Abstract
Background and purpose:
α-Humulene and trans-caryophyllene are sesquiterpene compounds identified in the essential oil of Cordia verbenacea which display topical and systemic anti-inflammatory effects in different experimental models. However, the molecular mechanisms through which they exert their anti-inflammatory activity still remain unclear. Here, we evaluate the effects of α-humulene and trans-caryophyllene on the acute inflammatory responses elicited by LPS.
Experimental approach:
The biological activities of α-humulene and trans-caryophyllene were investigated in a model of acute inflammation in rat paw, induced by LPS and characterized by paw oedema, neutrophil recruitment, cytokine production, activation of MAP kinases and NF-κB and up-regulated expression of kinin B1 receptors.
Key results:
Treatment with either α-humulene or trans-caryophyllene effectively reduced neutrophil migration and activation of NF-κB induced by LPS in the rat paw. However, only α-humulene significantly reduced the increase in TNF-α and IL-1β levels, paw oedema and the up-regulation of B1 receptors following treatment with LPS. Both compounds failed to interfere with the activation of the MAP kinases, ERK, p38 and JNK.
Conclusions and Implications:
Both α-humulene and trans-caryophyllene inhibit the LPS-induced NF-κB activation and neutrophil migration, although only α-humulene had the ability to prevent the production of pro-inflammatory cytokines TNF-α and IL-1β and the in vivo up-regulation of kinin B1 receptors. These data provide additional molecular and functional insights into the beneficial effects of the sesquiterpenes α-humulene and trans-caryophyllene isolated from the essential oil of Cordia verbenacea as agents for the management of inflammatory diseases.
Keywords: Cordia verbenacea, α-humulene, trans-caryophyllene, lipopolysaccharide, inflammation, B1 receptor, bradykinin, MAP kinases, nuclear factor-κB
Introduction
The use of herbal therapy or alternative medicine constitutes an attractive approach for the treatment of several inflammatory disorders (see, for review, Calixto et al., 2003, 2004a). Cordia verbenacea is a native Brazilian medicinal plant belonging to the Boraginaceae family, which has been widely used in folk medicine in the form of alcoholic extracts, decoctions and infusions for its antiulcer, antimicrobial, anti-inflammatory, antirheumatic, analgesic and tonic properties. The essential oil of C. verbenacea is composed mainly of mono- and sesquiterpenes and its major constituents are α-pinene, trans-caryophyllene and aloaromadendrene. Other constituents include α-humulene, espatulenol, β-gurjunene and epoxycaryophyllene (de Carvalho et al., 2004).
We have previously shown that the essential oil of C. verbenacea exhibits anti-inflammatory properties, when given orally, by inhibiting paw oedema caused by carrageenan and other phlogistic agents such as bradykinin, substance P, histamine and platelet-activating factor in mice. In addition, given orally, the essential oil of C. verbenacea significantly decreases tumour necrosis factor alpha (TNF-α) levels in carrageenan-injected rat paws. These anti-inflammatory properties were attributed to two sesquiterpene compounds, α-humulene and trans-caryophyllene (Passos et al., 2007).
Kinins represent a group of biologically active peptides generated at the sites of tissue damage, either in response to trauma or infection, or during the majority of inflammatory processes (Moreau et al., 2005). The role of kinins has been described in numerous systems and their main pharmacological effects consist of smooth muscle contraction and relaxation, vasodilatation, increase in vascular permeability and sensitization of nociceptive fibres (Regoli and Barabe, 1980; Leeb-Lundberg et al., 2005). Kinins exert most of their effects through the stimulation of two different G-protein-coupled receptors, classified as B1 and B2 (Regoli and Barabe, 1980). The B2 receptor is constitutively expressed in both the peripheral and central nervous systems, mediating most of the physiological actions evoked by kinins and exhibiting high affinity for BK and kallidin. In contrast, the B1 receptor shows higher affinity for the kinin metabolites des-Arg9-BK and des-Arg10-kallidin and is generally absent under normal conditions, although it may be strongly upregulated following tissue trauma, during certain inflammatory states or by the action of proinflammatory cytokines or bacterial products (for example, Escherichia coli endotoxin) (Regoli and Barabe, 1980; Calixto et al., 2000, 2004b; Leeb-Lundberg et al., 2005). Inappropriate B1 receptor expression has been associated with some pathological conditions, including inflammation, pain, atheromatous disease, asthma, cancer, septic shock and diabetes (Calixto et al., 2004b; Leeb-Lundberg et al., 2005).
In a recent paper (Passos et al., 2004), we have described a model of acute inflammation in which lipopolysaccharide (LPS) is administered into the rat paw, characterized by paw oedema, neutrophil recruitment, cytokine production, activation of nuclear factor-kappa B (NF-κB) and upregulation of the expression of kinin B1 receptors. In the present study, we examined the anti-inflammatory effects of the sesquiterpenes α-humulene and trans-caryophyllene, found in the essential oil of C. verbenacea, by employing this experimental model. We demonstrate that α-humulene, but not trans-caryophyllene, reduced the production of cytokines, paw oedema and the upregulation of kinin B1 receptors induced by local treatment with LPS. Moreover, both compounds suppressed the LPS-induced neutrophil recruitment and NF-κB activation, without interfering with the activation of mitogen-activated protein (MAP) kinases.
Methods
Animals
Experiments were conducted using male Wistar rats (140–180 g) kept in controlled room temperature (22±2°C) and humidity (60–80%) under a 12:12 h light–dark cycle (lights on 0600 h). At appropriate time intervals, rats were killed by isoflurane overdose. All procedures used in the present study complied with the guidelines on animal care of the UFSC Ethics Committee on the Use of Animals, which follows the ‘Principles of Laboratory Animal Care' from NIH publication No. 85-23.
Measurement of rat paw oedema
The animals received a 0.1 ml intraplantar (i.pl) injection in the right hind paw of phosphate-buffered saline (PBS; composition, 137 mM NaCl, 2.7 mM KCl and 10 mM phosphate buffer, pH 7.4) containing des-Arg9-bradykinin (des-Arg9-BK; 100 nmol per paw). The contralateral paw (left paw) received 0.1 ml of PBS and was used as the control. Oedema was measured with a plethysmometer (Ugo Basile, Comerio, Italy) at several time points (10, 20, 30, 60 and 120 min) after injection of des-Arg9-BK. The oedema is expressed in millilitres, as the difference between the right and left paws. To induce B1 receptor upregulation, animals were treated with LPS (1 μg per paw) 12 h before the injection of des-Arg9-BK at the same site (Passos et al., 2004).
