Abstract
Background and purpose:
Relaxation of corpus cavernosum, which is mediated by nitric oxide (NO) released from non-adrenergic non-cholinergic (NANC) neurotransmission, is critical for inducing penile erection and can be affected by many pathophysiological conditions. However, the peripheral effect of liver cirrhosis on erectile function is as yet unknown. The aim of the present study was to investigate the effect of biliary cirrhosis on NANC-mediated relaxation of rat corpus cavernosum and the possible roles of endocannabinoid and nitric oxide systems in this model.
Experimental approach:
Cirrhosis was induced by bile duct ligation. Controls underwent sham operation. Four weeks later, strips of corpus cavernosum were mounted in a standard organ bath and NANC-mediated relaxations were obtained by applying electrical field stimulation.
Key results:
The NANC-mediated relaxation was enhanced in corporal strips from cirrhotic animals. Anandamide potentiated the relaxations in both groups. Either AM251 (CB1 antagonist) or capsazepine (vanilloid VR1 antagonist), but not AM630 (CB2 antagonist), prevented the enhanced relaxations of cirrhotic strips. Either the non-selective NOS inhibitor L-NAME or the selective neuronal NOS inhibitor L-NPA inhibited relaxations in both groups, but cirrhotic groups were more resistant to the inhibitory effects of these agents. Relaxations to sodium nitroprusside (NO donor) were similar in tissues from the two groups.
Conclusions and implications:
Cirrhosis potentiates the neurogenic relaxation of rat corpus cavernosum probably via the NO pathway and involving cannabinoid CB1 and vanilloid VR1 receptors.
Keywords: cirrhosis, corpus cavernosum, NANC (non-adrenergic non-cholinergic) relaxation, nitric oxide (NO), cannabinoids, erection, rats
Introduction
Cirrhosis is associated with a host of haemodynamic and hydrodynamic abnormalities. The peripheral vasodilation seems to be the origin of many clinical manifestations in cirrhotic patients (García-Estañ et al., 2002). The exact aetiology of these problems is as yet elusive. However, a large body of evidence has suggested a possible role for the L-arginine-nitric oxide (NO) pathway in virtually all of these events. For instance, the involvement of both inducible NO synthase (iNOS) and endothelial NO synthase (eNOS) has been demonstrated in the pathogenesis of extrahepatic manifestations of cirrhosis (Martin et al., 1996, 1998; Morales-Ruiz et al., 1996; Wiest and Groszmann, 1999; Wiest et al., 1999). Recently, some studies have indicated that neuronal NOS (nNOS) protein expression and activity in aorta and liver is elevated in cirrhotic animals (Xu et al., 2000; Wei et al., 2002; Bieker et al., 2004), suggesting a possible role for nNOS-derived NO in this pathological condition. However, the exact role of nNOS in the pathophysiological changes occurring in cirrhosis has not yet been completely defined.
Besides NO, there is also convincing evidence for the role of endocannabinoids in the compromised haemodynamics seen in cirrhosis (Gabbay et al., 2005). The mechanism underlying this phenomenon appears to involve an increase in production of endocannbinoids in platelets and macrophages and a subsequent vasodilation, at least partially mediated by cannabinoid receptors subtype 1 (CB1) activation (Varga et al., 1998; Batkai et al., 2001). Moreover, it has been shown that circulating levels of anandamide is increased in patients with liver disease (Fernández-Rodriguez et al., 2004).
Penile tumescence (erection) and detumescence are regulated by a complex neurophysiological process of relaxation and contraction, respectively, of the corpus cavernosum (Lue and Tanagho, 1987). It is well known that NO is the most important factor mediating penile erection, which is mainly derived from nonadrenergic noncholinergic (NANC) neurotransmission (Burnett et al., 1992; Ignarro, 2002). We have recently shown that the endogenous cannabinoid anandamide may play a role in erectile function by its potentiating effect on NANC-mediated relaxation of rat corpus cavernosum possibly via the CB1 and vanilloid receptors and involving the NO pathway (Ghasemi et al., 2006). However, the exact role of endocannabinoid system in pathophysiological conditions affecting corpus cavernosum physiology and functioning is as yet unidentified.
In our previous study, we demonstrated that in cholestatic rats there was an alteration in neurogenic relaxation of corpus cavernosum and that the L-arginine-NO pathway may be a contributor to this condition (Sadeghipour et al., 2003). However, it has not been clear whether the cirrhotic state peripherally has any effect on this system or not. On the other hand, since penile erection is a neurovascular event, the evaluation of the effect of cirrhosis on the neurogenic relaxation of corpus cavernosum may be used as a model to study the role of interaction between endocannabinoids and nNOS in the pathophysiology of vascular dysfunction in cirrhosis. Therefore, the goals of this study were: (a) to investigate the effect of biliary cirrhosis on neurogenic relaxation of rat corpus cavernosum; (b) to assess the role of nNOS in neurogenic corporal relaxation by using selective nNOS inhibitors and immunoblotting in corpus cavernosum isolated from cirrhotic animals; and (c) to investigate the role of the endocannabinoid system by evaluating the effect of anandamide and cannabinoid and vanilloid receptor antagonists on neurogenic relaxation of corpus cavernosum from cirrhotic rats.
Methods
Animal model
The study, protocols were performed in accordance with the recommendations of the Ethics Committee of the University. Male Sprague–Dawley rats (Pasteur Institute, Tehran, Iran) weighing 200–250 g were used throughout the study. Rats had free access to rat chow and water and maintained in a 12-h light and dark cycle. Bile duct ligation was performed to induce cirrhosis. The surgical procedures for this cirrhotic model have been described in detail previously (Ma et al., 1999; Gaskari et al., 2005). Briefly, the common bile duct was exposed by a midline abdominal incision under general anaesthesia. The bile duct was doubly ligated and sectioned between the ligatures. Sham-operated rats were treated in the same manner as the above-mentioned group, except that the bile duct was visually inspected but not ligated. All studies were performed 4 weeks after the bile duct ligation or sham operation.
Preparation of rat corpus cavernosum strips
The rats were killed by cervical dislocation. Penises were surgically removed at the level of the crural attachments to the pubo-ischial bones and promptly placed in a petri dish containing Krebs-bicarbonate solution (containing in mM: NaCl 118.1, KCl 4.7, KH2PO4 1.0, MgSO4 1.0, NaHCO3 25.0, CaCl2 2.5 and glucose 11.1), bubbled with a mixture of 95% O2 and 5% CO2. After excising the glans penis and urethra, the corporal tissue was dissected free from the tunica albuginea. Corpora cavernosa were separated by cutting the fibrous septum between them. They were mounted separately in 20-ml organ chambers with one end tied to an electrode holder and the other to a wire connected to a force transducer (Narco F-60, Narco Biosystems, Houston, TX, USA). The chambers contained Krebs-bicarbonate solution (pH 7.4) at 37°C equilibrated with 95% O2 and 5% CO2. The strips were allowed to equilibrate under optimal resting tension of 0.5 g (Sadeghipour et al., 2003; Ghasemi et al., 2006) for 60 min, which was applied in all subsequent experiments. Electrical field stimulation (EFS) from a grass stimulator (Model S88) was applied via two parallel platinum electrodes on either side of the corporal strips. In experiments where EFS was used, guanethidine (5 μM) and atropine (1 μM) were always present in the bathing medium to obtain NANC conditions. In all the experiments, each strip was used only once.
