Abstract
Background and purpose:
The serine protease neutrophil elastase (NE) appears to regulate inflammatory responses at multiple levels but its role in leukocyte transmigration in vivo remains unclear. The present study aimed to address this issue by using both an NE inhibitor (ONO-5046) and NE deficient (NE−/−) mice.
Experimental approach:
A number of inflammatory mediators (LTB4, KC and PAF) were investigated in vitro for their ability to stimulate the release and the surface expression of NE by neutrophils. In addition, the role of NE in leukocyte migration elicited by topical LTB4 was investigated in vivo in mouse cremasteric venules as observed by intravital microscopy.
Key results:
Amongst the mediators tested in vitro, LTB4 was found to be a highly potent and efficacious inducer of NE cell surface expression on murine neutrophils. Furthermore, in wild-type mice (WT), LTB4-induced leukocyte transmigration was reduced by intravenous ONO-5046 (66% inhibition), an effect that appeared to occur at the level of the perivascular basement membrane. Interestingly, LTB4-induced responses were normal in NE−/− mice and, while ONO-5046 had no inhibitory effect in these animals, the broad-spectrum serine protease inhibitor aprotinin suppressed leukocyte transmigration in both WT and NE−/− mice.
Conclusions and implications:
The findings demonstrate the potent ability of LTB4 to induce cell-surface expression of NE and provide evidence for the involvement of NE in LTB4-induced neutrophil transmigration in vivo. The results also suggest the existence of compensatory mechanisms in NE−/− mice, highlighting the added value of investigating pharmacological blockers in parallel with genetic deletion.
Keywords: neutrophil elastase, inflammation, cell trafficking, basement membrane, intravital microscopy
Introduction
Neutrophil elastase (NE) is a 30 kD neutral serine protease stored in an active form in the azurophil granules of neutrophils. The potential substrates of NE include most components of the extracellular matrix (for example, collagen, fibronectin and laminin) as well as a wide range of other proteins such as cytokines, clotting factors, adhesion molecules and components of the complement cascade (Lee and Downey, 2001). In addition, NE has been shown to regulate the activity of certain enzymes such as activation of matrix metalloproteinase-9 (gelatinase B) from its pro-isoform (Delclaux et al., 1996). As a result of this broad substrate specificity and the ability of this enzyme to cause tissue damage, NE has been implicated in the pathogenesis of numerous inflammatory conditions (Weiss, 1989) and indeed it is frequently used as both a predictor and an indicator of inflammatory disease severity (for example, Groeneveld et al., 1995; Orem et al., 1997; Smith et al., 2000). Specifically, NE has been found in elevated levels in synovial fluid and/or blood in rheumatoid arthritis (Elsaid et al., 2003) and under conditions of ischemia/reperfusion injury (Vila et al., 1999). Furthermore, NE is found at high levels in bronchoalveolar lavage of patients with inflammatory lung disorders such as chronic obstructive pulmonary disease, acute lung injury, cystic fibrosis and adult respiratory distress syndrome, disorders in which it has been strongly implicated (Lee and Downey, 2001; Shapiro and Ingenito, 2005).
Considering the destructive nature of NE, a pathological role for this enzyme under inflammatory conditions where it is released inappropriately, in excess and/or in a prolonged manner, is entirely comprehensible (Lee and Downey, 2001; Shapiro and Ingenito, 2005). However, since NE is stored within neutrophil granules at high concentrations (estimated at ∼5 mM), it is also entirely appropriate to consider a physiological role for this enzyme in host defence (Barrick et al., 1999; Shapiro, 2002). In this context, there is much evidence for the antibactericidal activity of NE (Gabay and Almeida, 1993), a concept for which direct support has been obtained from studies using NE-deficient mice (Belaaouaj et al., 1998; Tkalcevic et al., 2000). There is also evidence to suggest that NE may play a critical role in regulating the bioavailability of inflammatory cytokines and chemokines in inflammation (Bank and Ansorge, 2001; Adkison et al., 2002; Young et al., 2004), thus contributing to the onset and/or termination of an inflammatory response. NE may also have additional roles in the process of wound healing (Barrick et al., 1999) but a controversial possibility relates to the potential role of NE in mediating leukocyte migration, specifically migration through venular walls, that is, through endothelial cells and/or its associated perivascular basement membrane (BM). In support of this, NE inhibitors have been shown to suppress neutrophil migration through BM-like structures (Delclaux et al., 1996; Delacourt et al., 2002) and to degrade or penetrate components of the venular BM (Heck et al., 1990; Steadman et al., 1993; Wang et al., 2005, 2006). Refuting a role for NE in leukocyte transmigration there are the findings of Huber and Weiss indicating that neutrophil migration through endothelial cell BM is not inhibited by a range of protease inhibitors (Huber and Weiss, 1989). Similar negative results were obtained in other studies employing NE inhibitors and investigating neutrophil migration through cultured endothelial cells or venular walls (Furie et al., 1987; Rosengren and Arfors, 1990; Mackarel et al., 1999). Furthermore, NE-deficient neutrophils exhibit a normal transendothelial cell migration response in vitro (Allport et al., 2002) and NE-deficient mice exhibit a largely normal profile of neutrophil transmigration in vivo (Belaaouaj et al., 1998; Tkalcevic et al., 2000; Hirche et al., 2004).
Despite the discrepancies detailed above, there remains a plausible possibility that NE may play a role in regulating neutrophil migration through venular walls. Of relevance, neutrophils can be stimulated in vitro to express NE on their cell surface, conditions under which the enzyme has been shown to remain active and resistant to endogenous inhibitors (Owen et al., 1995, 1997) and neutrophils migrating through endothelial cell junctions in vitro and transmigrated neutrophils in vivo have been shown to express NE on their cell surface (Cepinskas et al., 1999; Wang et al., 2005). In addition and of direct relevance, we have recently found that a specific NE inhibitor suppresses neutrophil migration through IL-1β-stimulated cremasteric venules at the level of the basement membrane (Wang et al., 2005), an effect that appears to be related to the ability of NE to facilitate migration of neutrophils through vulnerable regions within the venular BM (Wang et al., 2006). To extend these findings, the present study addressed the role of NE in neutrophil migration induced by topical LTB4 in the mouse cremaster muscle, as observed by intravital microscopy and investigated using both an NE inhibitor and NE-deficient mice. LTB4 was chosen as the stimulus under investigation since in initial studies this chemoattractant was found to be a potent and efficacious stimulus in inducing cell surface expression of NE on mouse neutrophils and in eliciting neutrophil transmigration in vivo. Collectively the findings of the present study provide evidence for the potent ability of LTB4 to induce cell surface expression of NE on murine neutrophils and for a specific NE inhibitor to suppress LTB4-induced neutrophil migration through the BM, though no such defect was seen in NE-deficient mice, possibly due to existence of compensatory mechanisms in the latter.