To assess the possible regulatory effect of the sesquiterpenes from C. verbenacea on LPS-induced kinin B1 receptor upregulation in vivo, animals were treated orally (p.o.) with α-humulene or trans-caryophyllene (50 mg kg−1), 1 h before LPS treatment. A separate experimental group received the anti-inflammatory steroid dexamethasone (0.5 mg kg−1, subcutaneously (s.c.)) 4 h before LPS injection and this group was used as a positive control (Passos et al., 2004). In a separate series of experiments, to assess the effect of these drugs directly on des-Arg9-BK-induced paw oedema following B1-receptor induction, animals were treated with α-humulene or with trans-caryophyllene (both 50 mg kg−1, p.o.) 1 h before des-Arg9-BK injection. The B1 receptor antagonist des-Arg9-[Leu8]BK (100 nmol per paw, 1 h before des-Arg9-BK) was used as a positive control (Passos et al., 2004). Control animals received saline solution (0.9% NaCl). The dose of α-humulene or trans-caryophyllene used in the present study was chosen on the basis of previous work (Passos et al., 2007).
Expression of B1 receptor mRNA in the rat paw
To assess the effect of α-humulene or trans-caryophyllene on B1-receptor mRNA expression, animals were pretreated with one of these compounds (50 mg kg−1), 1 h before LPS injection (1 μg/paw). PBS-injected paws were used as a control. The s.c. tissue of the paws was collected 6 h after LPS treatment and frozen under liquid nitrogen (Passos et al., 2004). The samples were homogenized, and total RNA was extracted using the TRIzol reagent (Life Technologies, Gaithersburg, Germany). One microgram of total RNA was reverse-transcribed using oligo(dT) as the primer (25 μg ml−1) and 200 U of reverse transcriptase (Life Technologies) in 20 μl of PCR buffer containing 0.5 mM dNTP, 10 mM dithiothreitol (DTT), 2.5 mM MgCl2, 50 mM KCl and 20 mM Tris-HCl, pH 8.4. The samples were incubated for 50 min at 42°C, heated for 15 min at 70°C and cooled using ice. After treatment with 2 U of RNase H (20 min, 37°C), cDNA amplification of a specific sequence of rat B1 receptor and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed by PCR using the following primers: for B1 receptor: sense, GAAGCTGTGAGCTCTTTG; and antisense, GCCAGTTGAAA CGGTTCCC; and for rat GAPDH: sense, CCACCCATGGC AAATTCCATGGCA; and antisense, CCACCTGGACTGG ACGGCAGATCT. GAPDH cDNA was used for standardization of the amount of RNA. Five microlitres of RT aliquots were mixed in a 20 mM Tris-HCl buffer (pH 8.4) containing 1.5 mM MgCl2, 300 μM dNTP, 2 μg ml−1 of each primer and 50 U ml−1 of Taq polymerase (Life Technologies) in a final volume of 100 μl. The cycling protocol used was the following: 4 min at 94°C, 36 cycles of 35 s at 94°C, 45 s at 60°C, 45 s at 72°C and finally, 7 min at 72°C. Aliquots of 25 μl were analysed by polyacrylamide gel electrophoresis and stained with silver salts.
Neutrophil myeloperoxidase (MPO) assay
Neutrophil recruitment was indirectly measured through assay of MPO activity. The s.c. tissue of the paws was collected 12 h after LPS treatment and frozen in liquid nitrogen (Passos et al., 2004). Samples were homogenized in 5% (w v−1) ethylenediaminetetraacetic acid (EDTA)/NaCl buffer (pH 4.7) and centrifuged at 12 500 g for 15 min at 4°C. The pellet was resuspended in 0.5% hexadecyltrimethyl ammonium bromide buffer (pH 5.4) and the samples were frozen and thawed three times in liquid nitrogen. Upon thawing, the samples were recentrifuged (12 500 g, 15 min, 4°C) and 25 μl of the supernatant was used for the MPO assay. The enzymatic reaction was assessed with 1.6 mM tetramethylbenzidine, 80 mM NaPO4 and 0.3 mM hydrogen peroxide. The absorbance was measured at 690 nm and the results were expressed as optical density per mg of tissue or per ml of exudate.
Measurement of interleukin-1β (IL-1β) and TNF-α levels in the rat paw
The s.c. tissue of the paws was collected 1 or 12 h after LPS treatment for quantification of TNF-α and IL-1β, respectively (Passos et al., 2004). Samples were homogenized in a PBS solution containing 0.05% Tween-20, 0.1 mM phenylmethylsulphonyl fluoride (PMSF), 0.1 mM benzamethonium chloride, 10 mM EDTA, 2 μg ml−1 aprotinin A and 0.5% bovine serum albumin (BSA). The samples were then centrifuged at 3000 g for 10 min, and the supernatant was stored at −70°C until further analysis. For the measurement of cytokine levels, a standard sandwich enzyme-linked immunosorbent assay technique was used according to the recommendations of the supplier (R&D Systems, Minneapolis, MN, USA).
Preparation of cytosolic and nuclear extracts
Tissues were homogenized in ice-cold 10 mM (4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid) HEPES (pH 7.4) containing 1.5 mM MgCl2, 10 mM KCl, 1 mM PMSF, 5 μg ml−1 leupeptin, 5 μg ml−1 pepstatin A, 10 μg ml−1 aprotinin, 1 mM sodium orthovanadate, 10 mM β-glycerophosphate, 50 mM sodium fluoride and 0.5 mM DTT (all from Sigma–Aldrich Corp., SP, Brazil). The homogenates were chilled on ice for 15 min and then vigorously shaken for 15 min in the presence of 0.1% Triton-X100. The nuclear fraction was precipitated by centrifugation at 10 000 g for 30 min. The supernatant containing the cytosolic fraction was stored at −70°C until use. The nuclear pellet was resuspended in 500 μl of high-salt extraction buffer (20 mM HEPES pH 7.4, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% (v v−1) glycerol, 1 mM PMSF, 5 μg ml−1 pepstatin A, 5 μg ml−1 leupeptin, 10 μg ml−1 aprotinin and 0.5 mM DTT) and incubated under continuous shaking at 4°C for 30 min. The nuclear extract was then centrifuged for 30 min at 10 000 g and the supernatant was aliquoted and stored at −70°C. Protein concentration was determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA).
Western blot analysis
Cell lysates (30 μg protein) were boiled in sodium dodecyl sulphate (SDS) sample buffer for 5 min before electrophoresis on 12% SDS-polyacrylamide gel. After transfer to polyvinylidene difluoride membrane (GE Healthcare, São Paulo, Brazil), the blots were blocked with 5% fat-free dry milk-TBST buffer (Tris-buffered saline containing 0.5% Tween-20) for 1 h at room temperature and then washed with TBST buffer. The membranes were incubated overnight at 4°C with 1:1000 dilutions of primary antibodies for extracellular-regulated kinase (ERK) (sc-94), phosphorylated-ERK (sc-7976), c-Jun N-terminal kinase (JNK) (sc-571), phosphorylated-JNK (sc-6254), p38 MAPK (sc-7149) and phosphorylated-p38 (sc-7975-R) (all from Santa Cruz Biotech Inc., Santa Cruz, CA, USA). Blots were washed three times with TBST at 5 min intervals followed by incubation with a 1:5000 dilution of appropriate horse raddish peroxidase-conjugated secondary antibodies for 1 h, after which they were washed three times in TBST once more. The transferred proteins were visualized with an ECL detection kit according to the manufacturer's instructions (GE Healthcare).
