Abstract
Background and purpose:
Cryptotanshinone, the major tanshinone isolated from Salvia miltiorrhiza Bunge, exhibits anti-inflammatory activity. However, there is no report on the effect of cryptotanshinone on recruitment of leukocytes to inflammatory sites. We therefore assessed the effects of cryptotanshinone on macrophage chemotaxis.
Experimental approach:
Macrophage migration induced by complement 5a (C5a) or macrophage inflammatory protein-1α (MIP-1α) was measured in vitro. Intracellular kinase translocation and phosphorylation was assessed by Western blotting.
Key results:
RAW264.7 cell migration towards C5a (1μg ml−1) was significantly inhibited by cryptotanshinone (1, 3, 10 and 30 μM) in a concentration-dependent manner. Primary human macrophages stimulated by C5a were similarly inhibited. C5a-evoked migration in RAW264.7 cells was significantly suppressed by wortmannin (phosphatidylinositol 3-kinase (PI3K) inhibitor), PD98059 (MEK1/2 inhibitor) and SB203580 (p38 mitogen-activated protein kinase (MAPK) inhibitor), but not by SP600125 (c-Jun N-terminal kinase (JNK) inhibitor), suggesting that activation of PI3K, ERK1/2 and p38 MAPK signal pathways was involved in responses to C5a. Western blotting revealed that cryptotanshinone significantly inhibited PI3K-p110γ membrane translocation and phosphorylation of Akt (PI3K downstream effector protein) and ERK1/2 induced by C5a. However, neither p38 MAPK nor JNK phosphorylation was affected by cryptotanshinone. Wortmannin significantly attenuated C5a-induced PI3K-p110γ translocation, Akt and ERK1/2 phosphorylation. PD98059 suppressed ERK1/2 phosphorylation but failed to modify PI3K-p110γ translocation by C5a stimulation. Furthermore, MIP-1α-induced cell migration and PI3K-p110γ translocation were also inhibited by cryptotanshinone in a concentration-dependent manner.
Conclusions and implications:
Inhibition of macrophage migration by cryptotanshinone involved inhibition of PI3K activation with consequent reduction of phosphorylation of Akt and ERK1/2.
Keywords: cryptotanshinone, chemotactic migration, C5a, PI3K-110γ, Akt, ERK1/2, MIP-1α
Introduction
Mononuclear phagocytes (macrophages) are ubiquitous cells which reside in the majority of tissues and accumulate in areas of inflammation in response to the secretion of appropriate chemotactic signals (Baggiolini and Loetscher, 2002). A variety of products present at the site of inflammation can act as chemotactic agents, including formylmethionyl peptides, platelet activating factor, anaphylatoxin complement 5a (C5a), and chemotactic cytokines (Taub and Oppenheim, 1994). In response to stimuli, activated macrophages display cytoskeletal rearrangement and subsequent chemotaxis. It has been shown that chemotactic activation is mediated by a seven-transmembrane-spanning receptor coupled to heterotrimeric G protein, resulting in transduction of signals to the interior of cells and phosphorylation of multiple proteins (Jacobs et al., 1995; Haribabu et al., 1999). Phosphatidylinositol 3-kinase (PI3K) activity plays a central role in cell signaling. One important role for PI3K in innate immunity is to respond to chemoattractants (Fruman and Cantley, 2002; Stephens et al., 2002). The activation of mitogen-activated protein kinase (MAPK) seems to be another key component in signal transduction associated with cell migration (English et al., 1999). Three distinct mammalian MAPKs have been identified, including extracellular signal-regulated kinase (ERK1/2 or p42/44 MAPK), p38 MAPK and c-Jun N-terminal kinase (JNK). MAPKs are a family of serine/threonine kinases that are themselves activated by a cascade of protein kinase reactions (Widmann et al., 1999).
Dried roots of Salvia miltiorrhiza Bunge (Danshen) have been used in traditional Chinese medicine for the treatment of several pathologies including coronary heart disease, hepatitis and chronic renal failure (Lu and Foo, 2002). Cryptotanshinone (Figure 1) and tanshinone IIA are two major tanshinones in this plant. Tanshinone was reported to show a variety of biological activities including anti-inflammation (Kim et al., 2002) and cytotoxicity against human tumor cell lines (Ryu et al., 1997; Lin and Chang, 2000; Yuan et al., 2003). For example, tanshinone IIA exhibited an inhibitory effect on leukocyte chemotactic migration (Gao, 1985; Zhou et al., 1997). Cryptotanshinone was also observed to possess diverse biological activities, such as anti-inflammatory, anti-oxidative, anti-mutagenic, anti-platelet aggregation, anti-cyclooxygenase II activities and displayed the most powerful antibacterial activity among tanshinones (Wang et al., 1989; Cao et al., 1996; Ryu et al., 1997; Lee et al., 1999; Mosaddik, 2003; Jin et al., 2006). Furthermore, Suh et al. (2006) pointed out that cryptotanshinone had anti-atherosclerosis and anti-neointimal formation activity through inhibition of smooth muscle cell migration. However, there is no related report about the effect of cryptotanshinone on inflammatory cell infiltration.