Materials and methods
Animals
Twenty to twenty-five gram male wild-type (WT) C57BL/6 mice (Harlan Olac, Bicester, UK) were purchased at least 2 weeks in advance of being employed in the studies. Mice deficient in neutrophil elastase (NE−/−), generated by targeted gene disruption as detailed previously (Belaaouaj et al., 1998) were a gift from Professor S Shapiro (Harvard Medical School, Boston, MA, USA) and were backcrossed onto a C57BL/6 background for six generations before their use. All experiments on animals have been reviewed and approved by the local ethical review panel of Imperial College, London, and by the UK Home Office.
Quantification of NE cell surface expression/release in vitro
Neutrophils were isolated from the bone marrow of WT and NE−/− mice as detailed previously (Young et al., 2004; Wang et al., 2005). Briefly, femurs from two mice per group were removed and flushed with sterile Hank's balanced salt solution. Neutrophils were purified using a discontinuous Percoll gradient and then washed before being resuspended in modified PBS containing 0.25% bovine serum albumin (BSA), 5 mM glucose, 1 mM Ca2+/Mg2+. The cell suspension purity was assessed by Kimura's stain and found to be typically >80% (contaminating cells consisting of mononuclear cells). For performing the assays, pooled neutrophils were added to BSA-coated (1 μg ml−1) 96-well plates (5 × 105 neutrophils per well) and were allowed to adhere for 15 min at 37°C before addition of stimuli. LTB4, PAF, the chemokine KC (CXCL1; considered to be a murine homologue of human IL-8/CXCL8) or modified PBS (control) was added to neutrophils and the plates were then incubated at 37°C for a further 30 min period. The plates were then centrifuged to allow for removal of supernatants for subsequent analysis of released elastase. The pelleted cells were then fixed using 3% paraformaldehyde/0.5% glutaraldehyde and finally resuspended in Tris buffer (0.2 M Tris base, 0.15 M NaCl, 0.02 M CaCl2 at pH8.5) for assay of cell surface NE as described previously (Owen et al., 1995; Young et al., 2004). Enzyme activity was assayed using a fluorogenic substrate specific for elastase, methoxysuccinyl-Ala-Ala-Pro-Val-7-amino-4-trifluoromethyl coumarin (MeOSuc-Ala-Ala-Pro-Val-AFC), as described by Owen et al. (1995). This substrate has previously been shown not to access intracellular stores of NE and hence, using fixed intact cells (to retain cell-associated NE), this substrate can be used to quantify cell-bound enzyme activity. Briefly, fixed intact cells, cell lysates (obtained following addition of 1% of Triton X-100 to cell pellets) or purified human NE (used to establish a standard curve) were incubated with 400 μM substrate in 0.2 M tris(hydroxymethyl)-aminomethane (pH 8.5) containing 0.15 M NaCl and 0.02 M CaCl2. After incubation at 37°C for 25 min in the dark, the level of liberated 7-amino-4-trifluoromethyl coumarin was quantified in a fluorescent plate reader (Millipore, Watford, UK) using excitation 409 nm and emission 530 nm. A standard curve, incorporated into each experiment, was established using commercially obtained purified human NE and was used to represent the results in terms of murine neutrophil enzyme activity equivalent to the activity detected in a known concentration (ng ml−1) of purified human NE.
Chemotaxis assay
Purified neutrophils (25 μl of 4 × 106 cells ml−1 solution) were placed on the top compartment of 3 μm diameter pore filtered chemotaxis chambers (NeuroProbe, Gaithersburg, MD, USA). The bottom wells contained 28 μl of PBS supplemented with 0.25% of BSA, 1 mM of Ca2+/Mg2+ and 5 mM of glucose in the presence or the absence of a range of concentrations of LTB4, PAF or KC. The chambers were then incubated at 37°C in a humidified atmosphere (5% CO2) for 4 h. Supernatant from the upper chambers (containing the non-transmigrated cells) was removed, the plates spun and the lower chamber supernatants harvested to count the number of migrated cells by microscopy using a Neubauer hematocytometer. Results are expressed as the number of transmigrated cells/chemotaxis well.
Intravital microscopy of murine cremasteric venules
Intravital microscopy was used to observe leukocyte responses within mouse cremasteric venules as previously described (Thompson et al., 2001). Briefly, after induction of anaesthesia with ketamine (100 mg kg−1) and xylazine (10 mg kg−1) by intraperitoneal injection, the carotid and jugular vein were cannulated for measurement of mean arterial pressure and the administration of drugs, respectively. Animals were maintained at 37°C on a custom-built heated Perspex microscope stage and the cremaster muscle was surgically exteriorized. The tissue was kept warm and moist throughout each experiment by superfusion of warmed Tyrode's balanced salt solution and leukocyte-endothelial cell interactions were observed on an upright fixed-stage microscope (Axioscop FS, Carl Zeiss, Welwyn Garden City, UK) fitted with water immersion objectives. Basal readings of leukocyte firm adhesion and transmigration were then quantified for 15 min at 5 min intervals, while the tissue was superfused with Tyrode's balanced salt solution. At time 0, LTB4 (10−8 M or 10−7 M), PAF (10−7 M) or KC (10−8 M) was added to the superfusion buffer and readings taken at 10–15 min intervals for 60 min. Some mice received the elastase inhibitor, ONO-5046 (Kawabata et al., 1991), the serine protease inhibitor, aprotinin, or the vehicle, saline, by a cannula inserted into the jugular vein. Owing to the short plasma half-life of these drugs, both were administered first as a bolus (50 mg kg−1 200 μl−1 for ONO-5046; 100 000KIU kg−1 200 μl−1 for aprotinin) at t=−10 min and then as a continuous infusion (50 mg kg−1 h−1 for ONO-5046; 100 000KIU kg−1 h−1 for aprotinin) until the end of the experiment. The dosing regimes employed were selected based on advice from manufacturers/suppliers and also based on previous experiments from our group (Wang et al., 2005, 2006). Firmly adherent leukocytes were considered as those remaining stationary for at least 30 s within a given 100 μm vessel segment. Transmigrated leukocytes were quantified as those in the extravascular tissue within 50 μm of the 100 μm vessel segments quantified. In each animal, all parameters were quantified in several vessel segments (4–5), within multiple venules (3–5) and averaged.