Responses to phenylephrine
In sham-operated and cirrhotic groups, concentration–response curves for phenylephrine (10 nM–1 mM) were obtained by the cumulative addition of phenylephrine to the chamber in half-log increments. The EC50 values for phenylephrine in two groups were compared.
Responses to electrical field stimulation
In two groups, corporal strips were precontracted with phenylephrine (7.5 μM; EC80) and when the contractions had stabilized, frequency–response curves for EFS were obtained, using consecutive 8 s stimulations (150 V, 3 ms duration, every 120 s) at the frequencies of 2, 5, 10 and 15 Hz (Sadeghipour et al., 2003; Ghasemi et al., 2006). In two separate groups of sham-operated and cirrhotic animals, the endocannabinoid anandamide (1 μM) was added after reaching the plateau response to phenylephrine and 20 min before EFS.
In corporal tissues of separate groups of sham-operated and cirrhotic animals, the cannabinoid CB1 receptor antagonist AM251 (10 μM) and the cannabinoid CB2 receptor antagonist AM630 (10 μM) (White et al., 2001) was added to the bathing medium 10 min before anandamide (1 μM) administration. In another separate group, the vanilloid receptor antagonist capsazepine (10 μM) (White et al., 2001), was added to the bathing medium 20 min before anandamide (1 μM) administration. Also, in separate groups, the effects of AM251 (10 μM), AM630 (10 μM) and capsazepine (10 μM) were investigated on NANC-mediated relaxation.
Effects of L-NAME and L-NPA on NANC-induced relaxation
For evaluating the effect of the nonselective NOS inhibitor L-NAME and the selective nNOS inhibitor L-NPA on the NANC-mediated relaxation, the corporal strips were pre-incubated for 30 min with either L-NAME (0.03, 0.1, 0.3, 1 and 10 μM) or L-NPA (0.1, 1, 10 and 100 μM) in separate groups of sham-operated and cirrhotic animals before constructing steady-state frequency–response curves for relaxation with EFS in the presence or absence of anandamide (1 μM).
Responses to sodium nitroprusside
In two experimental groups, corporal strips were precontracted with phenylephrine (7.5 μM, EC80) and when the contraction had stabilized, concentration–response curves for sodium nitroprusside (SNP) (1 nM–1 mM), were obtained by the cumulative addition of SNP to the chamber in half-log increments in the presence or absence of either of anandamide (1 μM), AM251 (10 μM), AM630 (10 μM) and capsazepine (10 μM).
Western blotting
Snap-frozen corporal tissues from cirrhotic and sham-operated animals (n=4 for each group) were homogenized in ice-cold radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors (protease inhibitor mixture from Roche, Mannheim, Germany), 50 mM Tris (pH 8), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS. Homogenates were then sonicated followed by centrifugation at 10 000 g for 5 min at 4°C. After determining the protein concentrations of the supernatants (Bradford assay with bovine serum albumin as standard), 10 μg protein of each sample was fractionated by sodiumdodecyl sulphate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking with Tris-buffered saline (10 mM Tris, 100 mM NaCl) containing 0.1% Tween-20 for 1 h, the membranes were incubated overnight with rabbit polyclonal anti-nNOS (Sigma, Poole, UK), VR1 or CB1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Primary antibodies were diluted as 1:1000, 1:200 and 1:200 for nNOS, VR1 and CB1, respectively. After rigorous washing, the membranes were incubated with anti-rabbit IgG alkaline phosphatase-linked antibody (1:5000 dilution, Perbio Science, Northumberland, UK). Alkaline phosphatase was detected using a BCIP/NBT developing kit (Promega, Madison, WI, USA). Developed immunoblot membranes were digitized and band optical densities were quantified using a computerized imaging system (Imaging densitometer model GS-670, Bio-Rad, Hercules, CA, USA). Densitometry data are expressed as relative optical densities (ROD) in arbitrary units.
Statistical analysis
The data are expressed as mean±s.e.m. Statistical analysis of the data was performed by one-way and two-way analysis of variance (ANOVA) followed by Newman–Keuls post hoc test. Differences between means were considered statistically significant when P<0.05.
Drugs
The following drugs were used: phenylephrine hydrochloride, anandamide (N-arachidonylethanolamine), AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide, Tocris, Bristol, UK), AM630 (6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl(4-methoxyphenyl)methanone, Tocris, UK), capsazepine, SNP, L-NAME (Nω-nitro-L-arginine methyl ester), L-NPA (Nω-propyl-L-arginine), guanethidine sulphate and atropine sulphate (Sigma, St Louis, MO, USA).
Anandamide was dissolved in 1:1:18 emulphore/ethanol/saline. AM251 and AM630 were dissolved in dimethyl sulphoxide (DMSO) and saline. Capsazepine was dissolved in ethanol. All other drugs were dissolved in distilled water.
Drugs were added to the bath in microlitre volumes, and experiments were controlled for the effects of the drug solvents. Neither ethanol nor DMSO nor saline in the final organ bath concentrations had a significant effect on the basal tension or on the relaxations observed.
Results
Responses to phenylephrine
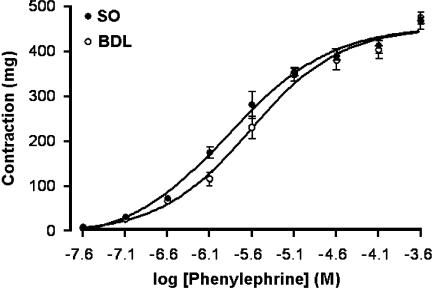
There was no significant difference between the maximal contractile responses to phenylephrine in sham-operated and cirrhotic rats (469±28 and 468±21 mg, respectively) or between the contractile responses to 7.5 μM phenylephrine (354±23 and 348±18 mg, respectively; Figure 1). Values for EC50 were significantly different between the two groups (1.32±0.19 and 2.4±0.315 μM, respectively; P<0.01; n=6).
Figure 1.
Dose–response relationship of contractions induced by phenylephrine in isolated corpus cavernosum of sham-operated (SO) and cirrhotic (BDL) groups. Each group consisted of six rats.
Responses to EFS
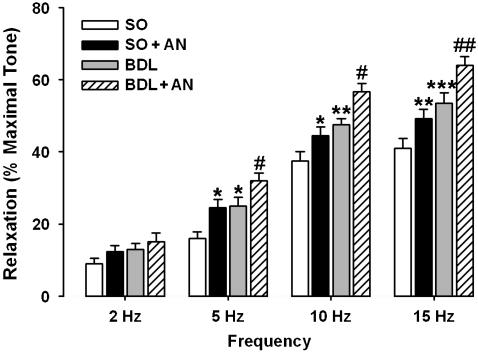
Precontracted corporal strips were relaxed in a frequency-dependent manner by EFS (Figure 2). Since guanethidine and atropine blocked the adrenergic and cholinergic nerve-mediated effects of EFS, the relaxation response of corporal strips induced by EFS was due to NANC mechanisms. Two-way ANOVA analysis revealed that in both sham-operated and cirrhotic groups, the precontracted strips were relaxed to EFS in a frequency-dependent manner (P<0.001) and also in cirrhotic animals relaxant responses to EFS was significantly (P<0.001) enhanced (Figures 2 and 3). Further analysis showed that administration of anandamide (1 μM) in each experimental groups of sham-operated and cirrhotic rats significantly (P<0.001) potentiated the relaxant responses to EFS (Figures 2 and 3).