Figure 1.
Chemical structure of cryptotanshinone.
The importance of C5a in several inflammatory diseases is demonstrated by the fact that agents that block the action of C5a also suppress inflammatory pathologies (such as multiple sclerosis and organ failure) in several animal models (Czermak et al., 1999; Heller et al., 1999). We hypothesized that cryptotanshinone may be one of the active components accounting for the anti-inflammatory activity of Salvia miltiorrhiza Bunge Danshen and suggested that a putative beneficial effect of this herb for the treatment of hepatitis and chronic renal failure might be mediated by interference with C5a-evoked, inappropriate recruitment of inflammatory cells. To evaluate this suggestion, C5a-induced chemotactic migration in RAW264.7 macrophages was used as an in vitro model to evaluate the anti-inflammatory property of cryptotanshinone. Furthermore, we also attempted to characterize whether interfering with protein kinase phosphorylation contributed to cryptotanshinone's effects on macrophage chemotaxis.
Methods
Cell culture conditions
RAW264.7 (American Type Culture Collection, TIB 71, Rockville, MD, USA) macrophage-like cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Biological Industries, Kibbutz Beit, Haemek, Israel), penicillin and streptomycin (Sigma Chemicals Co., St Louis, MO, USA) at 37°C in a humidified atmosphere in the presence of 5% CO2 (Chiou et al., 2003a, 2003b).
Primary human macrophages were prepared from healthy volunteers. In brief, peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by centrifugation over Ficoll–Hypaque gradients. PBMC at the interface were aspirated, diluted to 50 ml volume with phosphate-buffered saline (PBS), washed three times and centrifuged at 400 g for 10 min. After the final wash, PBMC were suspended in RPMI 1640 (Gibco BRL) containing 10% FCS, streptomycin and penicillin. The total number of viable PBMC in the suspension was determined by trypan blue dye exclusion. Then PBMC were plated onto 35-mm culture dishes and incubated overnight at 37°C, 5% CO2, in a humidified atmosphere to allow monocytes to adhere to the plate. Nonadherent cells were removed by gentle washing and the adherent monocytes were cultured in RPMI 1640 containing 10% FCS for 7 days before being used for migration experiments to allow differentiation to macrophages. The total number of macrophages was quantitated by detaching the macrophages by the addition of ice-cold 1 mM EDTA in PBS. Viable detached macrophages were counted by trypan blue dye exclusion.
Isolation and identification of cryptotanshinone
Cryptotanshinone was isolated by our laboratory (Sun et al., 2006). The dried roots of S. miltiorrhiza were purchased from a local herbal drug store in Taipei. The plant materials were identified by Mr Jun-Chih Ou, a former research fellow of National Research Institute of the Chinese Medicine (NRICM). A voucher specimen was deposited in the herbarium of NRICM. Briefly, slices of the dried roots of S. miltiorrhiza (5 kg) were extracted with ethanol (3 × 10 l) at room temperature. The combined ethanol extracts were concentrated in vacuo. The residue was then partitioned between ethyl acetate and H2O. The concentrated ethyl acetate extract was subjected to chromatography over silica gel and eluted with n-hexane/ethyl acetate (4:1), n-hexane/ethyl acetate (1:1) and ethyl acetate, successively. The first fraction was rechromatographed on silica gel using mixtures of n-hexane/ethyl acetate under gradient condition (10:1–2:1) to yield cryptotanshinone. The purity of cryptotanshinone and tanshinone IIA were >98% as judged by HPLC and 1H-NMR (Sun et al., 2006).
Chemotactic migration
Cell migration was assessed using a 24-well chemotaxis chamber with a membrane pore size of 5 μm (Transwell, Corning Costar, Lowell, MA, USA). Cell suspensions (90 μl; 2 × 107 cells ml−1) were added to each of the upper wells in the presence of 10 μl PBS or drugs (cryptotanshinone and protein kinase inhibitors) for 30 min, respectively. C5a (1 μg ml−1; Calbiochem, Darmstadt, Germany) or the chemokine, macrophage-inflammatory protein-1α (MIP-1α, (0.5 μg ml−1; Sigma) were added to the lower well of the chamber to assess chemoattractic activity. The entire chamber was then incubated at 37°C for 4 h to initiate migration. Nonmigrated cells were wiped off with a cotton swab and the filter was then fixed and stained with hematoxylin (Sigma) to define the cell nuclei. Chemotaxis was assessed by counting the number of migrated cells in five (at × 400 magnification) random microscopy fields per well (Chiou et al., 2003b). All experiments were performed in triplicate. Chemoattractant-induced cell migration was designated as 100% for each experiment.