Confocal microscopy
Cremaster muscles were dissected away from mice, fixed in 4% paraformaldehyde overnight and immunostained for components of venular walls to localize position of leukocytes, as observed by confocal microscopy, as detailed previously (Wang et al., 2005). Briefly, following a blocking/permeablilization step in PBS supplemented with 20% FCS serum and 0.5% Triton X-100 for 1 h at room temperature, tissues were incubated with primary antibodies against the endothelial cell basement membrane component laminin 10 (anti-laminin α5 chain polyclonal antibody 405, gift from Professor LM Sorokin, University Waldeyerstrasse, Muenster, Germany) (Sorokin et al., 1997) and the cytosolic neutrophil-specific protein MRP-14 (rat anti-mouse MRP-14 clone 2B10, gift from Dr Nancy Hogg, Cancer Research UK, London, UK) (Newton and Hogg, 1998) at room temperature, overnight. The samples were then incubated with goat anti-rabbit and goat anti-rat secondary antibodies directly conjugated to Alexa Fluor 488 and 633, respectively (Invitrogen, Paisley, UK) at room temperature, for 3 h. Following washes in PBS and a second blocking step in PBS supplemented with 20% FCS for 2 h at room temperature, a third immunostaining step was added using APC-conjugated primary antibody against the endothelial junctional molecule PECAM-1 (rat anti-mouse PECAM-1 clone Mec13.3, BD-Pharmingen, Oxford, UK) for 3 h. In all studies, appropriate control antibodies were used in parallel with the specific primary antibodies. Samples were viewed using a Zeiss LSM 5 PASCAL confocal laser-scanning microscope to detect the position of leukocytes in the vessel wall of immunostained tissues.
Statistical analysis
All results are expressed as mean±s.e.m. Statistical significance was assessed by one-way analysis of variance with Neuman–Keuls multiple comparison test. Where two variables were analysed a Student's t-test was used. P<0.05 was considered significant.
Reagents
The following reagents were obtained commercially: ketamine (Ketalar, Parke-Davis, Eastleigh, UK), xylazine (Rompun, Bayer, Bury St Edmunds, UK), methoxysuccinyl-Ala-Ala-Pro-Val-7-amino-4-trifluoromethyl coumarin (MeOSuc-Ala-Ala-Pro-Val-AFC) (Enzyme Systems Products, Livermore, CA, USA), human NE (Merck Biosciences, Nottingham, UK). All other general reagents were purchased from Sigma-Aldrich (Poole, UK). The synthetic and specific NE inhibitor ONO-5046 (Sivelestat) (Kawabata et al., 1991) was a kind gift from ONO Pharmaceuticals (Osaka, Japan).
Results
LTB4 is a potent inducer of murine neutrophil cell surface expression of NE in vitro
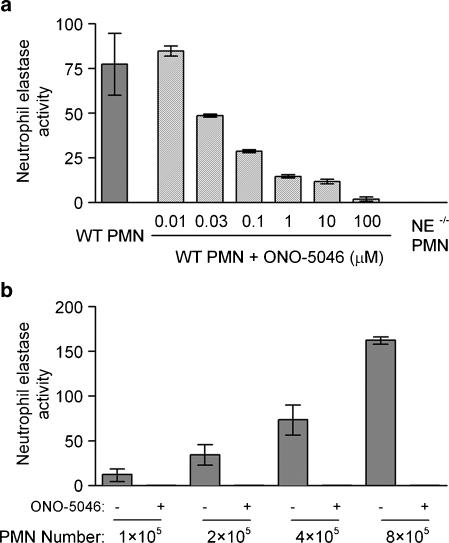
For NE to participate in leukocyte migration it must be mobilized from its intracellular azurophil granule stores to the cell surface or released locally into the extracellular environment in response to appropriate stimuli. To investigate the ability of a number of neutrophil chemoattractants to induce NE release and/or cell surface NE expression, an in vitro assay for detection of murine NE activity was developed as described previously (Young et al., 2004). The fluorogenic NE-specific substrate employed, methoxysuccinyl-Ala-Ala-Pro-Val-7-amino-4-trifluoromethyl coumarin (MeOSuc-Ala-Ala-Pro-Val-AFC), has previously been shown not to access intracellular stores of NE (Owen et al., 1995), and hence when using fixed intact cells (to retain cell-associated NE), the substrate can be used to quantify cell surface activity by quantifying the level of liberated 7-amino-4-trifluoromethyl coumarin. As this assay was originally developed for detection of human NE activity (Owen et al., 1995), its specificity for detection of murine NE activity was confirmed by the absence of detectable activity in lysates of neutrophils from NE-deficient mice and inhibition of activity by a specific NE inhibitor, ONO-5046 (Figure 1a and b).
Figure 1.
Characterization of an in vitro assay for detection of murine neutrophil elastase (NE) activity. Neutrophils from wild-type (WT) and NE−/− mice were purified from bone marrow as described in Materials and methods, seeded onto bovine serum albumin (BSA)-coated plates and lysed with 1% Triton X-100. Following centrifugation, supernatants were harvested and incubated for 25 min at 37°C with 400 μM of a fluorogenic NE-specific substrate (MeOsuc-Ala-Ala-Pro-Val-AFC). Murine NE activity was detected using a fluorescent plate reader (excitation: 405 nm, emission: 530 nm) and compared with a standard curve established using commercially available purified human NE. In some experiments, ONO-5046 (Sivelestat), a specific NE inhibitor, was added to the wells at the concentrations indicated. Results are expressed as murine NE activity equivalent to the activity detected from ng ml−1 of purified human NE. (a) The total amount of murine NE activity expressed by 4 × 105 lysed neutrophils from WT, ONO-5046-treated WT and NE−/− mice. (b) The increasing amount of NE activity relative to the number of neutrophils lysed, in the absence or presence of the NE inhibitor ONO-5046. Graphs shown are representative of n=3 experiments (two mice used per experiment).
The agonists KC (CXCL1), PAF and LTB4 were investigated for their ability to stimulate NE release/cell surface expression using murine neutrophils, purified from bone marrow. Figure 2a shows that with respect to both NE release and cell surface expression, LTB4 was more potent and efficacious than the other stimuli tested. Furthermore, LTB4 appeared to be more effective at eliciting cell surface expression of NE as opposed to inducing NE release and indeed in some studies, LTB4 was found to induce a small level of cell-associated NE at concentrations of less than 10−12 M (data not shown). Interestingly, LTB4 was also found to be the more potent and efficacious stimulus when tested in a chemotaxis assay (Figure 2b). Specifically, LTB4 caused significant leukocyte migration through Boyden chamber filters at all concentrations investigated in the range of 10−9–10−7 M, while a significant response to KC was only noted at 10−8 and 10−7 M and PAF failed to elicit a detectable chemotaxis response within the concentrations tested (10−10–10−7 M).
Figure 2.
