Abstract
Background and purpose:
Nitric oxide synthase (NOS) inhibitors cause vasoconstriction in pressurized arterioles with myogenic tone. This suggests either tonic production of NO modulates myogenic tone or a direct, NOS-independent effect of the NOS inhibitors. The nature of the contractile effect of the nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM) on pressurised arterioles was investigated.
Experimental approach:
Segments of rat cremaster muscle first-order arteriole were cannulated on glass micropipettes and maintained at an intraluminal pressure of 50, 70 or 120 mmHg.
Key results:
L-NAME and the related compound L-NA (100 μM) constricted pressurized vessels with myogenic tone. Removal of the endothelium did not cause constriction or alter myogenic tone, however the constrictor effect of L-NAME persisted. The constrictor effect of L-NAME was abolished by L-arginine (1 mM). Other NO and cGMP pathway inhibitors, including the nNOS inhibitor 7-nitroindazole (100 μM), the NO scavenger carboxy-PTIO (100 μM), the guanylate cyclase inhibitor ODQ (10 μM) and the cGMP inhibitor Rp-8CPT-cGMPS (10 μM) did not cause constriction of the arterioles. L-NAME caused a small (3-4 mV) but not statistically significant depolarization of the arteriolar smooth muscle at both pressures. The constrictor effect was not prevented by the K+-channel antagonist tetraethyl ammonium (TEA, 1 mM) or the KATP channel antagonist glibenclamide (1 μM).
Conclusions and implications:
These observations demonstrate that L-NAME causes an endothelium- and NOS-independent contraction of vascular smooth muscle in isolated skeletal muscle arterioles. It is suggested that the underlying mechanism relates to an arginine binding interaction.
Keywords: arteriole, myogenic tone, endothelium, L-NAME
Introduction
Nitrosylated L-arginine derivatives are used commonly as inhibitors of nitric oxide synthase (NOS; Rees et al., 1989). The arginine derivatives act as competitive inhibitors of the substrate L-arginine at the active site of NOS (Rees et al., 1989, 1990; Moore et al., 1990). Effects of the NOS inhibitors alone in a test system (i.e. in the absence of a coexisting stimulus for NO production) are usually interpreted as indicating endogenous NOS activity and basal production of NO. In studies of blood vessels, this interpretation is often used to suggest the tonic release of the vasodilator (Huang et al., 1997; Nguyen et al., 1999; Wang et al., 1999; Geary et al., 2000; Jarajapu et al., 2004; Szekeres et al., 2004).
Arginine-based NOS inhibitors, including Nω-nitro-L-arginine (commonly abbreviated as L-NOARG or L-NA) and Nω-nitro-L-arginine methyl ester (L-NAME), have been shown to constrict isolated, pressurized blood vessels possessing spontaneous myogenic tone in the absence of intraluminal flow (Huang et al., 1997; Nguyen et al., 1999; Wang et al., 1999; Geary et al., 2000; Undavia et al., 2003; Bai et al., 2004; Jarajapu et al., 2004; Szekeres et al., 2004). As mentioned above, such an effect is often ascribed to inhibition of spontaneous NO production in the preparation, thus suggesting a role for endothelium-derived NO in modulating myogenic tone. This is, however, problematic as myogenic tone was shown to be unaltered by damage to or removal of the endothelium (Falcone et al., 1991; Kuo et al., 1991; Undavia et al., 2003; for reviews see Meininger and Davis, 1992; Davis and Hill, 1999) suggesting no direct contribution of endothelial NO. Some investigators have ascribed this apparent disparity to a combination of constricting and dilating factors being released by the endothelium, with no net effect of endothelium removal upon myogenic tone (Nguyen et al., 1999; Undavia et al., 2003; Bai et al., 2004). Alternatively, the mechanisms underlying myogenic activity may compensate for the loss of the endothelium such that the same pressure–diameter relationship is maintained (Scotland et al., 2001). Vessel wall tension (by the Law of Laplace, the product of transmural pressure and lumen radius) is thought to be the physiological regulator of myogenic tone (Johnson, 1980; Zou et al., 1995). Removal of an inherent vasodilator component, such as the endothelium, would result in a reduction of lumen radius for a given transmural pressure, leading to a decreased wall tension and a resultant stimulus for myogenic dilation. Conceivably this would tend to restore the pressure-diameter relationship to the state before endothelium removal.
On the basis of above studies, the hypothesis of the present study was that the constrictor effect of the NOS inhibitor L-NAME on isolated, pressurized arterioles is independent of the vascular endothelium and NOS inhibition. The data obtained supported the hypothesis and further showed the effect to be a property of the arginine-based NOS inhibitors.
Materials and methods
Animals
Sprague–Dawley rats aged 6–9 weeks and weighing 160–300 g were housed in a dedicated facility maintained at 21±3°C and a relative humidity between 45% and 65%, with a 12:12-h light–dark cycle. Rats were provided with standard rat chow and drinking water ad libitum. The protocols and procedures were approved by the UNSW and RMIT Animal Experimentation and Ethics Committees.