Cell viability
Cell viability was monitored by Alamar Blue Assay. It is a nonisotopic colorimetric assay used to measure quantitatively the viable cells in culture. After incubation with or without cryptotanshinone or various protein kinase inhibitors for 24 h, Alamar Blue growth indicator dye (10% (v/v)) was added for another 4 h incubation at 37°C. The change in color was monitored with an ELISA reader at 620 nm. Cell viability correlates with optical density. Wells containing medium and Alamar Blue dye without cells were used as blanks. In each case, the experiments were performed in duplicate. All experiments were repeated at least twice with similar results. The mean absorbance for the duplicate cultures of each drug was calculated and the mean blank value was subtracted from these. Cell viability in control medium without any treatment was represented as 100%.
Preparation of membrane extracts for PI3K-p110γ translocation analysis
Cells were plated in T25 culture flasks and made quiescent at confluence by incubation in fresh DMEM for 24 h, which were then further stimulated with chemoattractants at 37°C for 10–15 min according to our previous findings (Tsai et al., 2004). When cryptotanshinone or inhibitors were used, they were applied 30 min before the addition of chemoattractants. After incubation, the cells were rapidly washed with ice-cold PBS, scraped and collected. Cell pellets were lysed with ice-cold solubilization buffer (0.5 ml) (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 0.1% aprotinin). The nuclear pellet was removed by centrifugation at 403 g for 5 min at 4°C. The postnuclear supernatant was centrifuged at 242 000 g for 30 min at 4°C to separate the cytosolic and membrane fraction. The membrane pellet was resuspended in radioimmunoprecipitation buffer (solubilization buffer with 1% NP-40) and lysed for 30 min at 4°C. The soluble proteins were separated by centrifugation at 10 000 g for 30 min and used as the membrane fraction. Protein (40 μg) was separated on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with antibody against p110γ (Santa Cruz Biotechnology, Delaware, CA, USA).
Preparation of cell extracts and Western blot analysis
After incubation, the cells were rapidly washed with ice-cold PBS, scraped and collected. Cell pellets were lysed with ice-cold lysis buffer containing 25 mM Tris-HCl at pH 7.4, 25 mM NaCl, 25 mM NaF, 25 mM sodium pyrophosphate, 1 mM sodium vanadate, 2.5 mM EDTA, 2.5 mM EGTA, 1 mM PMSF, 0.05% Triton X-100, 0.5% lauryl sulfate sodium salt (SDS), 0.5% deoxycholate, 0.5% nonylphenoxy polyethoxy ethanol (NP-40), 5 μg ml−1 leupeptin, and 5 μg ml−1 aprotinin (all from Sigma). The lysates were centrifuged at 45 000 g for 1 h at 4°C to yield the whole-cell extract in the supernatants. Protein concentration was determined using BCA reagents (Pierce; Rockford, IL, USA) according to the manufacturer's manual.
Protein (40 μg) was separated using 8% SDS-PAGE and transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked by incubating the membrane in TBS-T (20 mM Tris (pH 7.2); 150 mM NaCl; 0.1% Tween 20) with 5% bovine serum albumin for 1 h at room temperature. The membrane was incubated (overnight at 4°C) with rabbit polyclonal antibodies that specifically detect the total (that is, inactivated) and the phosphorylated (that is, activated) forms of p38 MAPK (1:1000), ERK1/2 (1:1000), JNK (1:1000) and Akt (1:10 000) (all purchased from Cell Signaling Technology, Beverly, MA, USA) at the indicated dilution, respectively. Then it was incubated with HRP anti-rabbit (Amersham, Buckinghamshire, UK) antibody and detected by ECL (Amersham). The results were evaluated by densitometry analysis.
Statistical analysis
All values in the text and figures represent mean±s.e.m. The data were analyzed by one-way analysis of variance (ANOVA) followed by post-hoc Dunnett's t-test for multiple comparisons. Values of P<0.05 were considered significant.
