the experiment the mice were anesthetized with chloral hydrate (380 mg kg−1, intraperitoneal (i.p.)). After midline laparotomy, the celiac and superior mesenteric arteries were isolated near their aortic origins. During this procedure, the intestinal tract was maintained at 37°C by placing it between gauze pads soaked with warmed 0.9% NaCl solution. Mice (n=10 for each group) were kept under visual examination by the operator for 30 min before either splanchnic ischemia or sham ischemia was performed. SAO shock was induced by clamping both the superior mesenteric artery and the celiac trunk, resulting in a total occlusion of these arteries for 45 min. After this period of occlusion, the clamps were removed. Mice were killed at different time points and intestinal tissues utilized for histological examination and for biochemical studies, as described below. To avoid the contribution of inducible nitric oxide synthase (iNOS)-derived NO, we have used experimental conditions of I/R in SAO shock, where iNOS is not induced, as described previously (Cuzzocrea et al., 1998a, 1998b, 2002).
Experimental groups
Mice were subjected to SAO shock and received, 15 min before reperfusion, one of three treatments: (i) 0.2 ml of saline solution; (ii) LY 294002 10, 30 or 100 mg kg−1 i.p. bolus in a volume of 0.2 ml of saline solution; and (iii) geldanamicyn (1 mg kg−1) bolus in a volume of 0.2 ml of saline solution. As a sham control group, we used mice that were subjected to identical surgical procedures as described above except that the blood vessels were not occluded. The mice were maintained under anesthesia for the same duration of the experiment and were given vehicle, LY 294002 or geldanamycin.
Western blots
Mice were killed with CO2 following 15, 30, 60 and 120 min of reperfusion and the ileum was homogenized in modified RIPA buffer (Tris-HCl 50 mM, pH 7.4, Triton 1%, sodium deoxycholate 0.25%, NaCl 150 mM, EDTA 1 mM, PMSF 1 mM, aprotinin 10 μg ml−1, leupeptin 20 μM, NaF 50 mM) using a Polytron homogenizer (two cycles of 10 s at maximum speed). After centrifugation of homogenates at 3000 g for 10 min, equal amounts (30 μg) of the denatured proteins were separated on 10% sodium dodecyl sulfate polyacrylamide gels and transferred to a nitrocellulose membrane. Membranes were blocked by incubation in phosphate-buffered saline (PBS) containing 0.1% (v/v) Tween 20 and 5% non-fat dry milk for 2 h, followed by a overnight incubation at 4°C with rabbit CAV-1 receptor polyclonal antibody (1:1000), rabbit anti phospho-eNOS (p-eNOS) -Ser1179 polyclonal antibody (1:1000), mouse eNOS monoclonal antibody (1:2000), rabbit Akt polyclonal antibody and Akt phospho-specific (Ser473) mouse monoclonal antibody. The filters were washed extensively in PBS containing 0.1% (v/v) Tween 20, before incubation for 2 h with anti horseradish peroxidase-conjugate secondary antibody. Membranes were then washed and developed using Enhanced chemiluminescence Substrate (ECL, Amersham Pharmacia Biotech, Piscataway, NY, USA).
Histological assessment of damage after SAO shock
Ileum biopsies were taken at 30 min after reperfusion. The biopsies were fixed in buffered formaldehyde solution (10% in PBS) at room temperature, dehydrated by graded ethanol and embedded in Paraplast (Sherwood Medical, Mahwah, NJ, USA). Tissue sections (thickness 7 μm) were deparaffinized with xylene, stained with hematoxylin/eosin and studied using light microscopy (Dialux 22 Leitz). Cellular damage in the tissue sections was quantified by a scoring system (Cuzzocrea et al., 2002). All the histological studies were performed without knowledge of the treatments.
Myeloperoxidase activity
Assessment of neutrophil infiltration in the intestinal tissues was performed, as described previously (Cuzzocrea et al., 1998a, 1998b), by measurement of the activity of myeloperoxidase (MPO), an enzyme specific to granulocyte lysosomes and, therefore, an indirect measurement of the presence of neutrophils. MPO activity was defined as the quantity of enzyme degrading 1 μmol of peroxide min−1 at 37°C and was expressed in U/g of weight of wet tissue.