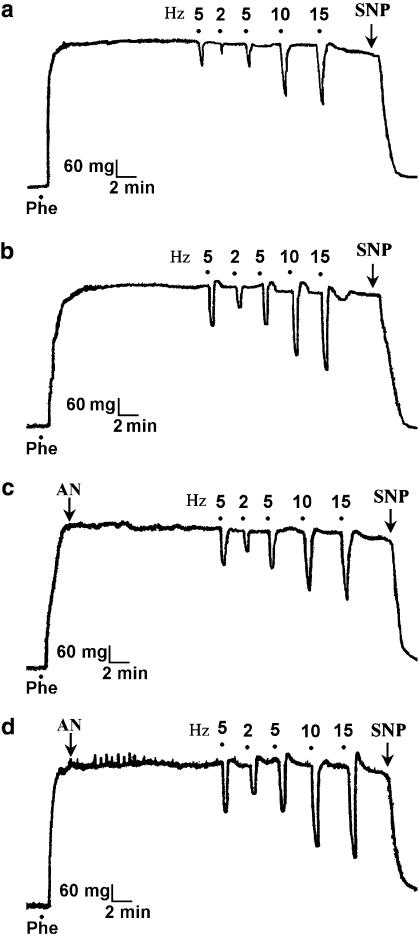
Figure 2.
Tracings of frequency-dependent relaxant responses to EFS in corporal strips precontracted with 7.5 μM phenylephrine (Phe) in the presence of guanethidine (5 μM) and atropine (1 μM). In comparison with sham-operated strips (a), relaxant responses to EFS were enhanced in corporal strips of biliary cirrhotic animals (b). Anandamide (AN, 1 μM) potentiated the NANC-mediated relaxations in both sham-operated (c) and cirrhotic (d) groups. EFS was applied at 2, 5, 10 and 15 Hz. EFS, electrical field stimulation; NANC, non-adrenergic non-cholinergic.
Figure 3.
EFS-induced relaxation in corporal strips precontracted with phenylephrine (7.5 μM) in the presence of guanethidine (5 μM) and atropine (1 μM) from sham-operated group in the absence (SO) or presence of 1 μM anandamide (SO+AN) and cirrhotic group in the absence (BDL) or presence of 1 μM anandamide (BDL+AN). The frequency-dependent relaxations were significantly enhanced in cirrhotic group as compared with sham groups. Anandamide potentiated the relaxant responses to EFS in both sham and cirrhotic groups. Each group consisted of six rats (*P<0.05, **P<0.001 and ***P<0.001 compared with sham group without anandamide; #P<0.05 and ##P<0.01 compared with cirrhotic group without anandamide). AN, anandamide; BDL, bile duct ligated; EFS, electrical field stimulation.
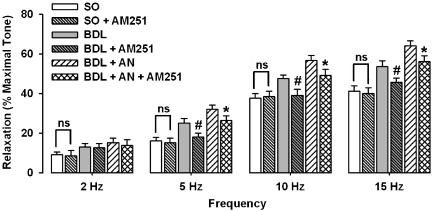
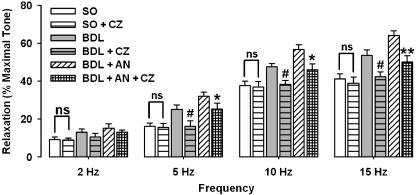
The enhanced responses to EFS in cirrhotic rats were significantly (P<0.01) prevented by the selective cannabinoid CB1 receptor antagonist AM251 (10 μM, Figure 4). But the selective cannabinoid CB2 receptor antagonist AM630 (10 μM) did not alter the NANC-induced relaxation in the cirrhotic rats (data not shown). Also, preincubation with capsazepine (10 μM) significantly (P<0.01) prevented the increased NANC-mediated relaxation in cirrhotic group (Figure 5). Further analysis in cirrhotic groups in which anandamide was administered revealed that the potentiating activity of anandamide on the responses to EFS in cirrhotic rats was significantly antagonized by either AM251 (Figure 4) or capsazepine (Figure 5), but not by AM630 (data not shown). Neither AM251 (10 μM) nor AM630 (10 μM) nor capsazepine (10 μM) had an influence on NANC-mediated relaxation in corpora cavernosum from sham-operated rats.
Figure 4.
Effect of AM251 (10 μM) on frequency-dependent relaxations in sham-operated (SO) and cirrhotic (BDL) groups in the presence or absence of 1 μM anandamide (AN). Each group consisted of six rats (*P<0.05 compared with cirrhotic group with anandamide; #P<0.05 compared with cirrhotic groups without anandamide, ns=nonsignificant difference). BDL, bile duct ligated.
Figure 5.
Effect of 10 μM capsazepine (CZ) on frequency-dependent relaxations in sham-operated (SO) and cirrhotic (BDL) groups in the presence or absence of 1 μM anandamide (AN). Each group consisted of six rats (*P<0.05, **P<0.01 compared with cirrhotic groups with anandamide; #P<0.05 compared with cirrhotic groups without anandamide; ns=nonsignificant difference). BDL, bile duct ligated.
Effects of L-NAME and L-NPA on NANC-induced relaxation
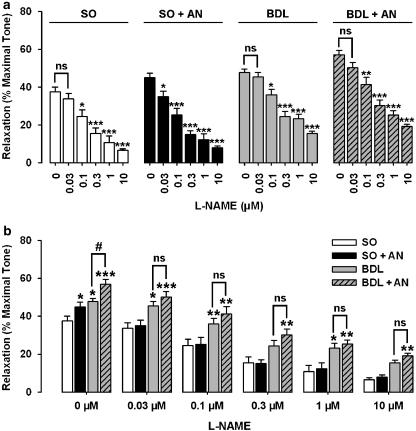
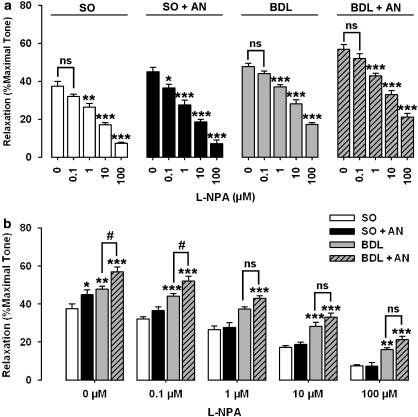
The addition of L-NAME and L-NPA caused a concentration-dependent inhibition of the relaxant responses to 10-Hz stimulation in sham and cirrhotic groups (Figures 6a and 7a), which suggests that NO (neuronal NO) is responsible for the NANC effect in this model. In sham animals, although either 30 nM L-NAME or 0.1 μM L-NPA had no significant effect on NANC-mediated relaxation, they significantly (P<0.05) prevented the potentiating effect of anandamide (1 μM) on relaxation to EFS (Figures 6a and 7a). However, L-NAME (at 30 nM) and L-NPA (at 0.1 μM) had no significant effect on NANC-mediated relaxation in cirrhotic rats in the presence or absence of anandamide (1 μM). In the presence of different concentrations of L-NAME and L-NPA, the potentiating activity of anandamide was prevented in both sham and cirrhotic groups (Figures 6b and 7b). As shown in Figures 6b and 7b, the highest concentrations of L-NAME (10 μM) and L-NPA (100 μM) caused a marked inhibition of neurogenic relaxation of corpus cavernosum in control animals. However, these agents could not block completely the neurally relaxations in cirrhotic animals.