Electrophoretic mobility shift assay (EMSA)
The s.c. tissue of the paws was collected 1 h after LPS treatment and frozen in liquid nitrogen (Passos et al., 2004). For EMSAs, an NF-κB double-stranded consensus oligonucleotide probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was end-labelled with [γ-32P]adenosine triphosphate in the presence of T4 polynucleotide kinase (10 U) for 10 min at 37°C. Unincorporated nucleotides were removed by passing the reaction mixture over a Sephadex G-25 spin column (GE Healthcare). In a total volume of 20 μl, nuclear extracts (10 μg) were incubated with gel shift binding buffer (10 mM Tris-HCl (pH 7.5), 1 mM MgCl2, 50 mM NaCl, 0.5 mM DTT, 0.5 mM EDTA, 4% glycerol and 1 μg of poly(dI-dC)) for 20 min at room temperature. Each sample was further incubated for 30 min at room temperature with 25 000 c.p.m. of 32P-labelled NF-κB consensus oligonucleotide. Protein–DNA complexes were resolved by nondenaturing 6% acrylamide/bis-acrylamide (37.5/1) in 0.25 × Tris-borate/EDTA buffer at 150 V for 2 h. Finally, the gel was dried and exposed to an X-ray film.
Quantitation
All Western blot, EMSA and RT-PCR experiments were scanned to acquire digital images using a Genius ColorPage Scanner (KYE Systems Corp., Taipei, Taiwan). Digital images were processed with the Photoshop software package (Adobe Systems, San Jose, CA, USA), complying with strict standards (Rossner and Yamada, 2004). Band density measurements were made using the Scion Image software package (Scion Corporation, Maryland, MD, USA).
Statistical analysis
The percentage inhibition in oedema experiments was calculated based on the area under the curves (AUC). All the results are presented as mean±s.e.m. The statistical significance between the group means was assessed by means of one-way analysis of variance (ANOVA) followed by Newman Keuls'post hoc test. The accepted level of significance for the tests was P<0.05. All tests were carried out using the Statistical software package (StatSoft Inc., Tulsa, OK, USA).
Drugs and reagents
The following drugs and reagents were used: E. coli LPS (serotype 0111:B4), PBS tablets, dexamethasone, pyrrolidinedithiocarbamate (PDTC), EDTA, hexadecyltrimethyl ammonium bromide, tetramethylbenzidine, DTT, PMSF, HEPES, BSA, benzamethonium chloride, aprotinin A, leupeptin, pepstatin A, soybean trypsin inhibitor, α-humulene (purity 98%) and trans-caryophyllene (purity 98%) (all from Sigma–Aldrich, St Louis, MO, USA); glycerol (Invitrogen, Carlsbad, CA, USA); Polyidet P-40 (Polyscience, Warrington, PA, USA); des-Arg9-bradykinin and des-Arg9-Leu8-bradykinin (Bachem Bioscience, King of Prussia, PA, USA); NaPO4, hydrogen peroxide, MgCl2, KCl, Tris-HCl, NaCl and Tween-20 (all from Merck, Haar, Germany). The stock solutions of des-Arg9-bradykinin, des-Arg9-Leu8-bradykinin, and LPS were prepared in PBS. All solutions were stored in siliconized plastic tubes, maintained at −20°C, and diluted to the desired concentration just before use. The other drugs were prepared daily in 0.9% (w v−1) NaCl solution, except for dexamethasone, which was diluted in 5% ethanol.
Results
Effect of α-humulene and trans-caryophyllene on des-Arg9-BK-induced paw oedema
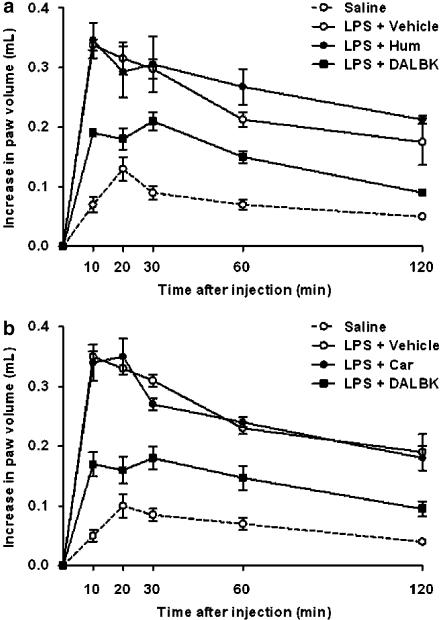
Several studies have shown the ability of LPS to induce B1 receptor upregulation in vivo. As described beforehand (Passos et al., 2004), the i.pl injection of the selective B1 receptor agonist des-Arg9-BK (100 nmol per paw) in naïve animals produced a very weak increase in rat paw oedema (Figure 1). The mean AUC found in des-Arg9-BK-treated rats was 8.45±1.15 (n=6). In contrast, the i.pl injection of des-Arg9-BK in rats that had been previously treated with an i.pl injection of LPS (1 μg per paw, 12 h earlier) induced a significant increase of rat paw oedema, that resulted in a mean AUC of 32.66±2.21 (n=6; Figure 1a). To detect any possible antagonistic effect of the two compounds from C. verbenacea on the oedematogenic response induced by the B1 agonist des-Arg9-BK, we treated rats with LPS (12 h earlier) to induce the upregulation of B1 receptor and then with α-humulene (50 mg kg−1, p.o), trans-caryophyllene (50 mg kg−1, p.o), dexamethasone (0.5 mg kg−1, s.c.) or des-Arg9-[Leu8]-BK (B1 antagonist, 100 nmol per paw) 1 h before des-Arg9-BK administration (11 h after LPS). As shown in Figure 1a, as expected, the selective B1 receptor antagonist des-Arg9-[Leu8]-BK significantly reduced des-Arg9-BK-induced paw oedema formation. The mean AUC found in the des-Arg9-[Leu8]-BK-treated animals was 17.37±1.18 (P<0.01; n=6). On the basis of AUC values, the antagonist caused 55% inhibition of the oedema response. In contrast, under the same conditions, neither α-humulene (Figure 1a) nor trans-caryophyllene (Figure 1b) had any significant effect on the oedema response to des-Arg9-BK. Moreover, the treatment with dexamethasone completely failed to affect des-Arg9-BK-induced oedematogenic response, when assessed in this experimental schedule of treatment (results not shown).
Figure 1.