Isolated arteriole preparation
Rats were anesthetized with pentothal sodium (100 mg kg−1, intraperitoneally), after which the cremaster muscles were excised and placed in a cooled (4°C) chamber containing dissection buffer (all concentrations in mM): 3-N-morpholino propanesulfonic acid, 145 NaCl, 5 KCl, 2.5 CaCl2, 1 MgSO4, 1 Na2HPO4, 0.02 ethylenediamine tetraacetic acid, 2 pyruvate, 5 glucose and 1% (w v−1) bovine serum albumin (see Duling et al., 1981). Segments of the main intramuscular arteriole were dissected as described previously (Potocnik et al., 2000). Vessel segments were cannulated with glass micropipettes, secured with 10–0 suture and mounted in a tissue superfusion chamber. The cannulated arterioles were continuously superfused (4 ml min−1) with a physiological salt solution (PSS, all concentrations in mM: 111 NaCl, 25.7 NaHCO3, 4.9 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 11.5 glucose and 10 HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid). Vessel segments were gradually pressurized to 70 mm Hg and warmed to 34°C over a 60-min equilibration period. During this time, the vessels were checked for pressure leaks and allowed to develop spontaneous myogenic tone. Vessels without spontaneous tone were discarded. The superfusion chamber containing the vessel was positioned on the stage of an inverted microscope and measurement of internal diameter was accomplished using an electronic video caliper.
Membrane potential measurements
Intracellular recordings of smooth muscle cell membrane potential were made in pressurized, cannulated arteriole preparations. Recordings were made using glass microelectrodes filled with 2 M KCl and tip resistances of 100–200 MΩ (Potocnik et al., 2000).
Protocols
Studies in arterioles with intact endothelium
As myogenic tone may be a stimulus for NO production, it was reasoned that the magnitude of any constrictor effect of L-NAME would vary corresponding to the level of myogenic tone. Different levels of myogenic tone were generated by varying transmural pressure. Arterioles were equilibrated at 70 mm Hg and the function of the endothelium assessed by responsiveness to 10 μM acetylcholine. After washout and recovery of baseline myogenic tone, the intraluminal pressure was either maintained at 70 mm Hg or adjusted to either 50 or 120 mm Hg, and the arteriole was allowed to equilibrate at this ‘test' pressure for 20 min. The vessel was then superfused with L-NAME (100 μM) until a maximum constriction was observed (approximately 20 min). Experiments examined the effect of L-arginine (1 mM) on the L-NAME-induced constriction; in these studies, L-arginine was added to the superfusate 20 min before the addition of L-NAME. The effects of the non-selective K+-channel inhibitor tetraethyl ammonium (TEA, 1 mM) or the ATP-sensitive potassium channel (KATP) inhibitor glibenclamide (1 μM) on responses to L-NAME were also examined. Adequate inhibition of KATP channels was established by examining the dilator response to the KATP channel activator pinacidil (1 μM) before and 20 min after the addition of glibenclamide. In studies with another arginine-based NOS inhibitor, L-NA, the inhibitor of neuronal NOS (nNOS), 7-NI, the soluble guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and the guanosine-cyclic 3′,5′-monophosphate (cGMP) antagonist Rp-8-[(4-chlorophenyl)thio]guanosine-cyclic 3′,5′-hydrogen phosphorothioate triethylammonium salt (Rp-8CPT-cGMPS), these were added to the arteriole 20 min before diameter being measured.
In a further series of experiments, concentration–response curves to the endothelium-dependent vasodilator acetylcholine (1 nM–1 μM) were performed in the arterioles, in the presence and absence of 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (carboxy-PTIO) (100 μM) to establish the effectiveness of carboxy-PTIO in inhibiting NO.
At the conclusion of the protocols, arterioles were rendered passive by superfusion with a nominally Ca2+-free PSS (containing 0 mM CaCl2 and 2 mM ethylene glycol bis (β-aminoethylether)-N,N,N',N',-tetraacetic acid (EGTA)) for 20 min. Passive diameters at 50, 70 and 120 mm Hg were measured to allow normalization of diameter data.
Studies in arterioles with the endothelium removed
Arterioles were equilibrated at 70 mm Hg and the functional integrity of the endothelium determined as described above; a dilator response to adenosine (10 μM) was also established. The endothelium was removed by passage of an air bolus through the lumen of the vessel. Passage of the air bolus was followed by washing of the lumen with PSS to remove cellular debris. Abolition of the dilator response to acetylcholine (1 μM) and retention of responsiveness to adenosine was taken as functional evidence of selective endothelium removal while not compromising smooth muscle function. L-NAME was then added to the vessels and observations made as described above. In experiments utilizing carboxy-PTIO (100 μM), performed at 70 mm Hg, this was added 15 min before the addition of L-NAME.
Effect of L-NAME on smooth muscle membrane potential
Arterioles were equilibrated at 70 mm Hg and the functional integrity of the endothelium tested as described above. The intraluminal pressure of the vessel was then altered to either 50 or 120 mm Hg, and the vessel was allowed to equilibrate at this test pressure for 20 min. Simultaneous recordings of diameter and smooth muscle cell membrane potential were made before and after superfusion of the vessel with 100 μM L-NAME for 20 min.
Statistical analysis
Diameter values (in μM) for each arteriole were expressed as a percentage of the passive diameter at the relevant test pressure (50 or 120 mm Hg). Data are expressed as means±s.e.m. Simple comparisons of means±s.e.m. were performed with the use of Student's t-test. Multiple comparisons were determined by using analysis of variance with the Bonferroni post hoc test. Values of P<0.05 were considered to be significant.
Drugs and chemicals
Acetylcholine hydrochloride, L-NA, L-NAME, L-arginine, adenosine, pinacidil hydrochloride, 7-nitroindazole (7-NI), carboxy-PTIO, tetraethyl ammonium, ODQ, Rp-8CPT-cGMPS and glibenclamide were all obtained from Sigma-Aldrich (St Louis, MO, USA). Carboxy-PTIO was dissolved initially in dimethyl sulfoxide and further diluted in PSS. All other drugs were initially dissolved in de-ionized water and diluted further in PSS.