Effect of soluble chemoattractants on cell surface expression and release of NE and on chemotaxis in vitro of murine neutrophils. Neutrophils were isolated from wild-type mouse bone marrow and differential counts performed to enable addition of 5 × 105 neutrophils per well to bovine serum albumin (BSA)-coated 96-well plates as detailed in Materials and methods. Cells were incubated in plates for 15 min before addition of LTB4, PAF and KC (10−10–10−7 M) for 30 min. Plates were spun and supernatants collected for assay of NE activity before the pelleted cells were fixed. Cells or supernatants were then incubated for 25 min at 37°C with 400 μM of the NE-specific fluorogenic substrate. NE activity was detected using a fluorescent plate reader and compared with a standard curve created using purified human NE as detailed in Materials and methods. (a) Stimulated released (left-hand graph) and cell surface expression of NE (right-hand graph). All data have been corrected for the small level of NE activity from unstimulated samples (3.61 and 3.20, released and cell surface NE, respectively). Results are from n=6–8 separate experiments. Significant released NE activity above basal levels were detected for LTB4 at 10−8 M (P<0.01) and 10−7 M (P<0.001) and for PAF at 10−8 M (P<0.05). Significant cell surface NE expression above basal levels were detected for LTB4 at 10−8 M (P<0.01) and 10−7 M (P<0.001) and for both PAF and KC at 10−8 M (P<0.01). For clarity, significant differences are not indicated on graphs by asterisks. (b) Stimulated murine neutrophil chemotactic responses. Bone marrow neutrophils (1 × 105 cells) were placed in the top wells of 3 μm filter 96-transwell NeuroProbe chemotaxis chambers and increasing concentrations of the chemoattractants LTB4, PAF and KC (or medium alone as control) were added to the bottom wells. The chambers were then incubated for 4 h at 37°C after which the number of cells in the bottom wells was counted as detailed in Materials and methods. Results are from n=3–10 separate experiments and all data have been corrected for the responses seen in control chambers (548 cells per well). Significant statistical differences from control samples are indicated by asterisks, *P<0.05, **P<0.01 and ***P<0.001.
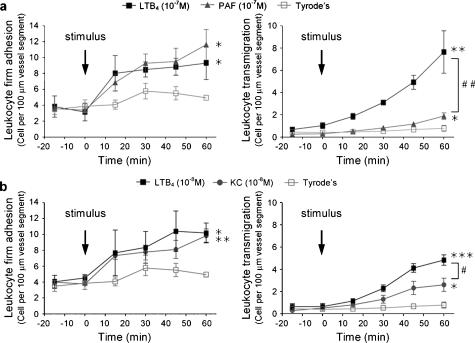
LTB4 is more effective than PAF and KC in inducing leukocyte transmigration through mouse cremasteric venules in vivo
Following on from the above in vitro results, we next sought to compare the abilities of LTB4, PAF and KC in inducing leukocyte migration in vivo. The in vivo model employed was leukocyte migration through mouse cremasteric venules, as induced by topical stimuli and observed by intravital microscopy. Topical application of LTB4 or PAF (both at 10−7 M) induced significant and comparable leukocyte firm adhesion responses as compared to the small levels detected following topical application of Tyrode solution (Figure 3a). Interestingly, in the same studies, LTB4-induced leukocyte transmigration was more pronounced than the transmigration response observed following topical PAF (Figure 3a). Similarly, while topical LTB4 and KC (10−8 M used for both stimuli due to limited availability of the chemokine KC) induced equivalent levels of leukocyte firm adhesion, LTB4 again elicited a significantly greater leukocyte transmigration response (Figure 3b).
Figure 3.
Effect of topical LTB4, PAF and KC on leukocyte responses in mouse cremasteric venules in vivo. Leukocyte firm adhesion (left-hand panels) and transmigration (right-hand panels) in mouse cremasteric venules in response to topical stimuli were investigated by intravital microscopy. The mouse cremaster muscle was surgically exteriorized, superfused with Tyrode's solution and basal leukocyte responses were quantified for 20 min, as detailed in Materials and methods. (a) LTB4 and PAF (both at 10−7 M) were applied topically at t=0 min to the exteriorized cremaster muscles and leukocyte responses were quantified at regular intervals for 60 min. (b) LTB4 and KC (both at 10−8 M) were applied topically at t=0 min to the exteriorized cremaster muscles and leukocyte responses were quantified at regular intervals for 60 min. Statistically significant differences between Tyrode's and stimulus-treated groups are shown by asterisks; *P<0.05, **P<0.01 and ***P<0.001. Additional statistical differences are indicated by lines and hatched symbols, #P<0.05, ##P<0.01 (n=4 mice per group).
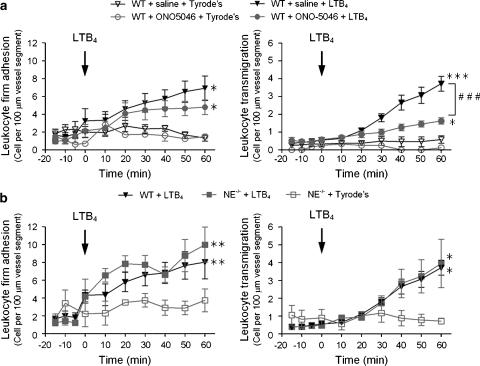
Leukocyte transmigration through LTB4-stimulated cremasteric venules is inhibited in mice treated with a specific NE inhibitor but not in NE-deficient mice
Since LTB4 was found to be a highly potent inducer of surface expression of NE and inducer of leukocyte transmigration in vivo, we next investigated the potential role of NE in LTB4-induced leukocyte migration through mouse cremasteric venules using a specific NE inhibitor ONO-5046 and NE-deficient mice. In these studies, leukocyte transmigration was elicited by topical 10−7 M LTB4.
Treatment of WT mice with ONO-5046 (50 mg kg−1 loading dose; 50 mg kg−1 h−1 infusion) had no inhibitory effect on LTB4-induced leukocyte adhesion, but caused significant inhibition of LTB4-elicited leukocyte transmigration with a 66% inhibition at 60 min (Figure 4a). In contrast to the effects seen with the NE inhibitor in WT mice, no significant inhibition in leukocyte responses induced by LTB4 was seen in NE−/− mice as compared to WT animals (Figure 4b).
Figure 4.
Effect of pharmacological inhibition and genetic deletion of neutrophil elastase (NE) on LTB4-induced leukocyte responses in mouse cremasteric venules. The mouse cremaster muscle was surgically exteriorized for investigations by intravital microscopy and leukocyte firm adhesion and transmigration were quantified following topical application of LTB4 (10−7 M), as detailed in Materials and methods. (a) Mice were treated intravenous with a bolus injection followed by a continuous infusion of ONO-5046 (Sivelestat), a specific NE inhibitor (50 mg kg−1 h−1) or with saline as control, before topical application of LTB4. (b) Leukocyte responses elicited by topical LTB4 in wild-type (WT) and NE−/− mice were compared. Results are from n=5–10 mice per group. Statistically significant differences between Tyrode's- and LTB4-treated groups are shown by asterisks; *P<0.05, **P<0.01 and ***P<0.001. Additional statistical differences are indicated by lines and hatched symbols, ###P<0.001.