Immunohistochemical localization of E-selectin, P-selectin, VCAM and ICAM-1
At 30 min after reperfusion, the ileum was fixed in 10% buffered formaldehyde and 8 μm sections were prepared from paraffin-embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 0.3% H2O2 in 60% methanol for 30 min. The sections were permeabilized with 0.1% Triton X-100 in PBS for 20 min. Non-specific adsorption was minimized by incubating the section in 2% normal goat serum in PBS for 20 min. Endogenous biotin- or avidin-binding sites were blocked by sequential incubation for 15 min with avidin and biotin. The sections were then incubated overnight with primary anti-E-selectin antibody (1:1000), anti-P-selectin antibody (1:500), anti-VCAM-1 antibody (1:500), anti-ICAM-1 antibody (1:500) or with control solutions. Controls included buffer alone or non-specific purified rabbit IgG. Immunocytochemistry photographs (n=5) were assessed by densitometry. The assay was carried out by using Optilab Graftek software on a Macintosh personal computer (CPU G3-266). All the immunocytochemistry analysis was carried out without knowledge of the treatments.
Statistical analysis
All values in the figures and text are expressed as mean±s.e.m. of n observations, where n represents the number of animals studied. In the experiments involving histology or immunohistochemistry, the figures shown are representative of at least three experiments performed on different experimental days. Data sets were examined by one- and two-way analysis of variance. Post-test analysis was performed by using Bonferroni's test. Non-parametric data were analyzed with the Fisher's exact test. A P-value <0.05 was considered significant.
Reagents
Biotin blocking kit, biotin-conjugated goat anti-rabbit IgG and avidin-biotin peroxidase complex were obtained from Vector Laboratories (Burlingame, CA, USA). Primary monoclonal P-selectin (CD62P) or ICAM-1 (CD54) for immunohistochemistry were purchased from Pharmingen (San Diego, CA, USA). Reagents and secondary and non-specific IgG antibody for immunohistochemical analysis were from Vector Laboratories inc. All antibodies utilized for Western blots were obtained from Calbiochem (San Diego, CA, USA). All other reagents and compounds used were obtained from Sigma Chemical Company (Milano, Italy). Geldanamycin was a generous gift from Professor Bill Sessa, Department of Pharmacology, Yale University School of Medicine.
Results
Effect of ischemia and reperfusion of the splanchnic organs on eNOS activation
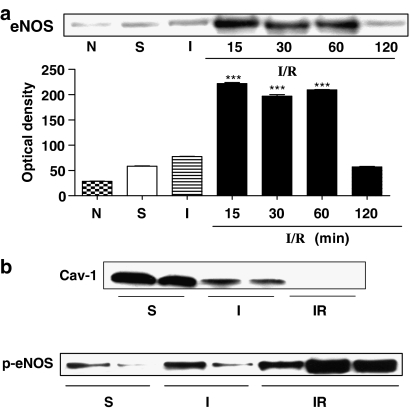
Mice were subjected to 45 min of occlusion followed by reperfusion of the superior mesenteric artery and celiac trunk. Reperfusion was interrupted at selected time points and the ileum taken for analysis by Western blotting. After the first 15 min of reperfusion, eNOS expression increased reaching almost a plateau at 30 min and returning to basal levels within 2 h of reperfusion (Figure 1a). To assess if the increased expression was coupled to an increased activation of eNOS, we evaluated the expression of CAV-1 and the relative level of p-eNOS. During ischemia, eNOS expression was unaltered but its activation was increased as demonstrated by a decreased expression of CAV-1 coupled to an increased phosphorylation at Ser1179 (Figure 1b). Conversely, during reperfusion, eNOS expression was increased, along with a further increase of its phosphorylation at Ser1179. The eNOS phosphorylation was maximal following 30 min of reperfusion (Supplementary Figure 1). The increased levels of phosphorylated eNOS following 30 min of reperfusion was coupled to a decrease in CAV-1 expression (Figure 1b).
Figure 1.
(a) eNOS expression was increased during reperfusion returning to basal levels within 120 min. (b) During ischemia, eNOS expression was unaltered but its activation was increased as assessed by the decreased expression of CAV-1 and the increased phosphorylation on eNOS Ser1179. Following reperfusion, eNOS was also significantly phosphorylated on Ser1179. The increased activation of eNOS was also confirmed by a decrease in CAV-1 expression during reperfusion. ***P<0.001 vs sham (S). The blot shown is representative of three different experiments.