Figure 6.
L-NAME inhibited the relaxant responses to 10-Hz stimulation in precontracted rat corpus cavernosum in a concentration-dependent manner. In the presence of L-NAME (30 nM), the potentiating activity of anandamide (AN) on relaxant responses to EFS was significantly inhibited in sham rats (SO). L-NAME (30 nM) alone did not cause a significant attenuation of NANC-mediated relaxation in other groups (a; *P<0.05, **P<0.01, and ***P<0.001 compared with corresponding groups without L-NAME). There was no significant difference in NANC-mediated relaxations between cirrhotic groups without anandamide (BDL) and cirrhotic groups with anandamide (BDL+AN) in the presence of different doses of L-NAME (b). Each groups consisted of six rats (b; *P<0.05, **P<0.01, and ***P<0.001 compared with corresponding sham groups without anandamide; #P<0.05 compared with corresponding cirrhotic groups without anandamide; ns=nonsignificant difference). BDL, bile duct ligated; L-NAME, Nω-nitro-L-arginine methyl ester; NANC, non-adrenergic non-cholinergic.
Figure 7.
Effect of L-NPA on the relaxant responses to 10-Hz stimulation in precontracted corpus cavernosum from sham-operated (SO) and cirrhotic (BDL) rats in the presence or absence of anandamide (AN, 1 μM). In the presence of L-NPA (0.1 μM), the potentiating activity of anandamide on relaxant responses to EFS was significantly inhibited in sham rats. L-NPA (0.1 μM) alone did not cause a significant attenuation of NANC-mediated relaxation in other groups (a; *P<0.05, **P<0.01, and ***P<0.001 compared with corresponding groups without L-NPA). There is no significant difference in NANC-mediated relaxations between cirrhotic groups without anandamide (BDL) and cirrhotic groups with anandamide (BDL+AN) in the presence of different doses (1–100 μM) of L-NPA (b). Each groups consisted of six rats (b; *P<0.05, **P<0.01, and ***P<0.001 compared with corresponding sham groups without anandamide; #P<0.05 compared with corresponding cirrhotic groups without anandamide; ns=nonsignificant difference). BDL, bile duct ligated; L-NPA, Nω-propyl-L-Arginine.
Responses to SNP
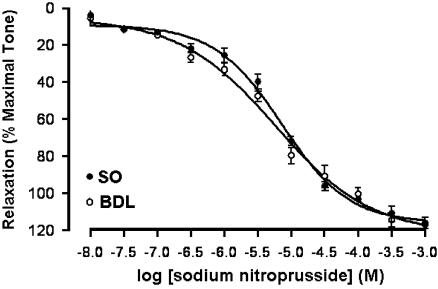
In phenylephrine-precontracted corporal strips, SNP caused concentration-dependent relaxation (Figure 8). There was no significant difference between the EC50s for control and cirrhotic groups (7.137±0.67 and 5.82±0.75 μM, respectively). Also, neither AM251 (10 μM) nor AM630 (10 μM) nor capsazepine (10 μM) nor anandamide (1 μM) had an influence on the concentration-dependent relaxation of SNP (data not shown.).
Figure 8.
Concentration-dependent relaxation in response to sodium nitroprusside in precontracted rat corporal smooth muscles of sham-operated (SO) and cirrhotic (BDL) groups. Each group consisted of six rats. BDL, bile duct ligated.
Western blotting
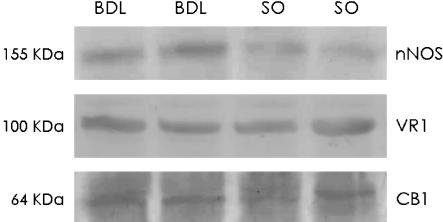
As shown in Figure 9, corporal tissues from both sham-operated and cirrhotic rats showed a band of approximately 64 and 100 kDa that was identified as CB1 and VR1 proteins, respectively. There was no significant alteration in the density of either the CB1 receptor band density (21.13±11.26 versus 33.53±5.85 ROD) or the VR1 receptor band density (7.03±2.19 versus 9.13±1.93 ROD), between sham-operated and cirrhotic rats respectively (n=4 in each group).
Figure 9.
Western blot of neuronal NO synthase (nNOS) enzyme, cannabinoid CB1 and vanilloid VR1 receptor protein in corporal tissue strips from two representative sham-operated (SO) and biliary cirrhotic (BDL) rats.
As shown in Figure 9, corporal tissues from both sham-operated and cirrhotic rats showed a band of approximately 155 kDa for nNOS protein. Cirrhosis was associated with a significant (P<0.05) increase in the relative density of nNOS protein compared with sham-operated rats (11.65±2.2 versus 22.3±2.7 ROD in sham-operated and cirrhotic rats respectively, n=4 in each group).
Discussion
In the present study, we demonstrated that NANC-mediated relaxation of corpus cavernosum was enhanced in cirrhotic rats. This result is in agreement with our previous studies which showed that the NANC-mediated relaxation of corpus cavernosum and anococcygeus muscles is increased in cholestatic rats (Dehpour et al., 2003; Sadeghipour et al., 2003). Either the nonselective NOS inhibitor L-NAME or the selective nNOS inhibitor L-NPA inhibited relaxation of corporal tissue in a dose-dependent manner, suggesting that this effect was mainly mediated via nitrergic neurotransmission. Moreover, in cirrhotic animals, the NANC-mediated relaxation was more resistant to the inhibitory effect of both L-NAME and L-NPA. Contractile responses to 7.5 μM phenylephrine (EC80) were indistinguishable between sham-operated and cirrhotic rats, which excludes the possibility that the increase in nitrergic relaxation might have been due to an alteration in responsiveness of the smooth muscle to 7.5 μM phenylephrine. However, our data showed that values for EC50s were significantly different between sham and cirrhotic groups. This result is in agreement with results of previous studies, which have shown that phenylephrine-induced vasoconstriction response of rats with obstructive cholestasis was impaired (Bomzon et al., 1985; Cioffi et al., 1986; Moezi et al., 2004). Our data also demonstrated that the increase in nitrergic relaxation was not due to changes in responsiveness of smooth muscle to NO, since concentration–response curves to SNP, an NO donor, in sham and cirrhotic animals were indistinguishable. Therefore, the increased resistance of the NANC relaxation to L-NAME and L-NPA might be due to an increased nitrergic neurotransmission.