Results
Arterioles maintained at intraluminal pressures of 50, 70 or 120 mm Hg developed spontaneous myogenic tone to 82.3±2.6 μm (54.5±1.9% of passive diameter, n=31), 83.3±2.8 μm (52.8±2.9%, n=18) and 71.9±2.8 μm (43.2±1.6%, n=30), respectively. Corresponding passive diameters, obtained after superfusion in Ca2+-depleted buffer containing 2 mM EGTA, were 152±4, 158±4 and 167±4 μm.
Effect of L-NAME and other agents interacting with the NOS, guanylate cyclase and cGMP system on arteriolar diameter
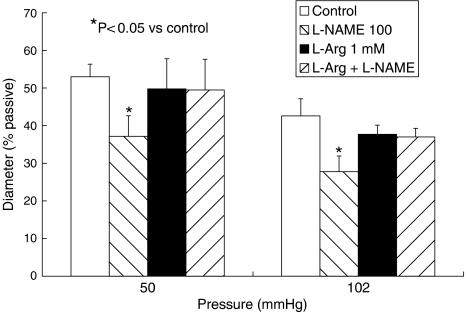
The NOS inhibitor L-NAME (100 μM) caused a significant, slowly developing constriction of arterioles maintained at either at 50 or 120 mm Hg (Figure 1), peaking approximately 20 min after the addition of L-NAME. The magnitude of the constriction was similar at either pressure, 23.2±6.0 μm or 15.9±2.8% of passive diameter at 50 mm Hg (n=6) and 23.4±7.2 μm or 14.8±2.6% of passive diameter at 120 mm Hg (n=6; Figure 1). L-NAME did not cause constriction of arterioles in the presence of L-arginine (1 mM), at either pressure (Figure 2). L-arginine alone did not alter arteriolar diameter (Figure 2).
Figure 1.
Effect of L-NAME (100 μM), L-NA (100 μM), 7-NI (100 μM), ODQ (10 μM), Rp-8CPT-cGMPS (10 μM) and cPTIO (100 μM) on the diameter of isolated, pressurized arterioles from rat cremaster muscle. Experiments were performed in vessels maintained at intraluminal pressures of 50, 70 or 120 mm Hg. Columns represent the mean±s.e.m. Open columns show the diameter of the vessel pre-drug (control) and hatched columns the diameter post-drug. Drug concentrations are shown in μM. 7-NI, 7-nitroindazole; cPTIO, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt; L-NA, Nω-nitro-L-arginine; L-NAME, Nω-nitro-L-arginine methyl ester; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; Rp-8CPT-cGMPS, Rp-8-[(4-chlorophenyl)thio]guanosine-cyclic 3′,5′-hydrogen phosphorothioate triethylammonium salt.
Figure 2.
Effect of L-arginine (L-Arg, 1 mM) on arteriolar diameter and responses to L-NAME (100 μM) in isolated, pressurized arterioles from rat cremaster muscle. Columns represent the mean±s.e.m. Open columns show the diameter of the vessel pre-drug (control), left-hatched columns the effect of L-NAME on diameter, closed columns the effect of L-arginine on diameter and right-hatched columns the effect of L-Arg plus L-NAME. Drug concentrations are shown in μM. L-NAME, Nω-nitro-L-arginine methyl ester.
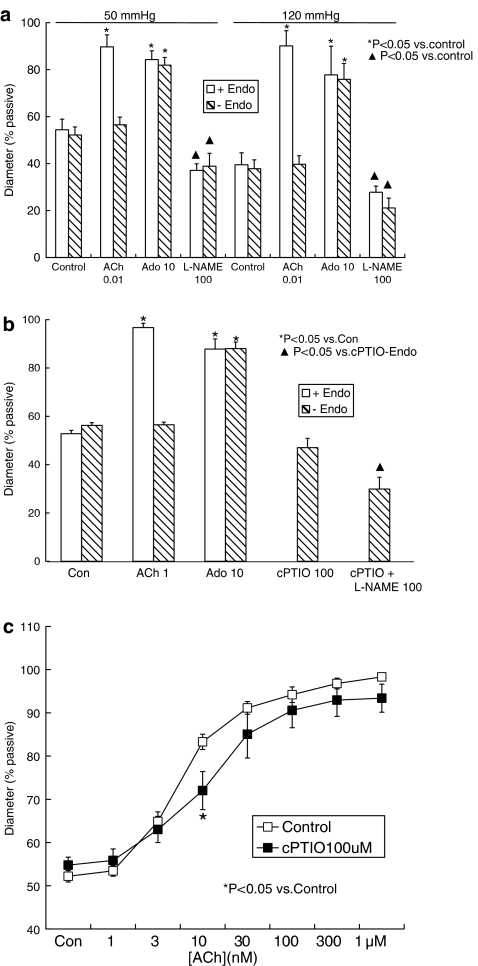
Another arginine-based NOS inhibitor, L-NA, also caused significant constriction of arterioles maintained at 50 or 120 mm Hg (Figure 1). A series of other (non-arginine-based) agents interacting with the NO, guanylate cyclase and cyclic GMP system did not cause constriction of the vessels. These included the relatively selective inhibitor of nNOS, 7-NI (100 μM), which caused dilation of pressurized arterioles; the guanylate cyclase inhibitor ODQ (10 μM), the cyclic GMP inhibitor Rp-8CPT-cGMPS (10 μM) and the NO scavenger compound carboxy-PTIO (100 μM), all of which had no effect of arteriolar diameter (see Figure 1).