Effect of α-humulene or trans-caryophyllene on the oedematogenic response induced by B1 receptor agonist des-Arg9-BK. Rats pretreated with LPS (1 μg per paw, 12 h prior) were treated orally with (a) α-humulene (50 mg kg−1, p.o.) or (b) trans-caryophyllene (50 mg kg−1, p.o.) 1 h before des-Arg9-BK (100 nmol per paw). As a positive control, B1 receptor antagonist des-Arg9-Leu8-BK (100 ng per paw) was administered 1 h before des-Arg9-BK (100 nmol per paw). Values represent the differences between volume (in millilitres) of vehicle-injected (0.1 ml of PBS solution) and drug-injected paws. Each point represents the mean±s.e.m. of six animals. The experiments were performed on three different experimental days. Hum, α-humulene; Car, trans-caryophyllene; DALBK, des-Arg9-Leu8-bradykinin.
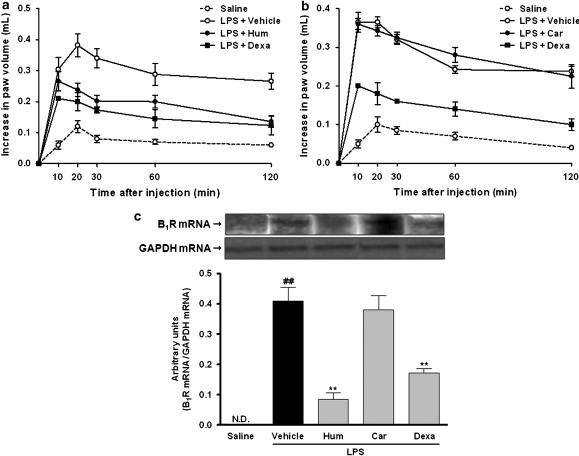
Effect of α-humulene and trans-caryophyllene on LPS-induced B1 receptor upregulation
Next, we investigated the effect of the two sesquiterpenes on LPS-induced B1 receptor upregulation in vivo. For this purpose, rats were orally treated with α-humulene or with trans-caryophyllene (50 mg kg−1), 1 h before LPS i.pl injection. In this set of experiments, the mean AUC found in saline-treated rats that received an i.pl injection of the B1 receptor agonist des-Arg9-BK (100 nmol per paw) was 8.37±1.22 (n=6). In contrast, the i.pl injection of des-Arg9-BK in rats that had been previously treated with an i.pl injection of LPS (1 μg per paw, 12 h prior) induced a significant increase of rat paw oedema, that resulted in a mean AUC of 34.60±3.20 (n=6; Figure 2a). The treatment with α-humulene, but not with trans-caryophyllene, significantly reduced des-Arg9-BK-induced paw oedema in rats that had previously received LPS (Figure 2). The mean AUC found in the α-humulene-treated animals was 22.16±0.97 (P<0.01; n=6). This inhibitory effect was similar to that obtained for the anti-inflammatory steroid dexamethasone (0.5 mg kg−1, s.c.), which resulted in a mean AUC of 17.79±2.58 (P<0.01; n=6). On the basis of AUC values, α-humulene and dexamethasone caused 47 and 64% inhibition, respectively, of the B1 receptor upregulation (Figure 2).
Figure 2.
Effect of α-humulene or trans-caryophyllene on LPS-induced B1 receptor upregulation. The oedematogenic response induced by B1 receptor agonist des-Arg9-BK was evaluated 12 h after LPS treatment in (a) α-humulene and (b) trans-caryophyllene pretreated rats. Rats were treated orally with α-humulene (50 mg kg−1, p.o.) or trans-caryophyllene (50 mg kg−1, p.o.) 1 h before LPS (1 μg per paw) i.pl injection. Dexamethasone (0.5 mg kg−1) was administered 4 h before LPS. Values represent the differences between volume (in millilitres) of vehicle-injected (0.1 ml of PBS solution) and drug-injected paws. Each point represents the mean±s.e.m. of six animals. The experiments were performed on three different experimental days. (c) Effect of α-humulene or trans-caryophyllene on levels of mRNA for B1 receptors induced by LPS. Rat paws were collected 1 h after LPS i.pl injection. Dexamethasone was used as a positive control. (Below graph) Quantification of B1 mRNA was normalized by GAPDH mRNA. Each point represents the mean±s.e.m. of three animals. Statistical analysis was carried out using one-way ANOVA followed by the Newman Keuls'post hoc test. ##P<0.01 compared to saline-treated animals. **P<0.01 compared to vehicle-treated animals. Hum, α-humulene; Car, trans-caryophyllene; Dexa, dexamethasone.
A previous study from our group has shown that LPS induces B1 receptor upregulation in the rat paw by a process which is dependent on mRNA synthesis (Passos et al., 2004). To gain further insights into the mechanisms involved in the inhibitory effects of α-humulene on the B1 receptor upregulation following LPS injection, we assessed the influence of this compound on B1 receptor mRNA expression by means of RT-PCR procedures. As shown in Figure 2c, no detectable levels of mRNA for B1 receptor were observed when the assay was carried out on saline-treated control paws. However, LPS treatment induced a marked increase in the B1 receptor mRNA levels according to evaluation 1 h after injection (P<0.01; n=3). Treatment with α-humulene, but not with trans-caryophyllene, significantly reduced the B1 receptor mRNA synthesis by LPS (P<0.01; n=3). The positive control – treatment with dexamethasone – also significantly reduced the B1 receptor mRNA expression (P<0.01, n=3). These results indicate that α-humulene prevents LPS-induced B1 receptor upregulation in rats, possibly at the transcriptional level.
Inhibitory effect of α-humulene and trans-caryophyllene on LPS induced of neutrophil influx
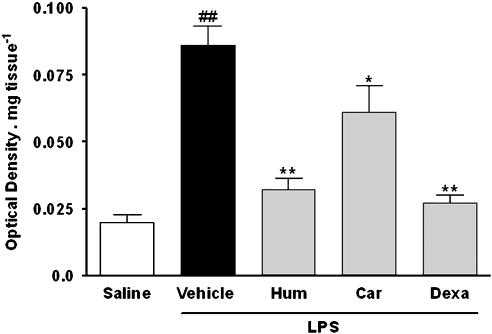
Migration of neutrophils to the rat paws in response to LPS injection was assessed indirectly by assaying MPO. We have previously shown that injection of LPS in rat paw induces a marked and time-related increase in MPO levels, which peaks at 12 h and remains significantly increased for up to 36 h (Passos et al., 2004). Data from Figure 3 shows that MPO levels were significantly increased in LPS-treated paws in comparison to those of the saline-treated group (P<0.01; n=4) 12 h after treatment. In addition, treatment 1 h before LPS with α-humulene (P<0.01; n=4) or with trans-caryophyllene (P<0.05 n=4) significantly reduced LPS-induced MPO levels in the rat paws (Figure 3). The inhibitions obtained for these compounds were 81 and 38%, respectively. A similar effect was observed when rats were treated 4 h before with dexamethasone (P<0.01; n=4; inhibition of 89%).