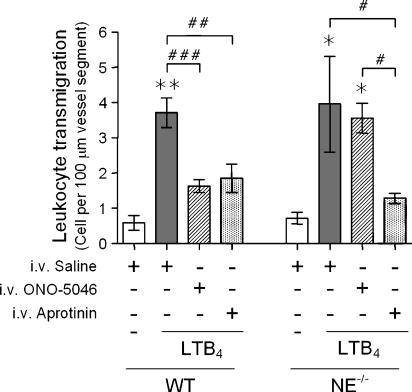
NE-deficient mice appear to exhibit compensatory mechanisms in regulation of leukocyte transmigration
To extend the above findings, we initially sought to investigate the potential specificity of the ONO-5046 compound as an NE inhibitor through its testing in NE−/− mice. Interestingly, in contrast to the inhibitory effects seen in WT animals, ONO-5046 had no inhibitory effects on LTB4-induced leukocyte transmigration in NE−/− mice, indicating the specificity of the compound in the present model (Figure 5). Since the lack of effect of the NE inhibitor suggested the existence of potential compensatory mechanisms in the NE-deficient animals, this was further investigated by testing the effect of the broad spectrum serine protease inhibitor aprotinin in both WT and NE−/− mice. For this purpose, using a dosing protocol extrapolated from that previously used in a rat model (Asimakopoulos et al., 2000; Pruefer et al., 2002), aprotinin (loading dose: 100 000KIU kg−1 and infusion: 100 000 KIU kg−1 h−1) was administered in the same manner as ONO-5046. In contrast to the findings with ONO-5046, aprotinin inhibited LTB4-induced leukocyte transmigration (Figure 5), but not firm adhesion (not shown), in both WT and NE−/− mice with an inhibition of 59.6 and 79.2%, respectively. Collectively these results suggest that as employed, the NE inhibitor used in the present studies, ONO-5046, is a specific NE inhibitor and that the NE-deficient mice appear to exhibit compensatory mechanisms through involvement of alternative serine proteases in the process of leukocyte transmigration elicited by topical LTB4.
Figure 5.
Effect of aprotinin on LTB4-induced leukocyte responses in cremasteric venules of wild-type (WT) and neutrophil elastase (NE−/−) mice. The cremaster muscle of WT and NE−/− mice was surgically exteriorized for analysis of LTB4-induced leukocyte transmigration by intravital microscopy as detailed in Materials and methods. Mice were pretreated with either intravenous saline (control), the NE inhibitor ONO-5046 or the broad-spectrum serine protease inhibitor aprotinin (see Materials and methods for dosing regime) before the topical application of LTB4 (10−7 M). Control mice were treated with topical Tyrode's solution. The graph shows leukocyte transmigration responses quantified at 60 min after application of LTB4. Data are from n=6 mice per group. Statistically significant differences between Tyrode's- and LTB4-treated mice are shown by asterisks; *P<0.05 and **P<0.01. Additional statistical comparisons are indicated by lines and by hatched symbols; #P<0.05, ##P<0.01 and ###P<0.001.
The NE inhibitor ONO-5046 inhibits LTB4-induced neutrophil transmigration at the level of the perivascular BM
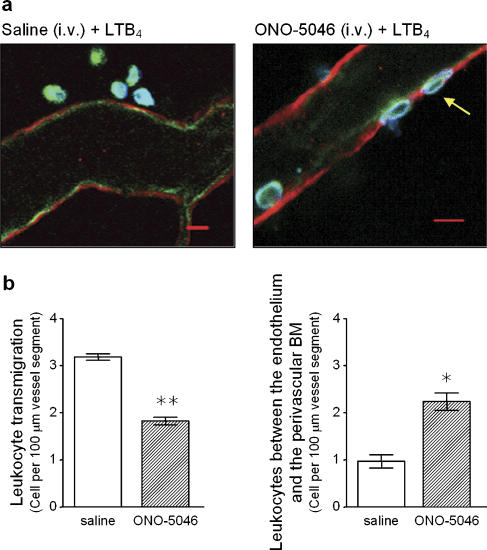
In a final series of experiments to delineate the stage in leukocyte transmigration at which the NE inhibitor ONO-5046 inhibited neutrophil transmigration, selected cremaster muscle tissues were further analysed by confocal microscopy. For this purpose, tissues were immunofluorescently stained with antibodies against PECAM-1 (as a marker for endothelial cells), laminin 10 (as a marker for venular basement membrane) and MRP-14 (as a marker for neutrophils) before being observed using a confocal microscope (Figure 6a), as detailed previously (Wang et al., 2005). Analysis of 1 μm optical sections running through whole intact venules from at least four random sections per tissue indicated an increased number of leukocytes within venular walls (that is, between endothelial cells and the venular basement membrane) in tissues from animals treated with ONO-5046 (56.8% increase; Figure 6b). Since suppression of LTB4-induced leukocyte transmigration by the NE inhibitor ONO-5046 was associated with an increase in number of leukocytes trapped in the venular wall, the findings suggest that treatment of mice with the NE inhibitor can suppress neutrophil transmigration at the level of the perivascular BM.
Figure 6.
Analysis of the site of arrest of leukocytes in mice treated with the neutrophil elastase (NE) inhibitor. Cremaster muscles from wild-type (WT) mice treated with intravenous saline (control) or with the NE inhibitor ONO-5046 (see Materials and methods for dosing regime) and stimulated with topical LTB4 (10−7 M) were dissected away from mice for immunostaining and analysis by confocal microscopy. Briefly, tissues were fixed in paraformaldehyde and immunostained overnight by using primary antibodies directed against mouse laminin 10 (as a marker for the endothelial cell basement membrane; red), PECAM-1 (as a marker for endothelial cells; green) and MRP14 (as a marker for neutrophils; blue), as detailed in Materials and methods. Samples were analysed by confocal microscopy as detailed in Materials and methods. (a) The figure shows representative images of postcapillary venules from saline-treated (left) or ONO-5046-treated mice (right) captured by confocal microscopy. While the image from the saline-treated mouse shows several leukocytes in the extravascular tissue, the tissue from ONO-5046-treated mouse shows clear evidence of leukocytes trapped within the vessel wall. (b) The graphs show quantification of leukocytes transmigrated into the surrounding tissue (left-hand graph) or trapped within the vessel wall (right-hand graph), as observed by confocal microscopy. Leukocyte transmigration (left panel) was quantified as number of cells in the tissue within 50 μm of the vessel wall across a 100 μm vessel segment. Within the same vessel segments, the number of leukocytes trapped between the basement membrane and the endothelial cells (right panel), following LTB4 stimulation (60 min), was quantified. Results represent a mean of 5–8 tissues analysed and a significant difference between saline- and ONO-5046-treated groups is indicated by asterisks; *P<0.05 and **P<0.01. (For colour figure see online version.)