In studies measuring arteriolar smooth muscle cell membrane potential (Table 1), the constriction caused by L-NAME was not accompanied by significant alteration of smooth muscle cell membrane potential, in arterioles maintained at 50 mm Hg (n=5; P>0.05, paired t-test). When arterioles were maintained at 120 mm Hg, the corresponding Em values were more depolarized; however, the NOS inhibitor did not significantly alter Em (n=4, P>0.05, paired t-test).
Table 1.
Effect of L-NAME (100 μM) on MP and diameter of isolated arterioles from rat cremaster muscle maintained at an intraluminal pressure of 50 or 120 mm Hg
| Pressure (mm Hg) | MP (mV) – control | MP (mV) – L-NAME (100 μM) | Diameter( % passive) – control | Diameter (% passive) – L-NAME (100 μM) |
|---|---|---|---|---|
| 50 | 38.8±2.4 | 34.8±2.7 | 55.8±6.4 | 40.8±7.7* |
| 120 | 28.3±0.9 | 25.3±1.8 | 48.3±3.0 | 38.3±3.7* |
Abbreviations: L-NAME, Nω-nitro-L-arginine methyl ester; MP, membrane potential.
P<0.05 vs control.
Values shown in the table are the mean±s.e.m. L-NAME did not cause a significant alteration of membrane potential at either pressure (P>0.05, paired t-test).
Removal of vascular endothelium and responses to L-NAME
Responses to L-NAME were examined in arterioles from which the endothelium had been removed by passage of an air bolus through the lumen of the vessel. At intraluminal pressures of either 50, 70 or 120 mm Hg, disruption of the endothelium was confirmed by abolition of the dilator response to acetylcholine (0.01 or 1 μM; Figure 3a and b) while maintained smooth muscle function was verified by a maintained dilator response to adenosine (10 μM; Figure 3a and b). Removal of the endothelium did not alter vessel diameter (i.e. myogenic tone) at any pressure examined; in these paired experiments, at 50 mm Hg, the diameter was 84.8±7.1 μm (54.4±4.5% of passive diameter) in endothelium-intact arterioles and 89.2±5.5 μm (57.2±3.4%) after removal of the endothelium (n=4, P>0.05, paired t-test); at 70 mm Hg, the corresponding values were 95.6±4.0 μm (56.9±1.2%) and 94.5±3.3 μm (56.3±1.1%; n=5, P>0.05, paired t-test) and at 120 mm Hg, diameter was 60.1±7.5 μm (39.5±5.1%) in endothelium-intact vessels and 60.2±5.8 μm (39.7±4.6%) after removal of the endothelium (n=4, P>0.05, paired t-test; see also Figure 3a and b).
Figure 3.
(a) Effect of endothelium removal on dilator responses to acetylcholine (ACh) and adenosine (Ado) and the constrictor effect of L-NAME in isolated, pressurized arterioles from rat cremaster muscle. The endothelium was removed by passage of an air bolus through the arteriole lumen. Experiments were performed in vessels maintained at intraluminal pressures of 50 or 120 mm Hg. (b) Effect of carboxy-PTIO (cPTIO, 100 μM) on responses to L-NAME (100 μM) in isolated, pressurized (70 mm Hg), endothelium-denuded arterioles from the rat cremaster muscle. Columns in (a and b) represent the mean±s.e.m. Open columns show experiments in vessels with an intact endothelium and hatched columns endothelium-denuded vessels. Drug concentrations are shown in μM. (c) Effect of carboxy-PTIO (100 μM) on responses to ACh in isolated, pressurized (70 mm Hg), endothelium-intact arterioles from the rat cremaster muscle. Points represent the mean±s.e.m. cPTIO, carboxy-2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt; L-NAME, Nω-nitro-L-arginine methyl ester.
The constrictor effect of L-NAME was not altered by endothelium removal, and was of similar magnitude to that observed in endothelium-intact vessels (Figure 3a). At 50 mm Hg, L-NAME caused a constriction of 28.7±4.2 μm or 18.3±2.3% of passive diameter (n=4); in arterioles maintained at 120 mm Hg, the L-NAME-induced constriction was 28.3±1.7 μm or 18.6±1.5% of passive diameter (n=4).
Further studies in endothelium-denuded vessels utilized the NO scavenger carboxy-PTIO (100 μM) to investigate a possible contribution of any NOS activity or NO remaining after the removal of the endothelium. These experiments were performed using arterioles pressurized to 70 mm Hg. Carboxy-PTIO did not prevent the L-NAME-induced constriction, nor did it significantly alter the baseline diameter of the vessels (Figure 3b). Similarly, in endothelium-intact vessels, carboxy-PTIO did not, itself, alter baseline diameter (see Figure 1). In endothelium-intact vessels, carboxy-PTIO did cause a modest but significant rightward shift of the concentration–dilation curve to acetylcholine (P<0.05, two-way analysis of variance followed by Bonferroni post hoc test, n=7; Figure 3c).