Figure 3.
Effect of α-humulene or trans-caryophyllene on LPS-induced neutrophil migration. Rats were treated orally with α-humulene (50 mg kg−1, p.o.) or trans-caryophyllene (50 mg kg−1, p.o.) 1 h before LPS (1 μg per paw) i.pl injection. Dexamethasone (0.5 mg kg−1) was administered 4 h before LPS. Rat paws were collected 12 h after LPS. Each point represents the mean±s.e.m. of four animals. Statistical analysis was carried out using one-way ANOVA followed by the Newman Keuls'post hoc test. ##P<0.01 compared to saline-treated animals. *P<0.05 and **P<0.01 compared to vehicle-treated animals. Hum, α-humulene; Car, trans-caryophyllene; Dexa, dexamethasone.
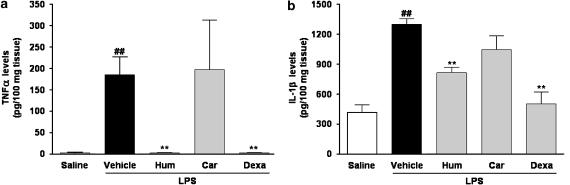
Effects of α-humulene and trans-caryophyllene on LPS-induced proinflammatory cytokine release
Cytokines are multifunctional molecules that play an important role in host defence, acute-phase reactions, immune response and haematopoiesis. Their production is upregulated by various factors, including LPS. As shown in Figure 4, only low levels of the cytokines, TNF-α and IL-1β, were detected in saline-treated paws (control group), but these were markedly increased at 1 and 12 h after LPS treatment. When administered systemically, α-humulene, but not trans-caryophyllene, prevented the increase in both cytokines in response to LPS (P<0.01; n=3), inhibiting the levels of TNF-α by 100% and those of IL-1β by 55%. A similar effect was observed in the group pretreated with dexamethasone (inhibition of 100 and 88%, for TNF-α and IL-1β, respectively).
Figure 4.
Effect of α-humulene or trans-caryophyllene on LPS-induced cytokine production. Levels of (a) TNF-α or (b) IL-1β were measured in the rat paws after 1 or 12 h of LPS treatment, respectively. Rats were treated orally with α-humulene (50 mg kg−1, p.o.) or trans-caryophyllene (50 mg kg−1, p.o.) 1 h before LPS (1 μg per paw) i.pl injection. Dexamethasone (0.5 mg kg−1) was administered 4 h before LPS. Each point represents the mean±s.e.m. of three animals. Statistical analysis was carried out using one-way ANOVA followed by the Newman Keuls'post hoc test. ##P<0.01 compared to saline-treated animals. **P<0.01 compared to vehicle-treated animals. Hum, α-humulene; Car, trans-caryophyllene; Dexa, dexamethasone.
Effects of α-humulene and trans-caryophyllene on LPS-induced MAPK activation
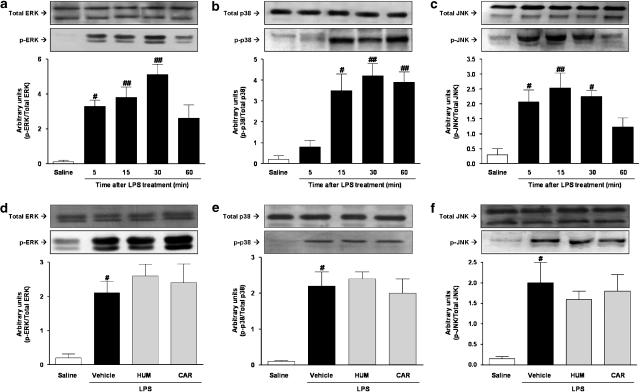
Recent evidence suggests that MAP kinases play an important role in B1 receptor upregulation, both in vitro and in vivo (Calixto et al., 2004b). Thus, it is reasonable to expect that α-humulene might interfere with the activation of these signalling pathways. Hence, we first assessed the temporal profile of LPS-induced activation of ERK, p38 MAPK and JNK in the rat paw. The data presented in Figure 5a–c indicates that no activation of ERK and only very low levels of JNK and p38 MAP kinase activation, was detected under basal conditions (saline-treated animals). Local (i.pl) treatment with LPS resulted in a marked activation of ERK, JNK and p38 MAPK. The maximal increase in the phosphorylation of MAP kinases was reached at 30 min. To our knowledge, this is the first evidence indicating that LPS may lead to the increased activation of MAP kinases in the rat paw. Next, we assessed the effects of α-humulene and trans-caryophyllene on LPS-induced MAP kinase activation. As shown in Figure 5d–f, neither α-humulene nor trans-caryophyllene was able to significantly inhibit LPS-induced MAP kinase activation.
Figure 5.
Effect of α-humulene or trans-caryophyllene on LPS-induced MAPK activation. Time-dependent activation of (a) ERK, (b) p38 MAPK and (c) JNK. Rats were treated with saline or LPS (1 μg per paw) and then the paws were isolated at the time points indicated. Rats were treated orally with α-humulene (50 mg kg−1, p.o.) or trans-caryophyllene (50 mg kg−1, p.o.), 1 h before LPS injection and then Western blotting was performed for (d) ERK, (e) p38 MAPK and (f) JNK. Rat paws were collected 30 min after LPS. (Below: sample Western blots). Quantification of p-ERK, p-p38 MAPK and p-JNK was normalized by total ERK, p38 MAPK and JNK, respectively. Each point represents the mean±s.e.m. of three animals. Statistical analysis was carried out using one-way ANOVA followed by the Newman Keuls'post hoc test. #P<0.05 and ##P<0.01 compared to the saline-treated group. Hum, α-humulene; Car, trans-caryophyllene.
Inhibitory effects of α-humulene and trans-caryophyllene on LPS-induced NF-κB activation
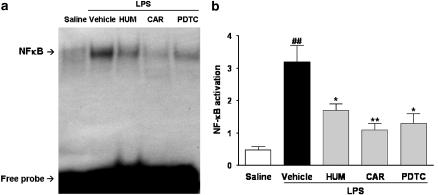
It is widely characterized that the 5′-flanking region of the B1 receptor gene promoter contains binding sequences for various transcription factors including NF-κB (Prado et al., 2002). Thus, we attempted to evaluate the effects of the two compounds on LPS-stimulated DNA binding of NF-κB in the rat paw. As reported previously (Passos et al., 2004), NF-κB activity was detectable at low levels in the nuclear proteins obtained from saline-treated preparations (control animals). However, DNA-binding activity was found to be significantly increased (P<0.01; n=3) in nuclear extracts as early as 1 h after local LPS treatment (Figure 6). Of note, LPS-induced NF-κB activation was markedly inhibited when rats were treated with α-humulene (P<0.05; n=3) or with trans-caryophyllene (P<0.01; n=3). The inhibition of NF-κB obtained with these compounds were 55 and 77%, respectively. Systemic treatment with the NF-κB inhibitor PDTC (100 mg kg−1; i.p.) also strikingly (70%) reduced LPS-induced NF-κB activation (Figure 6).