Endothelial NOS activation following I/R is dependent upon triggering of PI3K/Akt pathway
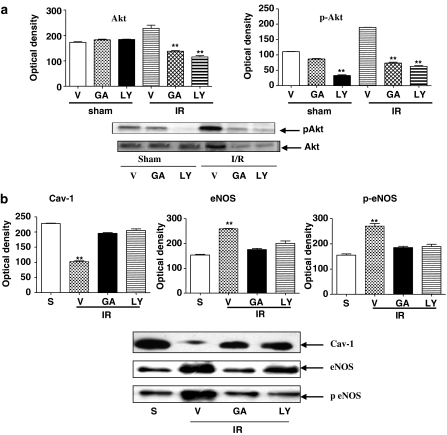
To understand if eNOS activation following I/R was dependent upon PI3K/Akt activation, we evaluated Akt expression and the relative level of phospho-AkT. As observed for eNOS, there was also an increase in Akt expression after 30 min reperfusion (data not shown). Western blots showed a significant amount of the phosphorylated form of Akt following I/R (Figure 2a). Ischemia did not affect Akt expression and activity (Figure 2a). When mice subjected to I/R were pretreated with LY-294002 (30 mg kg−1), a specific inhibitor of PI3K or with geldanamycin (1 mg kg−1), Western blots showed a reversal of the increased expression of both eNOS and Akt (Figure 2b and a), coupled with an increased CAV-1 expression (Figure 2b).
Figure 2.
(a) Akt expression was increased during reperfusion (30 min) and its phosphorylation significantly enhanced. When mice were pretreated with LY-294002 (30 mg kg−1) or geldanamycin (GA; 1 mg kg−1), Akt upregulation was reversed; S, sham; IR, ischemia-reperfusion; **P<0.01 vs vehicle (V). (b) Similarly, administration of LY-294002 (30 mg kg−1) or GA (1 mg kg−1) restored CAV-1 expression, but prevented the increase in eNOS expression and phosphorylation **P<0.01 vs sham (S). The blots are representative of three different experiments.
Effect of eNOS modulation at post-translational levels on intestinal injury associated with SAO shock
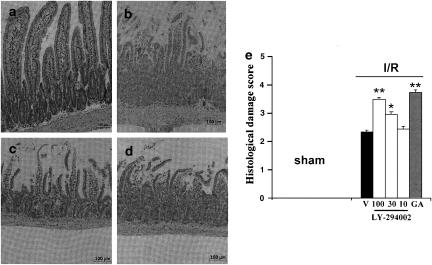
In vehicle-treated mice, SAO shock resulted in tissue injury mainly localized to the small intestine (Figure 3b) compared to sham-treated mice (Figure 3a). Further histological examination of the tissue demonstrated damage localized to the villi and associated with infiltration of inflammatory cells in the mucosa as well as tissue hemorrhage (Figure 3b). Treatment with LY 294002 (30–100 mg kg−1) given i.p. 15 min before reperfusion, significantly increased the extent and severity of the histological signs of ileum (Figure 3c) with a significant higher damage score. No significant difference was found between the effects of the lowest dose of LY 294002 (10 mg kg−1) and the effects of vehicle (Figure 3b). Similarly, the administration of geldanamycin (1 mg kg−1) 15 min before reperfusion was associated with a significant increase of tissue injury associated with ischemia and reperfusion (Figure 3d). Quantification of the histological damage (Cuzzocrea et al., 2002) showed that the damage scores induced by I/R and vehicle treatment were significantly increased by geldanamycin (1 mg kg−1) and LY-294002 (30–100 mg kg−1) (Figure 3e).
Figure 3.
Histological evaluation of damage to mouse ileum after reperfusion and SAO. Hematoxylin/eosin staining of sections of ileum from sham-treated mice (a). In (b–d), sections are from mice subjected to SAO shock, pretreated with (b), vehicle; (c) LY-294002 (30 mg kg−1); (d) geldanamycin (GA; 1 mg kg−1). (e) Histological damage score following treatment with vehicle (V), LY-294002 (10–100 mg kg−1) or GA (1 mg kg−1) 15 min before reperfusion *P<0.05; **P<0.01 vs vehicle.
Effect of eNOS modulation at post-translational levels on the expression of adhesion molecules and neutrophil infiltration
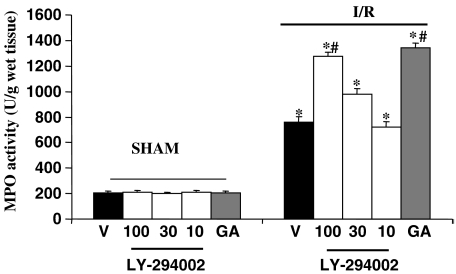
MPO activity in homogenates of ileum was significantly elevated after SAO shock in vehicle-treated mice (Figure 4). A further marked increase of MPO activity was observed in ileum of mice treated with LY 294002 (30–100 mg kg−1) after 30 min of reperfusion, whereas the dose of 10 mg kg−1 was ineffective (Figure 4). Similarly, the administration of geldanamycin (1 mg kg−1) before reperfusion caused a significant increase in MPO activity (Figure 4).