Discussion and conclusions
The role of leukocyte proteases in leukocyte transmigration remains a contentious issue (Shapiro, 2002; Yadav et al., 2003). Previous studies from our group have provided evidence to indicate a role for the serine protease NE in regulation of neutrophil transmigration in response to zymosan particles and IL-1β (Young et al., 2004; Wang et al., 2005, 2006), though the mechanisms through which NE mediated these responses appeared very different. With respect to zymosan particles, NE played a role in generation of endogenous inflammatory mediators and regulation of phagocytosis of zymosan particles (Young et al., 2004). With respect to neutrophil transmigration elicited by IL-1β, NE appeared to mediate migration of neutrophils through the venular basement membrane (Wang et al., 2005, 2006). To extend these studies further, we have now investigated the role of NE in regulation of leukocyte transmigration as elicited by the potent chemoattractant LTB4 (Ford-Hutchinson et al., 1980; Bray et al., 1981). LTB4 was chosen as the inflammatory mediator to be investigated as initial in vitro studies indicated its potent ability to induce cell surface expression of NE on murine neutrophils. Furthermore, LTB4 was found to be a highly efficacious stimulus at eliciting neutrophil migration through mouse cremasteric venules as observed in real-time by intravital microscopy. This response was selectively suppressed at the level of transmigration (apparently at the level of the venular basement membrane) by a specific NE inhibitor. Interestingly, no such defect was noted in NE-deficient mice and evidence was obtained to suggest the existence of compensatory mechanisms in these animals. Collectively, the findings demonstrate the potent ability of LTB4 to induce cell surface expression of NE on neutrophils and provide further evidence to suggest a role for NE in regulation of neutrophil migration in vivo.
NE is a serine protease that is stored within azurophil granules of neutrophils at high concentrations (Shapiro, 2002). Traditionally, enzymes of this class are believed to contribute primarily to intracellular degradation of microorganisms within phagolysosomes, serine proteases can also exert a wide range of extracellular proteolytic activities (Pham, 2006). For this to occur, enzymes such as NE need to be mobilized to the cell surface or released into the extracellular environment. With respect to the latter, released enzymes can be rapidly inhibited by plasma protease inhibitors present in the extracellular environment to protect against proteolytic tissue damage to the host (Owen and Campbell, 1999). In contrast, cell-associated enzymes appear to be protected from extracellular inhibitors (Owen and Campbell, 1999) and hence could contribute to the regulation of inflammatory and immune responses through degradation, regulation of activity and/or bioavailability of specific proteins (Pham, 2006). In this context, Owen et al. (1995, 1997) have previously demonstrated that inflammatory stimuli, such as PAF, fMLP and TNFα, can induce cell surface expression of NE on human neutrophils in vitro. Since we hypothesized that cell surface-associated NE may play a role in regulating neutrophil transmigration through venular walls, we first sought to investigate the ability of certain chemoattractants in inducing cell surface expression of the enzyme. For this purpose, we adapted an assay developed previously by Owen et al. (1995) for measuring cell surface-associated NE activity on human neutrophils for quantification of released/cell surface-associated murine NE. The specificity of the assay was confirmed by the lack of activity in lysates of NE-deficient murine neutrophils and also in lysates of WT neutrophils treated with the specific NE inhibitor ONO-5046 (Sivelestat) (Kawabata et al., 1991). Using this assay, NE activity, both released and cell associated, following stimulation of bone marrow-derived murine neutrophils with LTB4, PAF and KC (CXCL1) was quantified. The findings indicated that PAF- and KC-stimulated cells exhibited very low levels of NE release/cell surface expression and were also very weak at eliciting murine neutrophil chemotaxis. In contrast, in line with its potent chemotactic properties, LTB4 was a very potent inducer of cell surface expression of NE for murine neutrophils (significant responses detected at 10−9 and 10−8 M, respectively). Of interest, at all concentrations that induced degranulation, LTB4 was more effective at inducing cell surface expression of NE as opposed to the release of the enzyme. To our knowledge this is the first report on the potent ability of LTB4 to induce cell surface expression of NE on murine neutrophils. Since there is evidence to show that transmigrating neutrophils express NE on their cell surface in vitro (Cepinskas et al., 1999) and in vivo (Wang et al., 2005), we next investigated the role of NE in LTB4-induced neutrophil transmigration in vivo.
LTB4-induced neutrophil transmigration in vivo was investigated in the murine cremaster muscle as observed directly by intravital microscopy and the functional role of NE in this response was investigated using both a selective NE inhibitor, ONO-5046 (Sivelestat) and NE-deficient mice. Topical application of LTB4 (10−7 M) induced rapid leukocyte firm adhesion and transmigration (significant at 10–20 min and 20–40 min after application of LTB4, respectively) within mouse cremasteric venules. Treatment of WT mice with the NE-inhibitor had no effect on LTB4-induced leukocyte firm adhesion but significantly suppressed the associated leukocyte transmigration response (66% inhibition at 60 min after application of LTB4). In contrast, no defect in leukocyte transmigration (or adhesion) was noted in NE-deficient animals. To ensure that the NE inhibitor employed was specific for NE in our model, the effect of ONO-5046 was also tested in the NE-deficient mice and no inhibitory effects noted, strongly suggesting its specificity for NE in the present model. Collectively, the inhibitory effect of ONO-5046 on LTB4-induced neutrophil transmigration in WT but not NE-deficient mice and the normal leukocyte transmigration response observed in NE-deficient animals suggest (i) a role for NE in LTB4-induced transmigration and (ii) the NE-deficient mice exhibit compensatory mechanisms. The former is further supported by the fact that stimuli that were weak at inducing cell surface expression of NE on mouse neutrophils (PAF and KC) also elicited lower levels of neutrophil transmigration in vivo. Evidence for the latter possibility was obtained in studies showing that the broad-spectrum serine protease inhibitor aprotinin suppressed LTB4-induced leukocyte transmigration in both WT- and NE-deficient mice. The normal leukocyte migration response observed in NE-deficient animals in the present study is in line with previous work using this mouse strain where no defect in leukocyte migration was noted in numerous inflammatory models, for example neutrophil migration was elicited by bacterial infections (Belaaouaj et al., 1998), the non-specific inflammatory stimulus thioglycollate (Tkalcevic et al., 2000) and the cytokines IL-1β and TNFα (Young et al., 2004). Overall such findings suggest that NE does not play a critical role in mediating neutrophil transmigration in certain inflammatory models and that its functional role maybe compensated for by other molecules/mechanisms. Nonetheless, our findings do indicate that selective inhibition of NE does lead to suppression of neutrophil transmigration and so in a final series of experiments the precise site of arrest of the neutrophils under conditions of NE blockade was investigated.