Figure 6.
Effect of α-humulene or trans-caryophyllene on LPS-induced NF-κB activation. Rats were treated orally with α-humulene (50 mg kg−1, p.o.) or trans-caryophyllene (50 mg kg−1, p.o.) 1 h before LPS (1 μg per paw) i.pl injection. The NF-κB inhibitor, PDTC (100 mg kg−1, i.p.) was administered 1 h before LPS. Rat paws were collected 1 h after LPS. (a) EMSA and (b) summary of results for NF-κB activation. Each point represents the mean±s.e.m. of three animals. Statistical analysis was carried out using one-way ANOVA followed by the Newman Keuls'post hoc test. ##P<0.01 compared to the saline-treated group. *P<0.05 and **P<0.01 compared to the vehicle-treated group. Hum, α-humulene; Car, trans-caryophyllene.
Discussion and conclusions
The use of natural products, especially those derived from medicinal plants, is a traditional form of providing relief from illness. Over the years, natural products have contributed enormously to the development of important therapeutic drugs used currently in modern medicine (Cragg et al., 1997; De Smet, 1997; Surh et al., 2005). Recently, we have shown that the essential oil of C. verbenacea displays marked anti-inflammatory activity, on oral administration, in different models of inflammation, but at the molecular level, the mechanisms underlying its anti-inflammatory activity remain unclear (Passos et al., 2007). In the current study, we have shown for the first time, that oral administration of one component of the essential oil from C. verbenacea, namely α-humulene, but not trans-caryophyllene, prevented LPS-induced rat paw oedema, increase in the levels of TNF-α and IL-1β, as well as kinin B1 receptor upregulation in the rat paw in vivo. In addition, both α-humulene and trans-caryophyllene given orally to rats were capable of inhibiting neutrophil influx into the inflammatory site and NF-κB activation, but not ERK, p38 MAPK or JNK activation, induced by local LPS treatment.
In recent years, there has been considerable improvement in identifying the molecular events that are linked with the multistage process of chronic inflammatory and pain conditions. Because of the important relationship between inflammation and the kinin system, the B1 receptor has been considered as one of the potential targets for development of drugs for the treatment of inflammatory disorders (Calixto et al., 2004b; Marceau and Regoli, 2004; Campos et al., 2006). Evidence exists that the selective blockade of B1 receptors protects animals against inflammatory, infectious and painful stimuli (Calixto et al., 2004b; Marceau and Regoli, 2004). In this context, it has been reported that peptidergic and non-peptidergic selective B1 receptor antagonists inhibit des-Arg9-BK-induced paw oedema and capsaicin-induced ear oedema in mice, tissue destruction and neutrophil accumulation in the rat intestine following splanchnic artery occlusion/reperfusion, thermal hyperalgesia induced by UV irradiation and nociceptive response to formalin in rats, as well as neuropathic thermal pain induced by sciatic nerve constriction (Hoffmann et al., 1998; Gougat et al., 2004; Conley et al., 2005; Ferreira et al., 2005; Porreca et al., 2006). Our present results show that neither α-humulene nor trans-caryophyllene displayed a antagonistic effect directly at the bradykinin B1 receptor. This conclusion derives from our finding that, in contrast to what was observed for the selective B1 receptor antagonist des-Arg9-[Leu8]-BK, α-humulene and trans-caryophyllene completely failed to interfere with des-Arg9-BK-induced paw oedema formation in rats that had been treated 11 h after LPS injection.
Since B1 receptors are not constitutively present under normal conditions, and bearing in mind the fact that they might be overexpressed following some inflammatory stimuli, including LPS, we assessed the possible inhibitory effect of the two compounds on the upregulation of B1 receptors. Of interest, our data indicated that oral treatment with α-humulene, but not with trans-caryophyllene, markedly reduced des-Arg9-BK-induced paw oedema when the rats were treated 1 h before LPS injection. In addition, analysis of the B1 receptor mRNA levels in the rat paw indicated that α-humulene, but not trans-caryophyllene, like the anti-inflammatory steroid dexamethasone, reduced B1 receptor expression.
The inflammatory response involves a highly complex interplay between multiple factors at humoral and cellular levels (Ahluwalia and Perretti, 1999). Exposure to exogenous bacterial toxins such as LPS stimulates tissue resident cells to produce inflammatory cytokines, chemokines and adhesion molecules (Beutler and Rietschel, 2003). Reports regarding the effects of cytokines on the kinin system have indicated that certain cytokines, namely IL-1β and TNF-α, are involved in the process of B1 receptor upregulation in several tissues and under various conditions, both in vitro and in vivo (Campos et al., 1999; Passos et al., 2004; Rocha et al., 2005). Data presented herein confirm and also largely extends this evidence by demonstrating that α-humulene, but not trans-caryophyllene, inhibited the increase in the levels of TNF-α and IL-1β in rat paws treated with LPS. Such increases in cytokine levels might result in plasma protein extravasation and cellular infiltration into the inflammatory site (Rosenbaum and Enkel, 1987; Rosenbaum and Boney, 1991; Thorlacius et al., 1997; Derevianko et al., 1998; Grutkoski et al., 1999).
Our previous studies have demonstrated the importance of neutrophil influx for B1 receptor upregulation (Campos et al., 1999; Vianna et al., 2003; Passos et al., 2004) and our present results have revealed that oral treatment of rats with α-humulene and, to a lesser extent, with trans-caryophyllene, reduces cellular infiltration into the rat paw. These observations support the inhibitory properties of α-humulene in relation to B1-receptor upregulation by LPS, in both functional and molecular biology approaches. Compared with α-humulene, trans-caryophyllene displayed only a partial inhibition of neutrophil migration, as assessed by MPO activity, which might help to explain its lack of effect on cytokine production and consequently on kinin B1 receptor upregulation.
LPS is the major component of the outer membrane of Gram-negative bacteria and the transduction pathways activated by LPS include MAP kinases and IκB kinase, which control gene expression through transcriptional factors such as activator protein-1 and NF-κB (Hwang et al., 1997; Lawrence et al., 2001; Beutler, 2002; Miyake, 2004). Several lines of evidence have emphasized the importance of different members of the MAP kinase family for the upregulation of B1 receptors (Calixto et al., 2004b). In the present study, we show that i.pl injection of LPS leads to a time-dependent activation of ERK, JNK and p38 MAPK in the rat paw. However, both α-humulene and trans-caryophyllene failed to interfere with this LPS-induced MAP kinase activation.