Results
Effect of cryptotanshinone on C5a-induced chemotactic migration
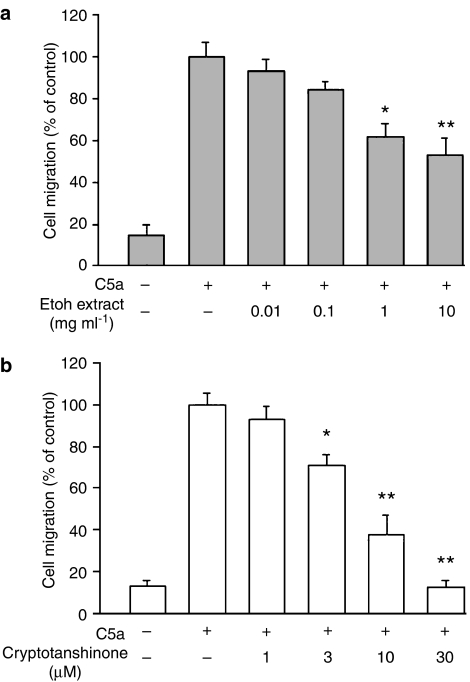
The standard chemotactic stimulus of C5a (1 μg ml−1) was chosen on the basis of our previous findings (Chiou et al., 2003a; Tsai et al., 2004). Nonstimulated control macrophages displayed a spontaneous migration with a total of 72±16 cells. The concentration gradient generated by 1 μg ml−1 of C5a induced an eightfold increase in cell migration, as compared with nonstimulated control and is represented as 100% in Figure 2. At noncytotoxic doses (0.01–10 mg ml−1), an ethanolic extract of Danshen exerted a consistent inhibitory effect on C5a-stimulated cell migration (Figure 2a). Cryptotanshinone (the major tanshinone isolated from Danshen) alone did not influence the spontaneous transmigration (data not shown), but significantly reduced the chemotactic migration in response to C5a in a concentration-dependent manner (IC50: 6.5±1.9 μM) (Figure 2b). We also compared the effect of cryptotanshinone on C5a-induced migration in human primary macrophages isolated from peripheral blood. Result showed that cryptotanshinone also has the ability to inhibit C5a-evoked chemotactic migration in primary macrophage cultures with an IC50 of 3.8±0.5 μM (four experiments performed in duplicate). It was important to establish whether exposure of cells to cryptotanshinone resulted in loss of viability. Both RAW264.7 cells and human primary macrophages were treated with cryptotanshinone for up to 24 h and the extent of cell death was monitored by Alamar Blue Assay. Results showed that none of the concentrations used for cryptotanshinone displayed significant cytotoxicity: cell viability in the presence of 30 μM cryptotanshinone in RAW264.7 cells and human primary macrophages were greater than 95% and 92%, respectively. As our results showed that the murine macrophage-like cell line and human primary macrophage cultures displayed the same sensitivity to cryptotanshinone, the RAW264.7 macrophages were used in all subsequent studies.
Figure 2.
Effects of ethanol extract (a) isolated from S. miltiorrhiza Bunge (Danshen) and cryptotanshinone (b) on complement 5a (C5a)-induced chemotactic migration in RAW264.7 macrophages. Cells pre-incubated with drugs for 30 min were plated onto the upper wells of the chamber. C5a (1 μg ml−1) was added to the lower wells for 4 h to induce cell migration. Migration was assessed by counting the number of migrated cells in five microscopic fields per well at × 400 magnification. C5a-induced cell migration was designated as 100%. Data reported are mean±s.e.m. of six independent experiments, each performed in triplicate. *P<0.05 and **P<0.01, indicate significance of difference as compared with samples receiving C5a alone.
Roles of PI3K and MAPKs in C5a-evoked chemotactic migration
We found that RAW264.7 macrophage migration to C5a was significantly inhibited from 100% to 81.1±11.2%, 42.3±9.5% and 23.6±10.1% by treatment with 0.01, 0.03 and 0.1 μM wortmannin, respectively. Furthermore, pre-incubation with a mouse embryonic kidney 1/2 (MEK1/2) inhibitor PD98059 (3 and 10 μM) or a p38 MAPK inhibitor SB203580 (3 and 10 μM) also caused a concentration-dependent inhibition of C5a-induced cell migration from 100% to 62.5±4.6% and 32.9±7.2%, and from 100% to 51.3±5.7% and 27.2±7.3%, respectively. In contrast, the JNK inhibitor SP600125 failed to decrease the response of C5a at the concentrations used (3, 10 and 20 μM). The concentrations used for all protein kinase inhibitors were non-cytotoxic to cells, cell viability after drug treatment were all greater than 95% as measured by Alamar Blue Assay. These results were consistent with our previous report (Tsai et al., 2004) and suggested that activation of PI3K, ERK1/2 and p38 MAPK signal pathways might be the main participants in the response to C5a.