To analyse the site of arrest of LTB4-stimulated emigrating neutrophils under conditions of NE inhibition, cremasteric tissues from control and ONO-5046-treated mice were immunostained with markers for endothelium, venular laminin and neutrophils and analysed by confocal microscopy. The results demonstrated that in ONO-5046-treated animals, suppression of neutrophil transmigration into the extravascular tissue was directly in line with an increase in the number of neutrophils trapped within the venular wall, suggesting a blockade at the level of the perivascular basement membrane. These results are in agreement with our previous findings indicating a role of NE in IL-1β-induced neutrophil migration through the basement membrane of cremasteric venules (Wang et al., 2005, 2006). Specifically, our previous results suggested that NE regulates neutrophil migration through permissive regions within certain components of the endothelial cell basement membrane (for example, laminin 10 and collagen IV), possibly via a contribution to the remodelling of these regions during the transmigration response (Wang et al., 2006). Although the precise mechanism by which NE may regulate such an event remains unclear, it is potentially possible that a similar phenomenon may contribute to the process of neutrophil transmigration as elicited by LTB4 in the present investigation. Of relevance, numerous in vitro studies have previously demonstrated the ability of this enzyme to degrade components of basement membranes (Heck et al., 1990; Delclaux et al., 1996; Delacourt et al., 2002; Shapiro, 2002; Wang et al., 2006). The broad substrate specificity and biological functions of NE does, however, strongly suggest that alternative modes of action of NE, for example, regulation of cell surface receptors, adhesion molecules and other enzymes as well as regulatory effects on intracellular signalling and cytoskeletal rearrangement (Pham, 2006), may also play a role in NE-dependent neutrophil transmigration.
In summary, the findings show that LTB4 is a potent inducer of cell surface expression of NE on murine neutrophils and that NE plays a role in mediating LTB4-induced neutrophil transmigration through the venular wall at the level of the endothelial cell basement membrane. While the mechanism through which NE regulates neutrophil transmigration remains unclear, the findings of this study highlight the need for further investigations into this unresolved aspect of leukocyte biology. Furthermore, the findings contribute to the growing list of functional discrepancies observed when studying molecules under conditions of pharmacological blockade as compared to genetic deletion.
Acknowledgments
This work was supported by The British Heart Foundation (BHF PG/03/123/16102) and The Wellcome Trust, UK (064920). We are grateful to Professor S Shapiro for providing the NE-deficient mice and to Professor L Sorokin for providing the anti-mouse laminin α5-chain mAb and Dr Nancy Hogg for providing the anti-mouse MRP-14 mAb.
Abbreviations
- BM
basement membrane
- IVM
intravital microscopy
- LTB4
leukotriene B4
- NE
neutrophil elastase
- PMN
polymorphonuclear neutrophil
Conflict of interest
The authors state no conflict of interest.
References
- Adkison AM, Raptis SZ, Kelley DG, Pham CTN. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109:363–371. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport JR, Lim Y-C, Shipley JM, Senior RM, Shapiro SD, Matsuyoshi N, et al. Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J Leukocyte Biol. 2002;71:821–828. [PubMed] [Google Scholar]
- Asimakopoulos G, Thompson R, Nourshargh S, Lidington EA, Mason JC, Ratnatunga CP, et al. An anti-inflammatory property of aprotinin detected at the level of leukocyte extravasation. J Thorac Cardiovasc Surg. 2000;120:361–369. doi: 10.1067/mtc.2000.106323. [DOI] [PubMed] [Google Scholar]
- Bank U, Ansorge S. More than destructive: neutrophil-derived serine proteases in cytokine bioactivity. J Leukocyte Biol. 2001;69:197–206. [PubMed] [Google Scholar]
- Barrick B, Campbell EJ, Owen CA. Leukocyte proteinases in wound healing: roles in physiologic and pathologic processes. Wound Repair Regen. 1999;7:410–422. doi: 10.1046/j.1524-475x.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, et al. Mice lacking neutrophil elastase reveal impaired host defence against gram negative bacterial sepsis. Nat Med. 1998;4:615–618. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- Bray MA, Ford Hutchinson AW, Smith MJH. Leukotriene B4: an inflammatory mediator in vivo. Prostaglandins. 1981;22:213–222. doi: 10.1016/0090-6980(81)90036-8. [DOI] [PubMed] [Google Scholar]
- Cepinskas G, Sandig M, Kvietys PR. PAF-induced elastase-dependent neutrophil transendothelial migration is associated with the mobilisation of elastase to the neutrophil surface and localisation to the migrating front. J Cell Sci. 1999;112:1937–1945. doi: 10.1242/jcs.112.12.1937. [DOI] [PubMed] [Google Scholar]
- Delacourt C, Herigault S, Delclaux C, Poncin A, Levame M, Harf A, et al. Protection against acute lung injury by intravenous or intratracheal pretreatment with EPI-HNE-4, a new potent neutrophil elastase inhibitor. Am J Respir Cell Mol Biol. 2002;26:290–297. doi: 10.1165/ajrcmb.26.3.4611. [DOI] [PubMed] [Google Scholar]
- Delclaux C, Delacourt C, d'Ortho M-P, Boyer V, Lafuma C, Harf A. Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am J Respir Cell Mol Biol. 1996;14:288–295. doi: 10.1165/ajrcmb.14.3.8845180. [DOI] [PubMed] [Google Scholar]
- Elsaid KA, Jay GD, Chichester CO. Detection of collagen type II and proteoglycans in the synovial fluids of patients diagnosed with non-infectious knee joint synovitis indicates early damage to the articular cartilage matrix. Osteoarthritis Cartilage. 2003;11:673–680. doi: 10.1016/s1063-4584(03)00151-1. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJH. Leukotriene B4, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286:264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Furie MB, Nalprestek BL, Silverstein SC. Migration of neutrophils across monolayers of cultured microvascular endothelial cells. J Cell Sci. 1987;88:161–175. doi: 10.1242/jcs.88.2.161. [DOI] [PubMed] [Google Scholar]