NF-κB is clearly one of the most important regulators of proinflammatory gene expression (Karin, 2005). A series of publications (using both in vivo and in vitro approaches) have provided convincing evidence of the relevance of NF-κB for the upregulation of B1 receptor among other inflammatory proteins (Ni et al., 1998; Schanstra et al., 1998; Campos et al., 1999; Medeiros et al., 2001, 2004; Sabourin et al., 2002; Passos et al., 2004; El Sayah et al., 2006). In fact, the postulated NF-κB binding site on the promoter for the B1 receptor seems to be mainly responsible for its inducibility in response to IL-1β, TNF-α and LPS (Ni et al., 1998). In the present study using EMSA, we have clearly shown that oral administration of α-humulene, like that of the NF-κB inhibitor PDTC, greatly reduced NF-κB activation in the LPS-treated paws. Surprisingly, a significant inhibition was also observed when animals were treated with trans-caryophyllene. Although at this stage of our work we cannot determine the exact mechanism(s) through which these compounds exert their inhibitory effect in the NF-κB activation, it is reasonable to surmise that these compounds might control the expression of several inflammatory proteins (for example, COX-2 and iNOS). However, it is important to mention that although trans-caryophyllene produced a marked inhibition of NF-κB activation, it was not able to inhibit B1 receptor upregulation, as demonstrated for α-humulene. It is tempting to suggest that blocking of multiple cellular pathways is necessary for preventing B1-receptor induction. Further investigation should provide additional biochemical data to elucidate the precise anti-inflammatory mechanisms of trans-caryophyllene.
Overall, the present findings provide consistent evidence that two compounds from C. verbenacea, α-humulene and trans-caryophyllene prevent LPS-induced inflammation through NF-κB inhibition. Additional studies are necessary to establish the exact anti-inflammatory properties of trans-caryophyllene on this process. However, it is possible now to suggest that α-humulene might constitute a relevant alternative for the control of B1-receptor upregulation. Nevertheless, we cannot discard the possibility that other mechanisms, besides B1 receptor modulation, might contribute to the in vivo anti-inflammatory actions of α-humulene. Further characterization of candidate targets for this compound would help to clarify the potential of these two sesquiterpene natural products, as therapeutic alternatives for the treatment of inflammatory pathologies, principally those where B1 receptors may be involved.
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Programa de Apoio aos Núcleos de Excelência (PRONEX), Fundação de Apoio a Pesquisa do Estado de Santa Catarina (FAPESC) (Brazil) and by Aché Laboratórios Farmacêuticos (Brazil). RM and GFP are post-graduate students in Pharmacology receiving grants from CNPq. JB Calixto has received research funding from Aché Laboratórios Farmacêuticos (Brazil).
Abbreviations
- ERK
extracellular-regulated kinase
- i.pl
intraplantar
- IL-1β
interleukin-1β
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAP kinases
mitogen-activated protein kinases
- MEK
mitogen-activated protein kinase kinase
- NF-κB
nuclear factor-κB
- PBS
phosphate-buffered saline
- PDTC
pyrrolidinedithiocarbamate
- PMSF
phenylmethylsulphonyl fluoride
- TNF-α
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Ahluwalia A, Perretti M. B1 receptors as a new inflammatory target. Could this B the 1. Trends Pharmacol Sci. 1999;20:100–104. doi: 10.1016/s0165-6147(99)01321-8. [DOI] [PubMed] [Google Scholar]
- Beutler B. LPS in microbial pathogenesis: promise and fulfilment. J Endotoxin Res. 2002;8:329–335. doi: 10.1179/096805102125000650. [DOI] [PubMed] [Google Scholar]
- Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Cabrini DA, Ferreira J, Campos MM. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Campos MM, Otuki MF, Santos AR. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004a;70:93–103. doi: 10.1055/s-2004-815483. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Medeiros R, Fernandes ES, Ferreira J, Cabrini DA, Campos MM. Kinin B1 receptors: key G-protein-coupled receptors and their role in inflammatory and painful processes. Br J Pharmacol. 2004b;143:803–818. doi: 10.1038/sj.bjp.0706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto JB, Otuki MF, Santos AR. Anti-inflammatory compounds of plant origin. Part I. Action on arachidonic acid pathway, nitric oxide and nuclear factor kappa B (NF-kappaB) Planta Med. 2003;69:973–983. doi: 10.1055/s-2003-45141. [DOI] [PubMed] [Google Scholar]
- Campos MM, Leal PC, Yunes RA, Calixto JB. Non-peptide antagonists for kinin B1 receptors: new insights into their therapeutic potential for the management of inflammatory and pain diseases. Trends Pharmacol Sci. 2006;27:646–651. doi: 10.1016/j.tips.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Campos MM, Souza GE, Calixto JB. In vivo B1 kinin-receptor upregulation. Evidence for involvement of protein kinases and nuclear factor kappaB pathways. Br J Pharmacol. 1999;127:1851–1859. doi: 10.1038/sj.bjp.0702715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley RK, Wheeldon A, Webb JK, DiPardo RM, Homnick CF, Bock MG, et al. Inhibition of acute nociceptive responses in rat spinal cord by a bradykinin B1 receptor antagonist. Eur J Pharmacol. 2005;527:44–51. doi: 10.1016/j.ejphar.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997;60:52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- de Carvalho PM, Jr, Rodrigues RF, Sawaya AC, Marques MO, Shimizu MT. Chemical composition and antimicrobial activity of the essential oil of Cordiaverbenacea DC. J Ethnopharmacol. 2004;95:297–301. doi: 10.1016/j.jep.2004.07.028. [DOI] [PubMed] [Google Scholar]
- De Smet PA. The role of plant-derived drugs and herbal medicines in healthcare. Drugs. 1997;54:801–840. doi: 10.2165/00003495-199754060-00003. [DOI] [PubMed] [Google Scholar]
- Derevianko A, Graeber T, D'Amico R, Simms HH. The role of neutrophil-derived oxidants as second messengers in interleukin 1beta-stimulated cells. Shock. 1998;10:54–61. doi: 10.1097/00024382-199807000-00010. [DOI] [PubMed] [Google Scholar]