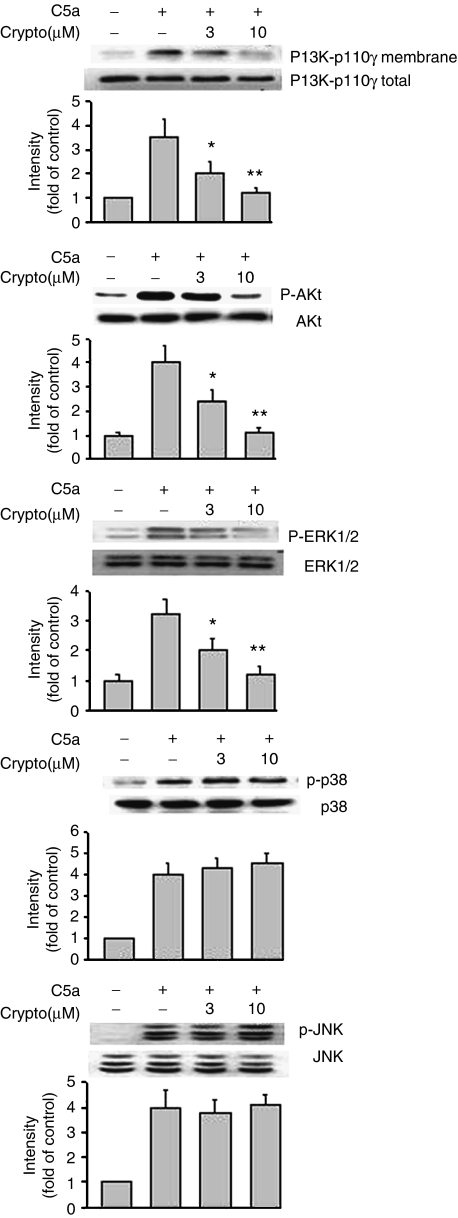
Effects of cryptotanshinone on C5a-induced PI3K-p110γ translocation and protein kinases phosphorylation
Figure 3 shows five representative immunoblot and pooled data from at least four independent experiments examining the membrane translocation of PI3K-p110γ and the phosphorylation of protein kinases by C5a stimulation, before and after cryptotanshinone treatment, respectively. First, we found the membrane distribution of PI3K-p110γ was markedly increased after stimulation of the cells with C5a for 15 min. Compared with unstimulated condition, C5a was able to induce significant phosphorylation of Akt, a downstream effector protein of PI3K. In the presence of cryptotanshinone (3 and 10 μM), both PI3K-p110γ membrane translocation and Akt phosphorylation were significantly attenuated. On the other hand, three MAPK phosphorylations were also significantly triggered by C5a stimulation. As shown in Figure 3 (middle trace), the ERK1/2 antibody recognized the two isoforms at 44 and 42 kDa and their phosphorylation were upregulated by C5a stimulation. Stimulation of RAW264.7 macrophages with C5a also activated p38 MAPK, as revealed by increased phosphorylation. Immunoblots analyzed for JNK in cells treated with C5a for 15 min showed expression of 45-kDa JNK2 and 54-kDa JNK1 isoforms and a cleavage product (immediately below JNK1). However, treating the cells with cryptotanshinone selectively interfered with phosphorylation of ERK1/2, but not that of p38 MAPK or JNK.
Figure 3.
Effects of cryptotanshinone on C5a-stimulated membrane translocation of PI3K-p110γ and protein phosphorylation of Akt, ERK1/2, p38 MAPK and JNK, respectively. Western blot analysis was performed as described in Methods. Similar results were obtained in four independent experiments. Bands were visualized by an ECL method and quantified with a densitometer. *P<0.05 and **P<0.01, indicate significance of difference as compared with samples receiving C5a alone. C5a, complement 5a; ERK1/2, extracellular signal-regulated kinase1/2; JNK, c-Jun N-terminal kinase; p38 MAPK, p38 mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase.