- Gabay JE, Almeida RP. Antibiotic peptides and serine protease homologs in human polymorphonuclear leukocytes: defensins and azurocidin. Curr Opin Immunol. 1993;5:97–102. doi: 10.1016/0952-7915(93)90087-9. [DOI] [PubMed] [Google Scholar]
- Groeneveld AB, Raijmakers PG, Hack CE, Thijs LG. Interleukin 8-related neutrophil elastase and the severity of the adult respiratory distress syndrome. Cytokine. 1995;7:746–752. doi: 10.1006/cyto.1995.0089. [DOI] [PubMed] [Google Scholar]
- Heck LW, Blackburn WD, Irwin MH, Abrahamson DR. Degradation of basement membrane laminin by human neutrophil elastase and cathepsin G. Am J Pathol. 1990;136:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- Hirche TO, Atkinson JJ, Bahr S, Belaaouaj A. Deficiency in neutrophil elastase does not impair neutrophil recruitment to inflamed sites. Am J Respir Cell Mol Biol. 2004;30:576–584. doi: 10.1165/rcmb.2003-0253OC. [DOI] [PubMed] [Google Scholar]
- Huber AR, Weiss SJ. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J Clin Invest. 1989;83:1122–1136. doi: 10.1172/JCI113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata K, Suzuki M, Sugitani M, Imaki K, Toda M, Miyamoto T. ONO-5046, a novel inhibitor of human neutrophil elastase. Biochem Biophys Res Commun. 1991;177:814–820. doi: 10.1016/0006-291x(91)91862-7. [DOI] [PubMed] [Google Scholar]
- Lee WL, Downey GP. Leukocyte elastase. Physiological functions and role in acute lung injury. Am J Respir Crit Care Med. 2001;164:896–904. doi: 10.1164/ajrccm.164.5.2103040. [DOI] [PubMed] [Google Scholar]
- Mackarel AJ, Cottell DC, Russell KJ, FitzGerald MX, O'Conner CM. Migration of neutrophils across human pulmonary endothelial cells is not blocked by matrix metalloproteinase or serine protease inhibitors. Am J Respir Cell Mol Biol. 1999;20:1209–1219. doi: 10.1165/ajrcmb.20.6.3539. [DOI] [PubMed] [Google Scholar]
- Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the {beta}2 integrin mac-1 on neutrophils. J Immunol. 1998;160:1427–1435. [PubMed] [Google Scholar]
- Orem A, Deger O, Cimsit G, Bahadir S. Plasma polymorphonuclear leukocyte elastase levels and its relation to disease severity in psoriasis. Clin Chim Acta. 1997;264:49–56. doi: 10.1016/s0009-8981(97)00072-7. [DOI] [PubMed] [Google Scholar]
- Owen CA, Campbell EJ. The cell biology of leukocyte-mediated proteolysis. J Leukocyte Biol. 1999;65:137–150. doi: 10.1002/jlb.65.2.137. [DOI] [PubMed] [Google Scholar]
- Owen CA, Campbell MA, Sannes PL, Boukedes SS, Campbell EJ. Cell-surface-bound elastase and cathepsin G on human neutrophils. A novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol. 1995;131:775–789. doi: 10.1083/jcb.131.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen CA, Campbell MA, Boukedes SS, Campbell EJ. Cytokines regulate membrane-bound leukocyte elastase on neutrophils: a novel mechanism for effector activity. Am J Physiol. 1997;272:385–393. doi: 10.1152/ajplung.1997.272.3.L385. [DOI] [PubMed] [Google Scholar]
- Pham CTN. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- Pruefer D, Buerke U, Khalil M, Dahm M, Darius H, Oelert H, et al. Cardioprotective effects of the serine protease inhibitor aprotinin after regional ischemia and reperfusion on the beating heart. J Thorac Cardiovasc Surg. 2002;124:942–949. doi: 10.1067/mtc.2002.123703. [DOI] [PubMed] [Google Scholar]
- Rosengren S, Arfors KE. Neutrophil-mediated vascular leakage is not suppressed by leukocyte elastase inhibitors. Am J Pathol. 1990;259:H1288–H1294. doi: 10.1152/ajpheart.1990.259.4.H1288. [DOI] [PubMed] [Google Scholar]
- Shapiro SD. Neutrophil elastase. Path clearer, pathogen killer, or just pathologic. Am J Respir Cell Mol Biol. 2002;26:266–268. doi: 10.1165/ajrcmb.26.3.f233. [DOI] [PubMed] [Google Scholar]
- Shapiro SD, Ingenito EP. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am J Respir Cell Mol Biol. 2005;32:367–372. doi: 10.1165/rcmb.F296. [DOI] [PubMed] [Google Scholar]
- Smith FB, Fowkes FG, Rumley A, Lee AJ, Lowe GD, Hau CM. Tissue plasminogen activator and leukocyte elastase as predictors of cardiovascular events in subjects with angina pectoris: Edinburgh Artery Study. Eur Heart J. 2000;21:1607–1613. doi: 10.1053/euhj.2000.2127. [DOI] [PubMed] [Google Scholar]
- Sorokin LM, Frieser M, Kroger S, Ohage E, Deutzmann R. Developmental regulation of the laminin α5 chain suggests a role in epithelial and endothelial cell maturation. Dev Biol. 1997;189:285–300. doi: 10.1006/dbio.1997.8668. [DOI] [PubMed] [Google Scholar]
- Steadman R, Irwin MH, St John PL, Blackburn WD, Heck LW, Abrahamson DR. Laminin cleavage by activated human neutrophils yields proteolytic fragments with selective migratory properties. J Leukocyte Biol. 1993;53:354–365. doi: 10.1002/jlb.53.4.354. [DOI] [PubMed] [Google Scholar]
- Thompson RD, Noble KE, Larbi KY, Dewar A, Duncan GS, Mak TW, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood. 2001;97:1854–1860. doi: 10.1182/blood.v97.6.1854. [DOI] [PubMed] [Google Scholar]
- Tkalcevic J, Novelli M, Phylactides M, Iredale JP, Segal AW, Roes J. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 2000;12:201–210. doi: 10.1016/s1074-7613(00)80173-9. [DOI] [PubMed] [Google Scholar]
- Vila N, Elena M, Deulofeu R, Chamorro A. Polymorphonuclear leukocyte elastase in patients with stroke. Acta Neurol Scand. 1999;100:391–394. doi: 10.1111/j.1600-0404.1999.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Wang S, Dangerfield JP, Young RE, Nourshargh S. PECAM-1, α6 integrins and neutrophil elastase co-operate in mediating neutrophil transmigration. J Cell Sci. 2005;118:2067–2076. doi: 10.1242/jcs.02340. [DOI] [PubMed] [Google Scholar]
- Wang S, Voisin M-B, Larbi KY, Dangerfield J, Scheiermann C, Tran M, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating PMN. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–375. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Yadav R, Larbi KY, Young RE, Nourshargh S. Migration of leukocytes through the vessel wall and beyond. Thromb Haemost. 2003;90:598–606. doi: 10.1160/TH03-04-0220. [DOI] [PubMed] [Google Scholar]
- Young RE, Thompson RD, Larbi K, La M, Roberts CE, Shapiro SD, et al. Neutrophil elastase (NE)-deficient mice demonstrate a non-redundant role for NE in neutrophil migration, generation of pro-inflammatory mediators, and phagocytosis in response to zymosan particles in vivo. J Immunol. 2004;172:4493–4502. doi: 10.4049/jimmunol.172.7.4493. [DOI] [PubMed] [Google Scholar]