Figure 4.
MPO activity in homogenates of ileum from mice subjected to SAO following treatment with vehicle (V), LY-294002 or geldanamycin (GA). All values are expressed as mean±s.e.m. *P<0.001 vs sham #P<0.001 vs I/R vehicle.
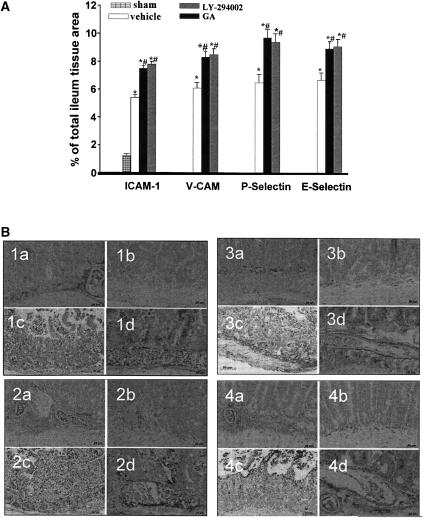
Neutrophil infiltration in the intestine after SAO shock was also associated with expression of adhesion molecules (Figure 5). Following 30 min reperfusion, a positive immunohistochemical staining for ICAM-1 (Figure 5; panel 1a), for VCAM-1 (Figure 5; panel 2a), for P-selectin (Figure 5; panel 3a) and for E-selectin (Figure 5; panel 4a) was found in sections of ileum from SAO-shocked mice. The positive immunostaining for ICAM-1 (panels 1c and d), for VCAM-1 (panels 2c and d), for P-selectin (panels 3c and d) and for E-selectin (panels 4c and d) was further significantly increased in sections from mice treated with LY 294002 (100 mg kg−1) or geldanamycin (1 mg kg−1).
Figure 5.
(A) Expression of E-selectin, P-selectin, VCAM and ICAM-1 in ileum obtained by sham mice or subjected to SAO following treatment with vehicle, LY-294002 (30 mg kg−1) or geldanamycin (GA) (1 mg kg−1). All values are expressed as mean±s.e.m. *P<0.001 vs sham #P<0.001 vs I/R vehicle. (B) Immunohistochemical localization of ICAM (panel 1), VCAM (panel 2), P-selectin (panel 3) and E-selectin (panel 4) following treatment with: (a) vehicle, (b) sham, (c) LY-294002 30 mg kg−1 and (d) GA (1 mg kg−1).
Discussion
SAO shock is a severe form of circulatory shock produced by ischemia and reperfusion of the splanchnic organs. This type of shock is characterized by a decrease in systemic blood pressure upon release of the splanchnic arteries, which leads to a fatal outcome (Altura et al., 1985; Carey et al., 1992; Zingarelli et al., 1992; Lefer and Lefer, 1993). SAO shock is induced in mice by clamping both the superior mesenteric artery and the celiac trunk, followed by release of clamps that can be performed at different time points. Ischemia progressively damages the cell structures and, following the restoration of blood flow (I/R), lesions produced are further exacerbated (McCord, 1985; Parks and Granger, 1986). Thus, SAO promotes complex interactions between endothelium and different cell types, leading to microvascular injury, cellular necrosis and/or apoptosis (Massberg and Messmer, 1998).
Here by using drugs that modulate, at different post-translational levels, the activation of eNOS, we have monitored in depth the role of eNOS in intestinal injury associated with splanchnic I/R. To avoid the possible contribution of NO derived from iNOS, we used I/R conditions in SAO, where iNOS is not yet induced (Cuzzocrea et al., 2002). When ischemia was induced by occlusion of the splanchnic artery, an increase in eNOS expression was already evident after the first 15 min of reperfusion reaching a plateau at 30 min returning to basal levels within 2 h of reperfusion. eNOS is located in different subcellular compartments; in particular eNOS is found primarily in the perinuclear region of cells and in discrete regions of the plasma membrane, suggesting a trafficking, in response to cell stimulation, of the protein from the Golgi to specialized plasma membrane structures, where it is bound to CAV-1 (Feron et al., 1998). It is now appreciated that the consequences of enzyme activation can be determined, to a large extent, by the intracellular localization of the signalling complex. The fact that we detected an increased level of eNOS by Western blot could be ascribed to an increased cytosolic form of eNOS. Interestingly, during reperfusion eNOS was also increasingly activated as demonstrated by a significant phosphorylation on Ser1179. The upregulation of eNOS activity was also confirmed by a decrease in the expression of the inhibitory protein CAV-1 during reperfusion. Thus, during I/R, eNOS was actively transformed in its active phosphorylated form, at the same time as the endogenous inhibitor binding protein, CAV-1, was reduced.