During the 1990s, a large body of evidence had indicated that NO production was enhanced in the vasculature of cirrhotic animals and humans, by measuring the systemic levels of nitrite and nitrate (Guarner et al., 1993; Hori et al., 1998), aortic and mesenteric NOS activity (Cahil et al., 1996; Martin et al., 1996), and the aortic cGMP level (Ros et al., 1995), since Vallance and Moncada (1991) proposed that NO could play a role in the pathogenesis of the haemodynamic abnormalities in cirrhosis. Although involvement of both iNOS and eNOS in these events has been suggested, the role of nNOS in this condition was unidentified until Xu et al. (2000) reported an elevated nNOS protein expression in aorta of cirrhotic rats. Also, Butterworth (2000) showed that the expression of gene coding for nNOS protein is increased in chronic liver disease. These findings led to a focus on the possible role of nNOS in vascular and even in neurological abnormalities in cirrhosis. However, some studies reported different results. Hernandez et al. (2004) reported an unchanged nNOS protein levels in the cerebellum of thioacetamide cirrhotic rats. Goh et al. (2006) indicated that there was no significant change in nNOS expression in cirrhotic human liver. Montes et al. (2006) even reported a diminished constitutive NO synthase activity in basal ganglia of biliary cirrhotic rats. In contrast to these studies, expression of nNOS mRNA and protein was enhanced in liver of rats with biliary cirrhosis (Wei et al., 2002), in the aorta of biliary cirrhotic mice (Bieker et al., 2004) and in hepatic arteries of cirrhotic patients (Bieker et al., 2004). By immunohistochemical methods, it was also shown that nNOS was localized in the cytoplasm of some hepatocytes of biliary cirrhotic rats, whereas none of the hepatocytes of sham rats were positively stained (Wei et al., 2002). In agreement with these studies, by immunoblotting using specific antibody, we also found that the amount of nNOS protein within the corpus cavernosum was increased in the cirrhotic group, compared with control sham animals. Therefore, according to these results and the data obtained from studies with the selective nNOS inhibitor (L-NPA), the enhanced NANC-mediated relaxation of corpus cavernosum in cirrhotic rats might be due to elevated levels of nNOS and consequently enhanced NO production in this tissue. However, more detailed studies are clearly needed to verify the underlying mechanisms involved in this condition.
Recently, we found that the endocannabinoid anandamide could potentiate the neurogenic relaxation of rat corpus cavernosum possibly through either CB1 or vanilloid receptors (Ghasemi et al., 2006). The present data also demonstrated that although neither the CB1 nor vanilloid receptor antagonists had an effect on NANC-mediated relaxation in control rats, they did prevent the enhanced neurogenic relaxations in cirrhotic animals. These results are in line with many studies, which showed that changes in the cannabinoid system might be one of the mechanisms responsible for the haemodynamic alterations in cirrhosis (Gabbay et al., 2005). Batkai et al. (2001) showed that in cirrhotic human livers there was a threefold increase in CB1 receptors on isolated vascular endothelial cells, indicating upregulation of these receptors in chronic liver disease. Also, CB1 receptor blockade increased arterial pressure and peripheral resistance in cirrhotic animals, but not in healthy ones (Ros et al., 2002). Moreover, Gaskari et al. (2005) indicated a pathogenic role for increased local (neuronal) production of endocannabinoids, mediated by a CB1-responsive pathway in cirrhotic cardiomyopathy. In another study, Domenicali et al. (2005) showed that mesenteric vessels of cirrhotic rats display greater sensitivities to anandamide and that either CB1 or vanilloid receptors were involved in this condition. They also demonstrated that CB1 and vanilloid receptor protein levels were higher in cirrhotic vessels (Domenicali et al., 2005). In contrast, in the present study, we were not able to demonstrate any alteration in CB1 and VR1 protein levels in cirrhotic tissues compared with controls. On the other hand, there is evidence that circulating levels of anandamide are elevated in patients with cirrhosis (Fernández-Rodriguez et al., 2004). Taking these earlier reports together with our results, it could be suggested that the endocannabinoid system may be involved in the enhanced neurally induced relaxation of cirrhotic corporal tissues. More detailed studies such as measurement of anandamide levels within the corpus cavernosum, as well as quantitative assessment of CB1 and VR1 mRNA expression in the corporal tissue from cirrhotic rats, are clearly needed to verify the exact underlying mechanisms of the observed effects.
In agreement with our previous study (Ghasemi et al., 2006), 30 nM L-NAME significantly reversed the potentiated relaxant responses resulting from 1 μM anandamide in the sham-operated group, although this concentration of L-NAME did not cause a significant inhibition of the relaxations to EFS individually. In addition, similar results were observed by using the selective nNOS inhibitor L-NPA at 0.1 μM. These data suggest that the potentiating activity of anandamide involves the nNOS-mediated component of the relaxant NANC responses in rat corpus cavernosum. In agreement with our finding, the close correlation between endocannabinoids and constitutive NO synthase has been reported in different cells and tissues, linked to stimulation of cannabinoid CB1 or vanilloid VR1 receptors (Deutsch et al., 1997; Stefano et al., 1998; Járai et al., 1999; Ledent et al., 1999; Howlett and Mukhopadhyay, 2000; Harris et al., 2002; Mukhopadhyay et al., 2002). For instance, Poblete et al. (2005) have shown that anandamide elicits an acute release of NO through endothelial VR1 receptor activation in the rat arterial mesenteric bed. In the present study, we also demonstrated that anandamide potentiated the NANC-mediated relaxation of corporal strips from cirrhotic rats. However, in the presence of different concentrations of L-NAME and L-NPA, the potentiating activity of anandamide in cirrhotic groups was prevented and also there was no significant difference between NANC-mediated relaxation in cirrhotic group with anandamide and cirrhotic group without anandamide. On the other hand, in comparison with cirrhotic groups without anandamide, the relaxations to EFS were more resistant to the inhibitory effects of both L-NAME and L-NPA in cirrhotic groups with anandamide. This finding might be explained by the probable involvement of the NO pathway in the effect of anandamide and also by the central role of NO in the enhanced NANC-mediated relaxations in cirrhosis. However, more studies are needed to explain in detail the interaction of endocannabinoids and the NO pathway in this model.