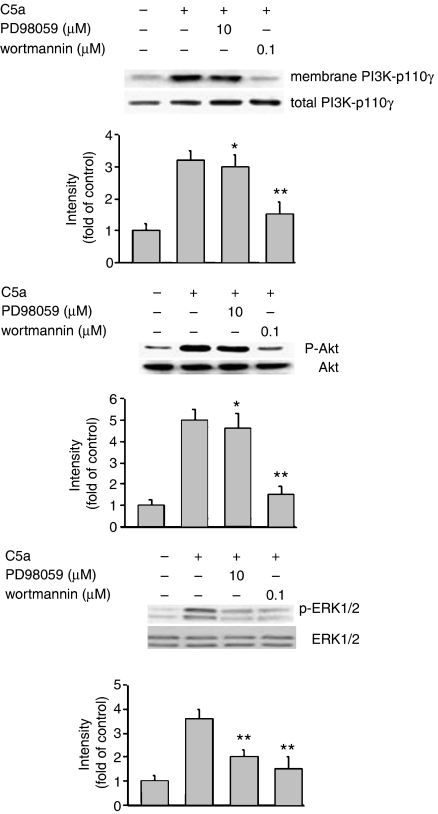
To elucidate the mechanism(s) of action of cryptotanshinone, we further investigated the signaling links between phosphorylation of protein kinases and cell migration, both mediated by C5a. Western blot analysis revealed that wortmannin significantly attenuated C5a-induced PI3K-p110γ translocation as well as Akt and ERK1/2 phosphorylation (Figure 4), whereas PD98059 only suppressed C5a-induced ERK1/2 phosphorylation. These findings demonstrated that C5a-stimulated phosphorylation of Akt and ERK1/2 might be mediated through upstream activation of PI3K-p110γ, suggesting that C5a may transduce the signal to PI3K through an undefined mechanism and subsequently phosphorylation of Akt and ERK1/2 for chemotaxis.
Figure 4.
Effects of PD98059 and wortmannin on PI3K-p110γ translocation and protein phosphorylation of Akt and p38 MAPK in response to C5a, respectively. Western blot analysis was performed as described in Methods. Similar results were obtained in four independent experiments. Bands were visualized by an ECL method and quantified with a densitometer. *P<0.05 and **P<0.01, indicate significance of difference as compared with samples receiving C5a alone. C5a, complement 5a; p38 MAPK, p38 mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase.
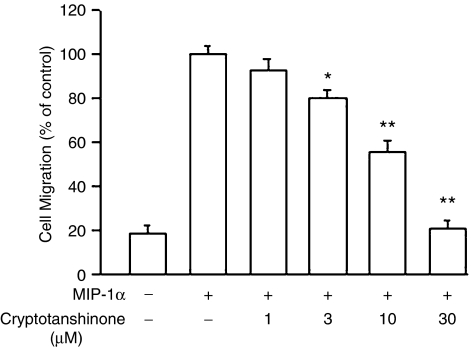
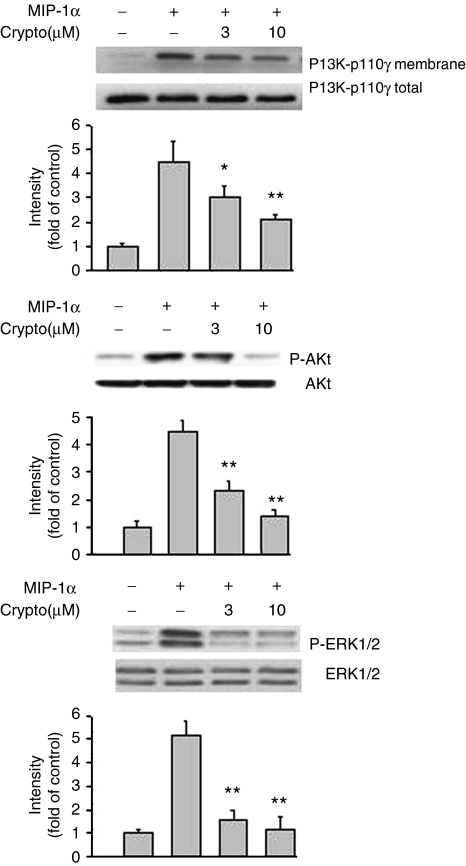
Effect of cryptotanshinone on MIP-1α-induced chemotactic migration, PI3K activation and MAPK phosphorylation
We also examined whether cryptotanshinone could affect the response of macrophages to agonists from different classes of chemotactic agents. Results shown in Figure 5 demonstrated that the chemokine, MIP-1α, at a concentration of 0.5 μg ml−1, could induce significant migration of RAW264.7 cells, to a total of 374±21 migrated cells (represented as 100%) during the 4-h migration period. In the presence of cryptotanshinone (1–30 μM), cell migration toward MIP-1α was concentration dependently inhibited from 100% to 92.4±5.6%, 80.3±3.5%, 55.4±6.7% and 21.2±3.3%, respectively (IC50: 11.3±2.4 μM). We also evaluated if cryptotanshinone could interfere with MIP-1α-induced PI3K translocation as well as Akt and ERK1/2 phosphorylation. Figure 6 showed that no significant band was seen in unstimulated cells, but stimulating the cells with MIP-1α for 15 min resulted in an increase in the membrane distribution of PI3K-p110γ (upper trace) and also upregulation of Akt and ERK1/2 phosphorylation. Both PI3K-p110γ translocation and protein kinase phosphorylation were clearly attenuated by cryptotanshinone.
Figure 5.