A key role in eNOS phosphorylation is played by the PI3K/Akt pathway. Activation of PI3K leads to phosphorylation of membrane phosphatidylinositol 3,4 biphosphate, which recruits Akt to the cell membrane leading to phosphorylation and activation of Akt. Activated Akt in turn promotes eNOS phosphorylation at Ser1179. We showed that, in our experimental setting, eNOS activation in reperfused intestinal tissue, was coupled to Akt activation. Next to assess the extent to which eNOS activation was dependent upon the PI3K/Akt pathway, we treated mice, in vivo, with LY-294002, a specific inhibitor of PI3K, before reperfusion. In another set of experiments, we used geldanamycin to interfere with eNOS activation. This compound binds to the ATP pocket of hsp90 and inhibits its calcium-dependent recruitment to eNOS (Garcia-Cardena et al., 1998). Geldanamycin also prevents hsp90 acting as an adaptor for Akt thereby preventing eNOS phosphorylation at Ser1179 (Brouet et al., 2001; Fontana et al., 2002). Western blots of intestinal tissue obtained from mice subjected to SAO following treatment with either LY-294002 or geldanamycin showed, as expected, a reversal of Akt and eNOS upregulation. Thus, the modulation of PI3K/Akt pathway by LY-294002 or geldanamycin implied that eNOS activation was dependent upon triggering of the Akt pathway following I/R injury. These molecular effects were mirrored in vivo by an exacerbation of the SAO-induced damage to the intestine following treatment with LY-249002 or geldanamycin. This macroscopic damage correlated with an increased neutrophil infiltration, as assessed by measuring MPO activity. From these data it is feasible to suggest that the role of eNOS during reperfusion is to act as an early protective trigger.
It is most likely this ‘protective' action involves modulation of the adhesive proteins expressed at the interface between the endothelium and neutrophils, such as ICAM-1, VCAM-1, P-selectin and E-selectin (Shreeniwas et al., 1992; Clark et al., 1995; Farhood et al., 1995). Indeed, our immunohistochemistry study clearly demonstrated that upon treatment in vivo with LY-294002 or geldanamycin before SAO shock, the expression of ICAM-I, VCAM, P- and E-selectin expression was increased. The mechanism underlying this effect could be linked to the activation of the PI3K/AKT pathway. Recently, evidence has accumulated indicating the PI3K/AKT pathway plays an important role in the modulation of the immune response. In this context, inhibition of PI3K activity increases plasma cytokine levels (e.g., TNFα, IL-6 and MCP-1) in endotoxemic mice, enhancing the recruitment of inflammatory cells into the liver and kidney and suggesting an indirect pro-inflammatory effect (Guha and Mackman, 2002; Schabbauer et al., 2004; Williams et al., 2004).
In conclusion, we have shown that pharmacological modulation of the PI3K/Akt/eNOS pathway caused an enhanced tissue injury. These data stress the concept that eNOS is involved at the early stages of I/R and plays a critical protective role in response to injury in intestinal inflammation. The most novel interesting observation of the present study was that the activation of the PI3K/Akt pathway in our experimental conditions accounted for many of the effects observed. These data suggest that the activation of the Akt pathway in ischemic regions of reperfused ileum is a protective event that is triggered to preserve the intestinal tissue from the I/R damage, through a fine tuning of the endothelial NO pathway.