According to the present data, the highest concentrations of L-NAME (10 μM) and L-NPA (100 μM) caused a marked inhibition of neurogenic relaxation of corpus cavernosum in control animals. However, these agents could not block completely the neurally induced relaxations in cirrhotic animals. This result might reflect the uncovering of a non-NO component of neurogenic relaxation of cavernosal tissue in cirrhotic animals. As mentioned above, there is good experimental evidence for neurogenic NO to be a mediator of penile erection, but even if NO probably is the most important factor for relaxation of corpus cavernosum, this does not exclude the possibility that other agents released from nerves may have a modulatory function in this process. Some investigators believe that vasoactive intestinal polypeptide (VIP) may be one of the neurotransmitters responsible for erection (Andersson et al., 1984; Mizusawa et al., 2001). VIP-immunoreactive nerve fibres have been identified within the cavernous trabeculae, and neurostimulation-induced cavernous smooth muscle relaxation has been shown to be blocked by VIP antagonist or anti-VIP serum (Ottesen et al., 1984; Yeh et al., 1994; Kim et al., 1995). Another neurotransmitter which may contribute to penile erection and corpus cavernosum functioning in dog, monkey, rat and human is calcitonin gene-related peptide (CGRP) (Stief et al., 1990, 1991, 1993; McNeill et al., 1992; Hauser-Kronberger et al., 1994). Gene transfer of CGRP can physiologically improve erectile function in either aged or diabetic rats (Bivalacqua et al., 2001; Xing et al., 2005). On the other hand, several studies have shown that patients with cirrhosis and those with acute liver disease or chronic illnesses with secondary hepatic involvement have a wide range of VIP levels with mean values significantly above that of normal individuals and patients with chronic illness and no liver involvement (Hunt et al., 1979; Henriksen et al., 1980, 1986; Li et al., 1990; Geraghty et al., 1994; Strauss et al., 2001). Moreover, Lee et al. (1996) reported elevated plasma levels of VIP in cirrhotic rats. Other studies have shown that circulating levels of CGRP as well as VIP are increased in the cirrhotic patients (Bendtsen et al., 1991; Gupta et al., 1992; Møller et al., 1996, 2001; Henriksen et al., 2001). Circulating CGRP relates to cardiac output, systemic vascular resistance, arterial compliance and liver dysfunction. In experimental studies, specific antagonists of CGRP partly reverse the vasodilatation and hyperdynamic circulation in cirrhosis (Henriksen et al., 2001; Møller et al., 2001). Taking our findings together with these reports, the enhanced neurogenic responses in cavernosal tissues from cirrhotic rats and their relative resistance to NOS inhibition might be, at least in part, due to the role of other neurotransmitters such as VIP and CGRP in this condition. In addition, the attenuation of relaxations in cirrhotic tissue by either the CB1 antagonist AM251 or the VR1 antagonist capsazepine might be via this non-nitrergic mechanism which could be possibly explained by the fact that vanilloid receptors on perivascular sensory nerves play a crucial role in mediating vascular responses to anandamide, as these receptors are activated by this endocannabinoid to cause a release of neuropeptides such as CGRP and vasorelaxation (Zygmunt et al., 1999; Ralevic et al., 2000; Mukhopadhyay et al., 2002). However, this general assumption has to await more detailed studies.
In summary, the present study demonstrated that NANC-mediated relaxation of corpus cavernosum was increased in biliary cirrhotic rats. L-NAME, in a concentration dependent manner, inhibited the neurogenic relaxation and also there was no difference in SNP-induced relaxation. Corporal nNOS protein level was increased in cirrhotic rats. These results suggested an increase in nitrergic neurotransmission in cirrhotic corpus cavernosum. We also found that either cannabinoid CB1 or vanilloid VR1 receptors may be involved in the increased NANC-mediated relaxation of corpus cavernosum in cirrhosis probably involving the NO pathway.
Abbreviations
- AN
anandamide
- BDL
bile duct ligated
- CB1
cannabinoid receptor subtype 1
- CB2
cannabinoid receptor subtype 2
- CGRP
calcitonin gene-related peptide
- CZ
capsazepine
- DMSO
dimethyl sulphoxide
- EFS
electrical field stimulation
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- L-NAME
Nω-nitro-L-arginine methyl ester
- NANC
non-adrenergic non-cholinergic
- nNOS
neuronal nitric oxide synthase
- L-NPA
Nω-propyl-L-Arginine
- Phe
phenylephrine
- PVDF
polyvinylidene fluoride
- ROD
relative optical densities
- SNP
sodium nitroprusside
- SO
sham-operated
- VIP
vasoactive intestinal polypeptide
- VR1
vanilloid receptor 1
Conflict of interest
The authors state no conflict of interest.
References
- Andersson PQ, Bloom SR, Mellander S. Haemodynamics of pelvic nerve induced penile erection in the dog: possible mediation by vasoactive intestinal polypeptide. J Physiol. 1984;350:209–224. doi: 10.1113/jphysiol.1984.sp015197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batkai S, Jarai Z, Wagner JA, Goparaju SK, Varga K, Liu J, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- Bendtsen F, Sckifter S, Henriksen JH. Increased circulating calcitonin gene-related peptide (CGRP) in cirrhosis. J Hepatol. 1991;12:118–123. doi: 10.1016/0168-8278(91)90920-7. [DOI] [PubMed] [Google Scholar]
- Bieker E, Neef M, Sägesser H, Shaw S, Koshy A, Reichen J. Nitric Oxide synthase 1 is partly compensating for nitric oxide synthase 3 deficiency in nitric oxide synthase 3 knock-out mice and is elevated in murine and human cirrhosis. Liver Int. 2004;24:345–353. doi: 10.1111/j.1478-3231.2004.0933.x. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Champion HC, Abdel-Mageed AB, Kadowitz PJ, Hellstrom WJ. Gene transfer of prepro-calcitonin gene-related peptide restores erectile function in the aged rat. Biol Reprod. 2001;65:1371–1377. doi: 10.1095/biolreprod65.5.1371. [DOI] [PubMed] [Google Scholar]
- Bomzon A, Gali D, Better OS, Blendis LM. Reversible suppression of the vascular contractile response in rats with obstructive jaundice. J Lab Clin Med. 1985;105:568–572. [PubMed] [Google Scholar]
- Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- Butterworth RF. Complications of cirrhosis III. Hepatic encephalopathy. J Hepatol. 2000;32 Suppl 1:171–180. doi: 10.1016/s0168-8278(00)80424-9. [DOI] [PubMed] [Google Scholar]
- Cahil PA, Redmond EM, Hodges R, Zhang S, Sitzmann JV. Increased endothelial nitric oxide synthase activity in the hyperemic vessels of portal hypertensive rats. J Hepatol. 1996;25:370–378. doi: 10.1016/s0168-8278(96)80124-3. [DOI] [PubMed] [Google Scholar]