Effect of KATP blockade on responses to L-NAME
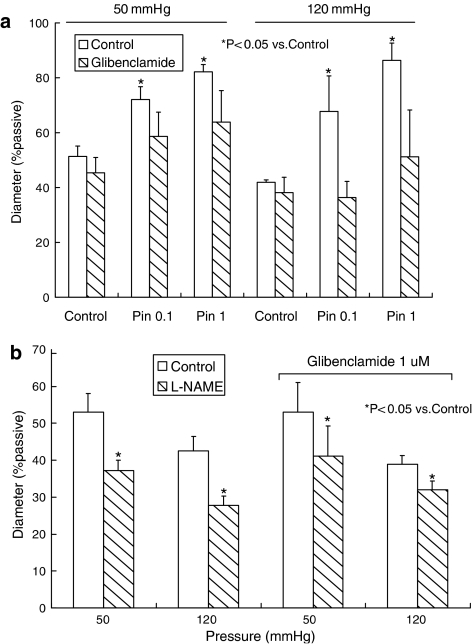
The role of KATP channels in the response to L-NAME was examined using the KATP channel inhibitor glibenclamide (1 μM), in endothelium-intact arterioles. Glibenclamide alone had no effect on arteriolar diameter, at either pressure (Figure 4a). At 50 mm Hg, control diameter was 79.0±3.4 μm (51.3±3.8% of passive diameter) and 71.7±7.4 μm (45.4±5.6%) in the presence of glibenclamide (n=5, P>0.05, paired t-test). At 120 mm Hg, control diameter was 73.2±4.6 μm (41.9±0.9%) and 64.7±5.6 μm (38.1±5.6%) after addition of glibenclamide (n=4, P>0.05, paired t-test). The effectiveness of glibenclamide in blocking KATP channels was, however, established by inhibition of dilator responses to the KATP channel activator pinacidil (100 nM and 1 μM; Figure 4a). In the presence of glibenclamide, L-NAME continued to cause significant constriction of arterioles (Figure 4b) suggesting that the arginine analogue was not exerting an effect through inhibition of KATP channels. At 50 mm Hg, the constriction caused by L-NAME was 18.5±4.4 μm or 12.0±2.7% of passive diameter (n=5); in arterioles maintained at 120 mm Hg, the L-NAME-induced constriction was 11.8±1.7 μm or 6.8±0.9% of passive diameter (n=4).
Figure 4.
(a) Effect of the KATP channel antagonist glibenclamide (1 μM) on dilator responses to pinacidil (Pin) in isolated, pressurized arterioles from rat cremaster muscle. (b) Effect of glibenclamide (1 μM) on constrictor responses to L-NAME (100 μM) in isolated, pressurized arterioles from rat cremaster muscle. In (a and b), experiments were performed in vessels maintained at intraluminal pressures of 50 or 120 mm Hg. Columns represent the mean±s.e.m. L-NAME, Nω-nitro-L-arginine methyl ester.
The wider role of K+ channels in the constrictor effect of L-NAME was investigated using the non-selective K+ channel inhibitor TEA (1 mM), in endothelium-intact arterioles maintained at 70 mm Hg. TEA alone caused a significant constriction of the arterioles, from 78.5±3.6 to 62.9±3.1 μm (n=6, P<0.05, paired t-test; Figure 5). Following TEA, L-NAME caused a further significant constriction of arterioles to 53.5±3.8 μm (n=6, P<0.05, paired t-test; Figure 5). In experiments in endothelium-denuded vessels, the constrictor effect of L-NAME persisted in the presence of TEA (n=2, data not shown).
Figure 5.
Effect of tetraethyl ammonium (TEA, 1 mM) on constrictor responses to L-NAME (100 μM) in isolated, pressurized (70 mm Hg) arterioles from rat cremaster muscle. Columns represent the mean±s.e.m. L-NAME, Nω-nitro-L-arginine methyl ester.
Discussion
Pressure-induced myogenic tone in arterioles is generally regarded as being endothelium-independent (for reviews see Meininger and Davis, 1992; Davis and Hill, 1999). Despite this, previous studies have demonstrated a constrictor effect of arginine-based NOS inhibitors on isolated, pressurized arterioles with myogenic tone (Huang et al., 1997; Nguyen et al., 1999; Wang et al., 1999; Geary et al., 2000; Jarajapu et al., 2004; Szekeres et al., 2004), implying a role for endothelium-derived NO in regulating pressure-induced constriction. In the present study, the arginine analogues and NOS inhibitors, L-NA and L-NAME, caused significant constriction of arterioles, decreasing vessel diameter by approximately 20% of its passive value. The L-NAME-induced constriction persisted in endothelium-denuded vessels, including those in the presence of the NO scavenger carboxy-PTIO suggesting no specific role for NO from either endothelial or other vascular wall cells in this effect. Removal of the endothelium itself did not alter arteriolar diameter.
In contrast with L-NAME, a number of other agents inhibiting various segments of the NO, guanylate cyclase and cyclic GMP signaling pathway did not cause constriction of the arterioles. These agents included the selective inhibitor of nNOS 7-NI, the NO scavenger carboxy-PTIO, the guanylate cyclase inhibitor ODQ and the cGMP antagonist Rp-8CPT-cGMPS. Taken together, these findings imply a constrictor action of L-NAME or other arginine-based NOS inhibitors in isolated, pressurized vessels should not be automatically interpreted as indicating a role for endogenous NO production in regulating myogenic tone. That this action of the inhibitors relates to their arginine analogue structure was confirmed by the action of exogenous L-arginine preventing the L-NAME-induced constriction.