Effects of cryptotanshinone on MIP-1α-induced chemotactic migration in RAW264.7 macrophages. Cells pre-incubated with cryptotanshinone for 30 min were plated onto the upper wells of the chamber. MIP-1α (0.5 μg ml−1) was added to the lower wells for 4 h to induce cell migration. Migration was assessed by counting the number of migrated cells in five microscopic fields per well at × 400 magnification. C5a-induced cell migration was designated as 100%. Data reported are mean±s.e.m. of six independent experiments, each performed in triplicate. *P<0.05 and **P<0.01, indicate significance of difference as compared with samples receiving MIP-1α alone. C5a, complement 5a; MIP-1α, macrophage-inflammatory protein-1α.
Figure 6.
Effects of cryptotanshinone on MIP-1α-induced PI3K-p110γ translocation and protein phosphorylation of Akt and ERK1/2 in RAW264.7 macrophages, respectively. Western blot analysis was performed as described in Methods. Similar results were obtained in four independent experiments. Bands were visualized by an ECL method and quantified with a densitometer. *P<0.05 and **P<0.01, indicate significance of difference as compared with samples receiving MIP-1α alone. ERK1/2, extracellular signal-regulated kinase1/2; MIP-1α, macrophage-inflammatory protein-1α.
Discussion
Cryptotanshinone was previously observed to possess potent antibacterial activity and had been used against inflammation (Lee et al., 1999). We report here that cryptotanshinone could inhibit chemotactic migration of macrophage, a crucial indicator of leukocyte trafficking in inflammation. Indeed, our results indicated that cryptotanshinone not only inhibited C5a-induced migration, but also inhibited cell migration in response to MIP-1α. These results suggested that cryptotanshinone may be one of the active components from S. miltiorrhiza and acts as an inhibitor to block a variety of inflammatory stimulation. Lee et al. (1999) had evaluated the antibacterial activity of cryptotanshinone and dihydrotanshinone I. They found that cryptotanshinone and dihydrotanshinone I generated superoxide radicals in Bacillus subtilis lysate and suggested that superoxide radical are important in the antibacterial actions of the agents. Nevertheless, Sato et al. (2001) had evaluated the direct effect of superoxide on fibronectin-induced fibroblast migration and found that superoxide generation did not significantly affect fibronectin-induced fibroblast migration. Based on these reports, we suggest that the anti-chemotactic effects of cryptotanshinone may be independent of its ability to generate superoxide radicals.
PI3K has been implicated as a signaling enzyme activated by chemoattractant receptors (Siddiqui and English, 2000). This pathway leads to activation of Akt (also known as PKB), a cytosolic serine/threonine kinase that acts downstream of PI3K (Matsui et al., 2003). Previous reports revealed that agonist binding to the C5a receptor can activate multiple signaling proteins including PI3K (Monsinjon et al., 2003). Some of the earliest studies of wortmannin and LY294002 (PI3K inhibitors) described inhibition of chemotaxis in macrophages treated with chemoattractants (Okada et al., 1994; Vlahos et al., 1995). There are two types of class I PI3Ks, both of which are heterodimeric molecules composed of a p110 catalytic subunit and a regulatory subunit (Hawkins et al., 2006). Class IA enzymes contain a p110α, β or δ catalytic subunit and an SH2 domain-containing adaptor subunit, p85α, p85β or p55γ. Class IB enzymes contain only one member PI3Kγ, which is composed of a p101 regulatory subunit and a p110γ catalytic subunit (Fruman and Cantley, 2002). PI3Kγ is a key player in the regulation of leukocyte functions such as chemotaxis and superoxide production (Katso et al., 2001). This enzyme is regulated by Gβγ subunits liberated upon activation of heterotrimeric G proteins. A great variety of stimuli activate PI3K, leading to the recruitment of p110γ to the cell membrane (Brock et al., 2003). In vivo migration of inflammatory cells was also impaired in the absence of p110γ. Studies of mice lacking PI3K-p110γ have shown that this isoform is essential for phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3) production and downstream Akt/PKB activation in macrophages exposed to C5a or IL-8 (Hirsch et al., 2000; Sasaki et al., 2000). Naccache et al. (2000) further observed that in resting cells, PI3Kγ is predominantly localized in the cytosol, whereas activation of G-protein-coupled receptors (GPCRs) induced an increase of PI3Kγ in the membrane fraction. This work has established p110γ as a critical PI3 K isoform linking ligands for GPCRs to chemotaxis.