- Cioffi WG, DeMeules JE, Kahng KU, Wait RB. Renal vascular reactivity in jaundice. Surgery. 1986;100:356–362. [PubMed] [Google Scholar]
- Dehpour AR, Seyyedi A, Rastegar H, Namiranian K, Moezi L, Sadeghipour H, et al. The nonadrenergic noncholinergic relaxation of anococcygeus muscle of bile duct-ligated rats. Eur J Pharmacol. 2003;445:31–36. doi: 10.1016/s0014-2999(02)01659-x. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HHO, Das SK, et al. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenicali M, Ros J, Fernández-Varo G, Cejudo-Martín P, Crespo M, Morales-Luis M, et al. Increased anandamide induced relaxation in mesenteric arteries of cirrhotic rats: role of cannabinoid and vanilloid receptors. Gut. 2005;54:522–527. doi: 10.1136/gut.2004.051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Rodriguez CM, Romero J, Petros TJ, Bradshaw H, Gasalla JM, Gutiérrez ML, et al. Circulating endogenous cannabinoid anandamide and portal, systemic and renal hemodynamics in cirrhosis. Liver Int. 2004;24:477–483. doi: 10.1111/j.1478-3231.2004.0945.x. [DOI] [PubMed] [Google Scholar]
- Gabbay E, Avraham Y, Ilan Y, Israeli E, Berry EM. Endocannabinoids and liver disease-review. Liver Int. 2005;25:921–926. doi: 10.1111/j.1478-3231.2005.01180.x. [DOI] [PubMed] [Google Scholar]
- García-Estañ J, Ortiz MC, Lee S. Nitric oxide and renal and cardiac dysfunction in cirrhosis. Clin Sci. 2002;102:213–222. [PubMed] [Google Scholar]
- Gaskari SA, Liu H, Moezi L, Li Y, Baik SK, Lee S. Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Br J Pharmacol. 2005;146:315–323. doi: 10.1038/sj.bjp.0706331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi M, Sadeghipour H, Mani AR, Tavakoli S, Hajrasouliha AR, Ebrahimi F, et al. Effect of anandamide on nonadrenergic noncholinergic-mediated relaxation of rat corpus cavernosum. Eur J Pharmacol. 2006;544:138–145. doi: 10.1016/j.ejphar.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Goh BJ, Tan BT, Hon WM, Lee KH, Khoo HE. Nitric oxide synthase and heme oxygenase expression in human liver cirrhosis. World J Gasteroenterol. 2006;12:588–594. doi: 10.3748/wjg.v12.i4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty JG, Angerson WJ, Carter DC. Splanchnic haemodynamics and vasoactive agents in experimental cirrhosis. HPB Surg. 1994;8:83–88. doi: 10.1155/1994/52975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner C, Soriano G, Tomas A, Bulbena O, Novella MT, Balanzo J, et al. Increased serum nitrite and nitrate levels in patients with cirrhosis: relationship to endotoxemia. Hepatology. 1993;18:1139–1143. [PubMed] [Google Scholar]
- Gupta S, Morgan TR, Gordan GS. Calcitonin gene-related peptide in hepatorenal syndrome. J Clin Gastroenterol. 1992;14:122–126. doi: 10.1097/00004836-199203000-00010. [DOI] [PubMed] [Google Scholar]
- Harris D, McCulloch AI, Kendall DA, Randall MD. Characterization of vasorelaxation responses to anandamide in the rat mesenteric arterial bed. J Physiol. 2002;539:893–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser-Kronberger C, Hacker GW, Graf AH, Mack D, Sundler F, Dietze O, et al. Neuropeptides in the human penis: an immunohistochemical study. J Androl. 1994;15:510–520. [PubMed] [Google Scholar]
- Henriksen JH, Møller S, Schifter S, Abrahamsen J, Becker U. High arterial compliance in cirrhosis is related to low adrenaline and elevated circulating calcitonin gene related peptide but not to activated vasoconstrictor systems. Gut. 2001;49:112–118. doi: 10.1136/gut.49.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen JH, Staun-Olsen P, Borg Mogensen N, Fahrenkrug J. Circulating endogenous vasoactive intestinal polypeptide (VIP) in patients with uraemia and liver cirrhosis. Eur J Clin Invest. 1986;16:211–216. doi: 10.1111/j.1365-2362.1986.tb01331.x. [DOI] [PubMed] [Google Scholar]
- Henriksen JH, Staun-Olsen P, Fahrenkrug J, Ring-Larsen H. Vasoactive intestinal polypeptide (VIP) in cirrhosis: arteriovenous extraction in different vascular beds. Scand J Gastroenterol. 1980;15:787–792. doi: 10.3109/00365528009181531. [DOI] [PubMed] [Google Scholar]
- Hernandez R, Martinez-Lara E, Del Moral ML, Blanco S, Canuelo A, Siles E, et al. Upregulation of endothelil nitric oxide synthase maintains nitric oxide production in the cerebellum of thioacetamide cirrhotic rats. Neuroscience. 2004;126:879–887. doi: 10.1016/j.neuroscience.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Hori N, Wiest R, Groszmann RJ. Enahnced release of nitric oxide in response to changes in flow and shear stress in the superior mesenteric arteries of portal hypertensive rats. Hepatology. 1998;28:1467–1473. doi: 10.1002/hep.510280604. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Mukhopadhyay S. Cellular signal transduction by anandamide and 2-arachidonoylglycerol. Chem Phys Lipids. 2000;108:53–70. doi: 10.1016/s0009-3084(00)00187-0. [DOI] [PubMed] [Google Scholar]
- Hunt S, Vaamonde CA, Rattassi T, Berian G, Said SI, Papper S. Circulating levels of vasoactive intestinal polypeptide in liver disease. Arch Intern Med. 1979;139:994–996. [PubMed] [Google Scholar]
- Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. PNAS. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Kim JH, Davies MG, Hagen PO, Carson CC., III Modulation of vasoactive intestinal polypeptide (VIP)-mediated relaxation by nitric oxide and prostanoids in the rabbit corpus cavernosum. J Urol. 1995;153:807–810. [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert J-F, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lee SS, Huang M, Ma Z, Rorstad O. Vasoactive intestinal peptide in cirrhotic rats: hemodynamic effects and mesenteric arterial receptor characteristics. Hepatology. 1996;23:1174–1180. doi: 10.1002/hep.510230536. [DOI] [PubMed] [Google Scholar]
- Li XN, Huang CT, Wang XH, Leng XS, Du RY, Chen YF, et al. Changes of blood humoral substances in experimental cirrhosis and their effects on portal hemodynamics. Chin Med J. 1990;103:970–977. [PubMed] [Google Scholar]
- Lue TF, Tanagho E. Physiology of erection and pharmacological management of impotence. J Urol. 1987;137:829–836. doi: 10.1016/s0022-5347(17)44267-4. [DOI] [PubMed] [Google Scholar]
- Ma Z, Zhang Y, Huet PM, Lee SS. Differential effects of jaundice and cirrhosis on beta-adrenoceptor signaling in three rat models of cirrhotic cardiomyopathy. J Hepatol. 1999;30:485–491. doi: 10.1016/s0168-8278(99)80109-3. [DOI] [PubMed] [Google Scholar]
- Martin PY, Ginès P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Eng J Med. 1998;339:533–541. doi: 10.1056/NEJM199808203390807. [DOI] [PubMed] [Google Scholar]
- Martin PY, Xu DL, Niederberger M, Weigert A, Tsai P, St John J, et al. Upregulation of endothelial constitutive NOS: a major role in the increased NO production in cirrhotic rats. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;270:F494–F499. doi: 10.1152/ajprenal.1996.270.3.F494. [DOI] [PubMed] [Google Scholar]