Experiments were conducted in arterioles with differing levels of pressure-induced myogenic tone, the reasoning being if increased intraluminal pressure in arterioles stimulated endothelial NOS, or the constrictor effect of L-NAME involved interaction with a pressure-dependent mechanism in the arterioles, this would be reflected in pressure-dependence of the effects of the inhibitor. Conceivably, this could result from myogenic tone increasing smooth intracellular [Ca2+] with subsequent communication to the endothelial cells via myo-endothelial gap junctions known to be present in this arteriolar preparation (McSherry et al., 2006). Consistent with these findings Dora et al. (1997) demonstrated such an interaction between smooth muscle and endothelial cells in preparations exposed to phenylephrine. However, in the present study, the constrictor effect of L-NAME was similar in magnitude at all of the pressures tested (50, 70 or 120 mm Hg), suggesting no pressure- or tone-dependent variable was involved. Further, this parallel shift in the pressure–diameter relation occurred in both endothelium-intact and denuded preparations.
Earlier studies have shown that in vivo dilation of feline cerebral arterioles mediated by KATP channel activators required L-arginine or L-lysine (Kontos and Wei, 1998). Under conditions in which the channels were activated, such as hypercapnia, the arginine-based NOS inhibitor L-NA or L-NOARG caused constriction of these arterioles and inhibited dilator responses to KATP activators (Kontos and Wei, 1996). The effect was prevented by superfusion of the arterioles with L-arginine and was not observed in the presence of the KATP channel inhibitor glyburide (glibenclamide). In the present study, however, the KATP antagonist glibenclamide did not prevent the constrictor effect of L-NAME at either 50 or 120 mm Hg. The presence of functional KATP channels in the preparation was confirmed by demonstrating dilator responses to pinacidil, which were inhibited by glibenclamide (see also Hill and Meininger, 1994). Glibenclamide itself did not have any effect on arteriolar diameter, at either pressure, suggesting KATP channels were not active in this preparation under basal conditions; indeed, KATP channels in many blood vessels are commonly active only under conditions of metabolic stress, typically hypoxia or ischemia (Quayle et al., 1997; Brayden, 2002).
Voltage-sensitive K+ and Ca2+ channels are known to be active in arterioles possessing myogenic tone (Brayden and Nelson, 1992; Knot and Nelson, 1998; Davis and Hill, 1999). In the present study L-NAME-induced constriction of the cremaster muscle arterioles was not associated with a significant change in smooth muscle membrane potential, suggesting K+-channel inhibition or enhancement of voltage-sensitive Ca2+-channel activity was unlikely to be involved in the effect. In further support of this hypothesis, the non-selective K+-channel antagonist TEA caused constriction of the arterioles, supporting a role for K+ channels (presumably smooth muscle large-conductance, Ca2+-sensitive potassium channel (BKCa); Brayden and Nelson, 1992; Knot and Nelson, 1998; Kotecha and Hill, 2005) in modulating myogenic tone, without altering the constrictor effect of L-NAME. It may be argued that the degree of depolarization induced by L-NAME in the arterioles (3–4 mV), although not statistically significant, may be sufficient to cause a constriction of the magnitude observed. This seems unlikely, however, given the relationship between membrane potential and arteriolar diameter observed in the preparation (Kotecha and Hill, 2005). From this previously established relationship, a 3 mV depolarization (at an intraluminal pressure of 50 mm Hg) would result in a contraction of about 20 μm, similar to that caused by L-NAME at this pressure. At 120 mm Hg, however, where owing to the sigmoidal shape of the Em–myogenic tone relationship a change of 3 mV would only be predicted to result in a diameter change of 1 or 2 μm, L-NAME still caused a constriction of about 20 μm (Kotecha and Hill, 2005).
Importantly, the findings of this study are not to suggest that constrictor effects of arginine-based NOS inhibitors always occur independently of NOS inhibition in isolated, pressurized arterioles, rather that such an observation alone is not conclusive evidence of endogenous NOS activity and NO production contributing to the observed level of myogenic tone. In rabbit mesenteric arterioles, a large constrictor effect of L-NOARG was significantly attenuated (although not abolished) by removal of the endothelium (Nguyen et al., 1999). In some disease models such as chronic hypoxia (Earley and Walker, 2002) or aged rats (Shipley and Muller-Delp, 2005), both L-NAME and removal of the endothelium increased myogenic tone; interestingly, in the latter study, L-NAME constricted mesenteric arterioles from young and old rats, whereas removal of the endothelium was effective only in aged rats, in which an increase in endothelial nitric oxide synthase (eNOS) enzyme expression was also demonstrated. Similarly, constrictor effects of both endothelium removal and L-NAME in wild-type mouse mesenteric arteries were not observed in an eNOS-knockout model (Scotland et al., 2001). These studies clearly associated a constrictor effect of L-NAME or L-NOARG with inhibition of NOS.
Other investigators have reconciled the seemingly contradictory observations of a constrictor effect of L-NNA or L-NAME on isolated, pressurized arterioles with no effect of endothelium removal by suggesting the endothelium releases a combination of constricting and dilating factors, such that there is no net effect of endothelium removal (Undavia et al., 2003). There is some support for this idea; in the studies of Nguyen et al. (1999) and Bai et al. (2004), in mouse-isolated cerebral arterioles, L-NOARG and L-NAME, respectively, constricted the vessels while the endothelin-1 antagonist bosentan caused dilation, suggesting simultaneous release of vasodilatory NO and constricting endothelin-1. It seems unlikely the contribution of constricting and dilating factors to myogenic tone would always balance in response to different intraluminal pressures. Alternatively, heterogeneity may exist between vessels from different tissues in the overall importance of NO as a mediator of vasodilation; indeed, the modest effects of carboxy-PTIO (shown in this study) and L-NAME on acetylcholine-induced dilation in the cremaster muscle arteriole suggest that NO plays a minor role as a dilator in this vessel (Murphy et al., 2002; McSherry et al., 2006), although earlier studies did implicate a more significant role for NO in this vessel (Kaley et al., 1992; Koller et al., 1993). A key finding of this study, however, is that L-NAME-induced constriction of the cremaster muscle arterioles occurred in the absence of the endothelium, clearly demonstrating its effect does not involve inhibition of eNOS.