- El Sayah M, Medeiros R, Fernandes ES, Campos MM, Calixto JB. Mechanisms underlying lipopolysaccharide-induced kinin B1 receptor up-regulation in the pig iris sphincter in vitro. Mol Pharmacol. 2006;69:1701–1708. doi: 10.1124/mol.105.021097. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Beirith A, Mori MA, Araujo RC, Bader M, Pesquero JB, et al. Reduced nerve injury-induced neuropathic pain in kinin B1 receptor knock-out mice. J Neurosci. 2005;25:2405–2412. doi: 10.1523/JNEUROSCI.2466-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougat J, Ferrari B, Sarran L, Planchenault C, Poncelet M, Maruani J, et al. SSR240612 [(2R)-2-[((3R)-3-(1,3-benzodioxol-5-yl)-3-[[(6-methoxy-2-naphthyl)sulfonyl]amino]propanoyl)amino]-3-(4-[[2R,6S)-2,6-dimethylpiperidinyl]methyl]phenyl)-N-isopropyl-N-methylpropanamide hydrochloride], a new nonpeptide antagonist of the bradykinin B1 receptor: biochemical and pharmacological characterization. J Pharmacol Exp Ther. 2004;309:661–669. doi: 10.1124/jpet.103.059527. [DOI] [PubMed] [Google Scholar]
- Grutkoski PS, D'Amico R, Ayala A, Simms HH. IL-1beta stimulation induces paracrine regulation of PMN function and apoptosis. Shock. 1999;12:373–381. doi: 10.1097/00024382-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Hoffmann TF, Kubler J, Messmer K. Selective bradykinin B1 receptor block in ischemia/reperfusion of the rat pancreas. Langenbecks Arch Chir Suppl Kongressbd. 1998;115:443–446. [PubMed] [Google Scholar]
- Hwang D, Jang BC, Yu G, Boudreau M. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide: mediation through both mitogen-activated protein kinase and NF-kappaB signaling pathways in macrophages. Biochem Pharmacol. 1997;54:87–96. doi: 10.1016/s0006-2952(97)00154-8. [DOI] [PubMed] [Google Scholar]
- Karin M.Inflammation-activated protein kinases as targets for drug development Proc Am Thorac Soc 20052386–390.discussion 394–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Marceau F, Regoli D. Bradykinin receptor ligands: therapeutic perspectives. Nat Rev Drug Discov. 2004;3:845–852. doi: 10.1038/nrd1522. [DOI] [PubMed] [Google Scholar]
- Medeiros R, Cabrini DA, Calixto JB. The ‘in vivo' and ‘ex vivo' roles of cylcooxygenase-2, nuclear factor-kappaB and protein kinases pathways in the up-regulation of B1 receptor-mediated contraction of the rabbit aorta. Regul Pept. 2001;97:121–130. doi: 10.1016/s0167-0115(00)00186-5. [DOI] [PubMed] [Google Scholar]
- Medeiros R, Cabrini DA, Ferreira J, Fernandes ES, Mori MA, Pesquero JB, et al. Bradykinin B1 receptor expression induced by tissue damage in the rat portal vein: a critical role for mitogen-activated protein kinase and nuclear factor-kappaB signaling pathways. Circ Res. 2004;94:1375–1382. doi: 10.1161/01.RES.0000128404.65887.08. [DOI] [PubMed] [Google Scholar]
- Miyake K. Innate recognition of lipopolysaccharide by Toll-like receptor 4-MD-2. Trends Microbiol. 2004;12:186–192. doi: 10.1016/j.tim.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38. doi: 10.1254/jphs.srj05001x. [DOI] [PubMed] [Google Scholar]
- Ni A, Chao L, Chao J. Transcription factor nuclear factor kappaB regulates the inducible expression of the human B1 receptor gene in inflammation. J Biol Chem. 1998;273:2784–2791. doi: 10.1074/jbc.273.5.2784. [DOI] [PubMed] [Google Scholar]
- Passos GF, Fernandes ES, Campos MM, Araujo JG, Pesquero JL, Souza GE, et al. Kinin B1 receptor up-regulation after lipopolysaccharide administration: role of proinflammatory cytokines and neutrophil influx. J Immunol. 2004;172:1839–1847. doi: 10.4049/jimmunol.172.3.1839. [DOI] [PubMed] [Google Scholar]
- Passos GF, Fernandes ES, Cunha FM, Ferreira J, Pianowski LF, Campos MM, et al. Anti-inflammatory and anti-allergic properties of the essential oil and active compounds from Cordia verbenacea. J Ethnopharmacol. 2007;21:323–333. doi: 10.1016/j.jep.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Porreca F, Vanderah TW, Guo W, Barth M, Dodey P, Peyrou V, et al. Antinociceptive pharmacology of N-[[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]methyl]-2-[2-[[(4-methoxy-2,6-dimethylphenyl) sulfonyl]methylamino]ethoxy]-N-methylacetamide, fumarate (LF22-0542), a novel nonpeptidic bradykinin B1 receptor antagonist. J Pharmacol Exp Ther. 2006;318:195–205. doi: 10.1124/jpet.105.098368. [DOI] [PubMed] [Google Scholar]
- Prado GN, Taylor L, Zhou X, Ricupero D, Mierke DF, Polgar P. Mechanisms regulating the expression, self-maintenance, and signaling-function of the bradykinin B2 and B1 receptors. J Cell Physiol. 2002;193:275–286. doi: 10.1002/jcp.10175. [DOI] [PubMed] [Google Scholar]
- Regoli D, Barabe J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- Rocha AC, Fernandes ES, Passos GF, Calixto JB, Campos MM. Assessment of TNFalpha contribution to the functional up-regulation of kinin B(1) receptors in the mouse paw after treatment with LPS. Int Immunopharmacol. 2005;5:1593–1600. doi: 10.1016/j.intimp.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JT, Boney RS. Use of a soluble interleukin-1 receptor to inhibit ocular inflammation. Curr Eye Res. 1991;10:1137–1139. doi: 10.3109/02713689109024131. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JT, Enkel H. Stimulus-specific effects of endotoxin on superoxide production by rabbit polymorphonuclear leukocytes. Yale J Biol Med. 1987;60:391–396. [PMC free article] [PubMed] [Google Scholar]
- Rossner M, Yamada KM. What's in a picture? The temptation of image manipulation. J Cell Biol. 2004;166:11–15. doi: 10.1083/jcb.200406019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin T, Morissette G, Bouthillier J, Levesque L, Marceau F. Expression of kinin B(1) receptor in fresh or cultured rabbit aortic smooth muscle: role of NF-kappa B. Am J Physiol Heart Circ Physiol. 2002;283:H227–H237. doi: 10.1152/ajpheart.00978.2001. [DOI] [PubMed] [Google Scholar]
- Schanstra JP, Bataille E, Marin Castano ME, Barascud Y, Hirtz C, Pesquero JB, et al. The B1-agonist [des-Arg10]-kallidin activates transcription factor NF-kappaB and induces homologous upregulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J Clin Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005;135:2993S–3001S. doi: 10.1093/jn/135.12.2993S. [DOI] [PubMed] [Google Scholar]
- Thorlacius H, Lindbom L, Raud J. Cytokine-induced leukocyte rolling in mouse cremaster muscle arterioles in P-selectin dependent. Am J Physiol. 1997;272:H1725–H1729. doi: 10.1152/ajpheart.1997.272.4.H1725. [DOI] [PubMed] [Google Scholar]
- Vianna RM, Ongali B, Regoli D, Calixto JB, Couture R. Up-regulation of kinin B1 receptor in the lung of streptozotocin-diabetic rat: autoradiographic and functional evidence. Br J Pharmacol. 2003;138:13–22. doi: 10.1038/sj.bjp.0704999. [DOI] [PMC free article] [PubMed] [Google Scholar]