- McNeill DL, Papka RE, Harris CH. CGRP immunoreactivity and NADPH-diaphorase in afferent nerves of the rat penis. Peptides. 1992;13:1239–1246. doi: 10.1016/0196-9781(92)90035-2. [DOI] [PubMed] [Google Scholar]
- Mizusawa H, Hedlund P, Hakansson A, Alm P, Andersson KE. Morphological and functional in vitro and in vivo characterization of the mouse corpus cavernosum. Br J Pharmacol. 2001;132:1333–1341. doi: 10.1038/sj.bjp.0703938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moezi L, Rezayat M, Samini M, Shafaroodi H, Mehr SE, Ebrahimkhani MR, et al. Potentiation of anandamide effects in mesenteric beds isolated from bile duct-ligated rats: role of nitric oxide. Eur J Pharmacol. 2004;486:53–59. doi: 10.1016/j.ejphar.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Møller S, Bendtsen F, Henriksen JH. Splanchnic and systemic hemodynamic derangement in decompensated cirrhosis. Can J Gastroenterol. 2001;15:94–106. doi: 10.1155/2001/603012. [DOI] [PubMed] [Google Scholar]
- Møller S, Bendtsen F, Schifter S, Henriksen JH. Relation of calcitonin gene-related peptide to systemic vasodilatation and central hypovolaemia in cirrhosis. Scand J Gastroenterol. 1996;31:928–933. doi: 10.3109/00365529609052004. [DOI] [PubMed] [Google Scholar]
- Montes S, Pérez-Severiano F, Vergara P, Segovia J, Rios C, Muriel O. Nitric oxide production in striatum and pallidum of cirrhotic rats. Neurochemical Research. 2006;31:11–20. doi: 10.1007/s11064-005-9005-7. [DOI] [PubMed] [Google Scholar]
- Morales-Ruiz M, Jimenez W, Perez-Sala D, Ros J, Leivas A, Lamas S, et al. Increased nitric oxide synthase expression in arterial vessels of cirrhotic rats with ascites. Hepatology. 1996;24:1481–1486. doi: 10.1053/jhep.1996.v24.pm0008938184. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Chapnick BM, Howlett AC. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am J Physiol Heart Circ Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- Ottesen B, Wagner G, Virag R, Fahrenkrug J. Penile erection: possible role for vasoactive intestinal polypeptide as a neurotransmitter. Br Med J (Clin Res Ed) 1984;288:9–11. doi: 10.1136/bmj.288.6410.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poblete IM, Orliac ML, Briones R, Adler-Graschinsky E, Huidobro-Toro JP. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J Physiol. 2005;568:539–551. doi: 10.1113/jphysiol.2005.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Zygmunt PM, Movahed P, Högestä ED. Vanilloid receptors on capsaicin-sensitive sensory arterial bed. Br J Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros J, Jimenez W, Lamas S, Claria J, Arroyo V, Rivera F, et al. Nitric oxide production in arterial vessels of cirrhotic rats. Hepatology. 1995;21:554–560. [PubMed] [Google Scholar]
- Ros R, Claria J, To-Figueras J, Planaguma A, Cejudo-Martín P, Fernández-Varo G, et al. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122:85–93. doi: 10.1053/gast.2002.30305. [DOI] [PubMed] [Google Scholar]
- Sadeghipour H, Dehghani M, Dehpour AR. Role of opioid and nitric oxide systems in the nonadrenergic noncholinergic-mediated relaxation of corpus cavernosum in bile duct-ligated rats. Eur J Pharmacol. 2003;460:201–207. doi: 10.1016/s0014-2999(02)02946-1. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Salzet M, Magazine HI, Bilfinger TV. Antagonism of LPS and INF-(induction of iNOS in human saphenous vein endothelium by morphine and anandamide by NO inhibition of adenylate cyclase. J Cardio Pharmacol. 1998;31:813–820. doi: 10.1097/00005344-199806000-00003. [DOI] [PubMed] [Google Scholar]
- Stief CG, Benard F, Bosch RJ, Aboseif SR, Lue TF, Tanagho EA. A possible role for calcitonin gene-related peptide in the regulation of the smooth muscle tone of the bladder and penis. J Urol. 1990;143:392–397. doi: 10.1016/s0022-5347(17)39972-x. [DOI] [PubMed] [Google Scholar]
- Stief CG, Benard F, Bosch RJ, Aboseif SR, Wetterauer U, Lue TF, et al. Calcitonin gene-related peptide: possibly neurotransmitter contributes to penile erection in monkeys. Urology. 1993;41:397–401. doi: 10.1016/0090-4295(93)90608-d. [DOI] [PubMed] [Google Scholar]
- Stief CG, Wetterauer U, Schaebsdau FH, Jones U. Calcitonin gene-related peptide: a possible role in human penile erection and its therapeutic application in impotent patients. J Urol. 1991;146:1010–1014. doi: 10.1016/s0022-5347(17)37989-2. [DOI] [PubMed] [Google Scholar]
- Strauss GI, Edvinsson L, Larsen FS, Møller K, Knudsen GM. Circulating levels of neuropeptides (CGRP, VIP, NPY) in patients with fulminant hepatic failure. Neuropeptide. 2001;35:174–180. doi: 10.1054/npep.2001.0861. [DOI] [PubMed] [Google Scholar]
- Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide. Lancet. 1991;337:776–778. doi: 10.1016/0140-6736(91)91384-7. [DOI] [PubMed] [Google Scholar]
- Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet and macrophage derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- Wei CL, Khoo HE, Lee KH, Hon WM. Differential expression and localization of nitric oxide synthases in cirrhotic livers of bile duct-ligated rats. Nitric Oxide. 2002;7:91–102. doi: 10.1016/s1089-8603(02)00103-9. [DOI] [PubMed] [Google Scholar]
- White R, Ho WS, Bottrill FE, Ford WR, Hiley CR. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br J Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest R, Das S, Cadelina G, Garcia-Tsao G, Milstien S, Groszmann RJ. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest. 1999;104:1223–1233. doi: 10.1172/JCI7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest R, Groszmann RJ. Nitric oxide and portal hypertension: its role in the regulation of intrahepatic and splanchnic vascular resistance. Semin Liver Dis. 1999;19:411–426. doi: 10.1055/s-2007-1007129. [DOI] [PubMed] [Google Scholar]
- Xing JP, Cui XF, Sun JH, Qiu SD. Effect of secretory human calcitonin gene-related peptide recombinant AAV on possible erection in streptozocine-induced diabetic rat. Zhonghua Nan Ke Xue. 2005;11:775–779. [PubMed] [Google Scholar]
- Xu L, Carter EP, Ohara M, Martin PY, Rogachev B, Morris K, et al. Neuronal nitric oxide synthase and systemic vasodilation in rats with cirrhosis. Am J Physiol Renal Physiol. 2000;279:F1110–F1115. doi: 10.1152/ajprenal.2000.279.6.F1110. [DOI] [PubMed] [Google Scholar]
- Yeh KH, Aoki H, Matsuzaka J, Ra S, Sato F, Fujioka T, et al. Participation of vasoactive intestinal polypeptide (VIP) as a humoral mediator in the erectile response of canine corpus cavernosum penis. J Androl. 1994;15:187–193. [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgård M, Di Marzo V, et al. Vanilloid receptors on sensory nerve mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]