The mechanism underlying the observed constrictor effect of L-NAME is unclear. While the evidence from the present study suggests an L-arginine binding site is involved, there did not appear to be an interaction with a pressure-sensitive mechanism related to the generation of myogenic tone, but rather an effect on a parallel mechanism capable of constricting vascular smooth muscle. For example, increased intracellular arginine causes inhibition of the plasma membrane Na+, K+ exchange pump in neuronal tissue (da Silva et al., 1999). In some studies, in blood vessels with pressure-induced myogenic tone the Na+, K+ pump inhibitor ouabain has been reported to cause constriction (Aalkjaer and Mulvany, 1985; Zhang et al., 2005).
In summary, this study demonstrates an endothelium-independent constrictor effect of the arginine-based NOS inhibitor L-NAME on isolated, pressurized arterioles from rat cremaster muscle. Caution should, therefore, be taken against assuming that a constrictor effect of arginine-based NOS inhibitors automatically implies a role for NOS or NO in regulating arteriolar myogenic tone, and that there is a tonic release of NO in the absence of physiological stimuli such as shear stress. Further, the data support earlier studies demonstrating that the myogenic response occurs independently of the endothelium (Falcone et al., 1991; Kuo et al., 1991; Undavia et al., 2003).
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia.
Abbreviations
- 7-NI
7-nitroindazole
- BKCa
large-conductance, Ca2+-sensitive potassium channel
- carboxy-PTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt
- cGMP
guanosine-cyclic 3′,5′-monophosphate
- Em
membrane potential
- eNOS
endothelial nitric oxide synthase
- KATP
ATP-sensitive potassium channel
- L-NA
Nω-nitro-L-arginine
- L-NAME
Nω-nitro-L-arginine methyl ester
- L-NOARG
Nω-nitro-L-arginine
- nNOS
neuronal nitric oxide synthase
- NOS
nitric oxide synthase
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PSS
physiological salt solution
- Rp-8CPT-cGMPS
Rp-8-[(4-chlorophenyl)thio]guanosine-cyclic 3′,5′-hydrogen phosphorothioate triethylammonium salt
- TEA
tetraethyl ammonium
Conflict of Interest
The authors state no conflict of interest.
References
- Aalkjaer C, Mulvany MJ. Effect of ouabain on tone, membrane potential and sodium efflux compared with [3H]ouabain binding in rat resistance vessels. J Physiol. 1985;362:215–231. doi: 10.1113/jphysiol.1985.sp015672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai N, Moien-Afshari F, Washio H, Min A, Laher I. Pharmacology of the mouse-isolated cerebral artery. Vasc Pharmacol. 2004;41:97–106. doi: 10.1016/j.vph.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2002;29:312–316. doi: 10.1046/j.1440-1681.2002.03650.x. [DOI] [PubMed] [Google Scholar]
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Da Silva CG, Parolo E, Streck EL, Wajner M, Wannmacher CMD, De Souza Wyse AT. In vitro inhibition of Na+,K+-ATPase activity from rat cerebral cortex by guanidino compounds accumulating in hyperargininemia. Brain Res. 1999;838:78–84. doi: 10.1016/s0006-8993(99)01671-6. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci USA. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duling BR, Gore RW, Dacey RG, Jr, Damon DN. Methods for isolation, cannulation and in vitro study of single microvessels. Am J Physiol. 1981;241:H108–H116. doi: 10.1152/ajpheart.1981.241.1.H108. [DOI] [PubMed] [Google Scholar]
- Earley S, Walker BR. Endothelium-dependent blunting of myogenic responsiveness after chronic hypoxia. Am J Physiol. 2002;283:H2202–H2209. doi: 10.1152/ajpheart.00125.2002. [DOI] [PubMed] [Google Scholar]
- Falcone JC, Davis MJ, Meininger GA. Endothelial independence of the myogenic response in isolated skeletal muscle arterioles. Am J Physiol. 1991;260:H130–H135. doi: 10.1152/ajpheart.1991.260.1.H130. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. Am J Physiol. 2000;279:H511–H519. doi: 10.1152/ajpheart.2000.279.2.H511. [DOI] [PubMed] [Google Scholar]
- Hill MA, Meininger GA. Calcium entry and myogenic phenomena in skeletal muscle arterioles. Am J Physiol. 1994;267:H1085–H1092. doi: 10.1152/ajpheart.1994.267.3.H1085. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Koller A, Kaley G. Gender difference in myogenic tone of rat arterioles is due to estrogen-induced, enhanced release of NO. Am J Physiol. 1997;272:H1804–H1809. doi: 10.1152/ajpheart.1997.272.4.H1804. [DOI] [PubMed] [Google Scholar]
- Jarajapu YP, Grant MB, Knot HJ. Myogenic tone and reactivity of the rat ophthalmic artery. Invest Ophthalmol Vis Sci. 2004;45:253–259. doi: 10.1167/iovs.03-0546. [DOI] [PubMed] [Google Scholar]