External data objects
Abbreviations
- CAV-1
caveolin-1
- eNOS
endothelial nitric oxide synthase
- I/R
ischemia/reperfusion
- iNOS
inducible nitric oxide synthase
- MPO
myeloperoxidase
- p-eNOS
phospho-eNOS
- PI3K
phosphatidylinositol 3-kinase
- SAO
splanchnic artery occlusion
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura BM, Gebrewolda A, Burton RW. Reactive hyperaemic responses of single arterioles are attenuated markedly after intestinal ischemia, endotoxemia and traumatic shock: possible role of endothelial cells. Microcirc Endothel Lymph. 1985;2:3–14. [PubMed] [Google Scholar]
- Brouet A, Sonveaux P, Dessy C, Balligand JL, Feron O. Hsp90 ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of the endothelial nitric-oxide synthase in vascular endothelial growth factor-exposed endothelial cells. J Biol Chem. 2001;276:32663–32669. doi: 10.1074/jbc.M101371200. [DOI] [PubMed] [Google Scholar]
- Carey C, Siegfried MR, Ma SL, Weyrich AS, Lefer AM. Antishock and endothelial protective actions of a NO donor in mesenteric and reperfusion. Circ Shock. 1992;38:209–216. [PubMed] [Google Scholar]
- Clark WM, Lauten JD, Lessov N, Woodward W, Coull BM. Time course of ICAM-1 expression and leukocyte subset infiltration in rat forebrain ischemia. Mol Chem Neuropathol. 1995;26:213–230. doi: 10.1007/BF02815139. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Prabal K, Mazzon E, Dugo L, De Sarro A, Van de Loo F, et al. Role of induced nitric oxide in the initiation of the inflammatory response after postischemic injury. Shock. 2002;18:169–176. doi: 10.1097/00024382-200208000-00014. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Zingarelli B, Caputi AP. Role of peroxynitrite and poly (ADP-ribosyl) synthetase activation in cardiovascular derangement induced by zymosan in the rat. Life Sci. 1998a;63:923–933. doi: 10.1016/s0024-3205(98)00350-6. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Zingarelli B, Caputi AP. Role of constitutive nitric oxide synthase activation and peroxynitrite production in a rat model of splanchnic artery occlusion shock. Life Sci. 1998b;63:789–800. doi: 10.1016/s0024-3205(98)00334-8. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Farhood A, Mcguire GM, Manning AM, Miyasaka M, Smith CW, Jaeschke H. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J Leukoc Biol. 1995;57:368–374. [PubMed] [Google Scholar]
- Feron O, Saldana F, Michel J, Michel T. The Endothelial nitric oxide synthase-caveolin regulatory cycle. J Biol Chem. 1998;273:3125–31258. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]
- Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, et al. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res. 2002;90:866–873. doi: 10.1161/01.res.0000016837.26733.be. [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, et al. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic. Cells J Biol Chem. 2002;35:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- Izuishi K, Tsung A, Hossain MA, Fujiwara M, Wakabayashi H, Masaki T, et al. Ischemic preconditioning of the murine liver protects through the Akt kinasepathway. Hepatology. 2006;44:573–580. doi: 10.1002/hep.21298. [DOI] [PubMed] [Google Scholar]
- Lefer AM, Lefer DJ. Pharmacology of the endothelium in ischemia-reperfusion and circulatory shock. Annu Rev Pharmacol Toxicol. 1993;33:71–90. doi: 10.1146/annurev.pa.33.040193.000443. [DOI] [PubMed] [Google Scholar]
- Massberg S, Messmer K. The nature of ischemia/reperfusion injury. Transplant Proc. 1998;30:4217–4223. doi: 10.1016/s0041-1345(98)01397-9. [DOI] [PubMed] [Google Scholar]
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- O'Neill BT, Abel ED. Akt1 in the cardiovascular system: friend or foe. J Clin Invest. 2005;115:2059–2064. doi: 10.1172/JCI25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250:G749–G753. doi: 10.1152/ajpgi.1986.250.6.G749. [DOI] [PubMed] [Google Scholar]
- Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. PI3K/Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol. 2004;24:1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Woodgett JR. PKB/Akt: functional insights from genetic models. Nat Rev. 2001;2:760–768. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- Shreeniwas R, Koga S, Karakurum M, Pinsky D, Kaiser E, Brett J, et al. Hypoxia-mediated induction of endothelial cell interleukin-1 alpha. An autocrine mechanism promoting expression of leukocyte adhesion molecules on the vessel surface. J Clin Invest. 1992;90:2333–2339. doi: 10.1172/JCI116122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth A, Kovacs K, Deres P, Halmosi R, Czopf L, Hanto K, et al. Impact of a novel cardioprotective agent on the ischaemia-reperfusion-induced Akt kinase activation. Biochem Pharmacol. 2003;66:2263–2272. doi: 10.1016/j.bcp.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, et al. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- Zingarelli B, Squadrito F, Ioculano MP, Altavilla D, Bussolino F, Campo GM, et al. Platelet activating factor in splanchnic artery occlusion shock. Eur J Pharmacol. 1992;222:13–19. doi: 10.1016/0014-2999(92)90456-e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.