- Johnson PC.The myogenic response Handbook of Physiology. The Cardiovascular System. Section II, Volume 2, Vascular Smooth Muscle 1980American Physiological Society: Bethesda, MD; 409–442.In: Bohr D, Somlyo A and Sparks H (eds). [Google Scholar]
- Kaley G, Koller A, Rodenburg JM, Messina EJ, Wolin MS. Regulation of arteriolar tone and responses via L-arginine pathway in skeletal muscle. Am J Physiol. 1992;262:H987–H992. doi: 10.1152/ajpheart.1992.262.4.H987. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller A, Sun D, Messina EJ, Kaley G. -arginine analogues blunt prostaglandin-related dilation of arterioles. Am J Physiol. 1993;264:H1194–H1199. doi: 10.1152/ajpheart.1993.264.4.H1194. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP. Arginine analogues inhibit responses mediated by ATP-sensitive K+-channels. Am J Physiol. 1996;271:H1498–H1506. doi: 10.1152/ajpheart.1996.271.4.H1498. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP. Cerebral arteriolar dilations by KATP channel activators need L-lysine or L-arginine. Am J Physiol. 1998;274:H974–H981. doi: 10.1152/ajpheart.1998.274.3.H974. [DOI] [PubMed] [Google Scholar]
- Kotecha N, Hill MA. Myogenic contraction in rat skeletal muscle arterioles: smooth muscle membrane potential and Ca2+ signaling. Am J Physiol. 2005;289:H1326–H1334. doi: 10.1152/ajpheart.00323.2005. [DOI] [PubMed] [Google Scholar]
- Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine resistance vessels. Am J Physiol. 1991;261:H1706–H1715. doi: 10.1152/ajpheart.1991.261.6.H1706. [DOI] [PubMed] [Google Scholar]
- McSherry IN, Sandow SL, Campbell WB, Falck JR, Hill MA, Dora KA. A role for heterocellular coupling and EETs in dilation of rat cremaster arterioles. Microcirc. 2006;13:119–130. doi: 10.1080/10739680500466400. [DOI] [PubMed] [Google Scholar]
- Meininger GA, Davis MJ. Cellular mechanisms involved in the vascular myogenic response. Am J Physiol. 1992;263:H647–H659. doi: 10.1152/ajpheart.1992.263.3.H647. [DOI] [PubMed] [Google Scholar]
- Moore PK, Al-Swayeh OA, Chong NW, Evans RA, Gibson A. -NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990;99:408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TV, Tran H, Kotecha N, Hill MA.Endothelium-independent constriction of rat cremaster arterioles by L-NAME Proc Aust Health Med Res Congr 20021447–6010.(1160); Melbourne: ISSNAbs. 431
- Nguyen T-D, Véquad P, Thorin E. Effects of endothelin receptor antagonists and nitric oxide on myogenic tone and α-adrenergic-dependent contractions of rabbit resistance arteries. Cardiovasc Res. 1999;43:755–761. doi: 10.1016/s0008-6363(99)00170-4. [DOI] [PubMed] [Google Scholar]
- Potocnik SJ, Murphy TV, Kotecha N, Hill MA. Effects of mibefradil and nifedipine on arteriolar myogenic responsiveness and intracellular Ca2+ Br J Pharmacol. 2000;131:1065–1072. doi: 10.1038/sj.bjp.0703650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Rees DD, Palmer RM, Hodson HF, Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989;96:418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterisation of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland RS, Chauhan S, Vallance PJT, Ahluwalia A. An endothelium-derived hyperpolarizing factor-like factor moderates myogenic constriction of mesenteric resistance arteries in the absence of endothelial nitric oxide synthase-derived nitric oxide. Hypertension. 2001;38:833–839. doi: 10.1161/hy1001.092651. [DOI] [PubMed] [Google Scholar]
- Shipley RD, Muller-Delp JM. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res. 2005;66:374–383. doi: 10.1016/j.cardiores.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Szekeres M, Nadasy GL, Kaley G, Koller A. Nitric oxide and prostaglandins modulate pressure-induced myogenic responses of intramural coronary arteries. J Cardiovasc Pharmacol. 2004;43:242–249. doi: 10.1097/00005344-200402000-00012. [DOI] [PubMed] [Google Scholar]
- Undavia SS, Berger V, Kaley G, Messina EJ. Myogenic responses of isolated adipose tissue arterioles. Microvasc Res. 2003;66:140–146. doi: 10.1016/s0026-2862(03)00042-6. [DOI] [PubMed] [Google Scholar]
- Wang HX, Davis MJ, Rajanayagam MA, Potocnik SJ, Hill MA. Myogenic activity of rat epineurial arterioles: potential role in local vasoregulatory events. Am J Physiol. 1999;277:H144–H151. doi: 10.1152/ajpheart.1999.277.1.H144. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lee MY, Cavalli M, Chen L, Berra-Romani R, Balke CW, et al. Sodium pump alpha-2 subunits control myogenic tone and blood pressure in mice. J Physiol. 2005;569:243–256. doi: 10.1113/jphysiol.2005.091801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Ratz PH, Hill MA. Role of myosin phosphorylation and [Ca2+]i in myogenic reactivity and arteriolar tone. Am J Physiol. 1995;269:H1590–H1596. doi: 10.1152/ajpheart.1995.269.5.H1590. [DOI] [PubMed] [Google Scholar]