In this experiment, the possible involvement of PI3K in C5a-induced chemotactic migration in RAW264.7 macrophage was first established. We identified that C5a can activate PI3K-110γ membrane translocation and Akt phosphorylation in RAW264.7 cells. We demonstrated that wortmannin, a specific PI3K inhibitor, significantly suppressed cell migration in response to C5a, emphasizing the importance of this enzyme as part of the C5a receptor-activated signal cascade leading to chemotactic migration of macrophages. Our results showed that cryptotanshinone significantly attenuated not only C5a-induced migration, but also C5a-stimulated PI3K-p110γ translocation and Akt phosphorylation. This finding suggested that interfering with PI3K pathway may contribute to cryptotanshinone's antagonism of the chemotactic response induced by C5a.
The chemotactic process appears to be also highly regulated by MAPKs (ERK1/2, JNK and p38 MAPK) and each with a unique signaling pathway. Previous studies also showed that MAPK inhibitors decrease cell migration in response to chemoattractants (Boehme et al., 1999; Ayala et al., 2000). Although the chemotaxis process is the result of multiple signaling pathways (Wenzel-Seifert and Seifert, 2001), it is likely that activation of ERK1/2 and p38 MAPK pathways, but not JNK, contributes mainly to the chemotactic migration evoked by C5a in RAW264.7 macrophages, as the MEK1/2 inhibitor (PD98059) and a p38 MAPK inhibitor (SB203580), but not the JNK inhibitor (SP600125), clearly suppressed the chemotactic response. MAPKs were among the first kinases to be implicated in the synthesis of pro-inflammatory cytokines and several inhibitors of cytokine production exert their activity by blocking MAPKs activation (Mantovani et al., 2000). Thus, MAPK inhibitors have been shown to be of significant therapeutic benefit in a number of models of inflammation, including endotoxin shock, arthritis and pulmonary inflammation (Badger et al., 1996; Nick et al., 2000). Results obtained from this study demonstrated that cryptotanshinone selectively abolished C5a-stimulated ERK1/2 phosphorylation, suggesting that cryptotanshinone acts by blocking this pathway to suppress cell recruitment. Suh et al. (2006) reported that cryptotanshinone significantly attenuated TNF-α-induced migration of human aortic smooth muscle cells by inhibiting ERK1/2, p38 and JNK MAPK phosphorylation. We suggest that there is no real discrepancy between these and our results for at least two reasons. First, two very different cell types were used. Second, Suh et al. (2006) used a higher concentration (10 μg ml−1) of cryptotanshinone, equal to about 33 μM (MW of cryptotanshinone is 296.4). At such a higher concentration, a nonselective effect of cryptotanshinone on phosphorylation of MAPKs may be more likely.
Whether the phosphorylation of ERK1/2 by C5a is linked to PI3K activation was not clear. We further characterized the interaction between these two signaling molecules. Western blot analysis showed that wortmannin pre-treatment clearly blocked not only C5a-induced PI3K-110γ translocation, but also ERK1/2 phosphorylation. In contrast, PD98059 (an MEK1/2 inhibitor) affected only ERK phosphorylation. It was postulated that C5a-mediated activation of PI3K is necessary for ERK1/2 activation and that C5a promoted the phosphorylation of ERK downstream of PI3K pathway. Nevertheless, our results did not show if there is crosstalk between ERK1/2 and Akt signaling. According to the above observation, we speculated that cryptotanshinone might inhibit C5a-induced cell migration by interfering with P13K activation and subsequently ERK1/2 phosphorylation.
Chemoattractants (such as C5a) and chemokines (such as MIP-1α), although act through different receptors (such as C5a receptor and C-C chemokine receptors), can activate intracellular protein kinase cascades to mediate cell migration (Mukherjee and Pasinetti, 2001; Lentzsch et al., 2003). Our results confirmed that exposure of macrophages to MIP-1α increased the translocation levels of PI3K-110γ. Migration assays with the selective PI3K inhibitor wortmannin further revealed that PI3K also plays a pivotal, but possibly not an essential, role in mediating MIP-1α-induced migration. Hence, it is not surprising that cryptotanshinone simultaneously exerts its inhibitory activity against the cell response to C5a and MIP-1α. In summary, it is concluded that interfering with PI3K activation and thus reducing the phosphorylation of Akt and ERK1/2 may account for the antagonism of cell migration shown by cryptotanshinone, suggesting that cryptotanshinone may be used as an effective antimigratory drug against inflammatory disorders by limiting the early phases of macrophage infiltration.
Acknowledgments
This work was supported by National Research Institute of Chinese Medicine (NRICM95-DBCMR-01), Taipei, Taiwan, ROC.
Abbreviations
- C5a
complement 5a
- ERK1/2
extracellular signal-regulated kinase1/2
- JNK
c-Jun N-terminal kinase
- MIP-1α
macrophage inflammatory protein-1α
- p38 MAPK
p38 mitogen-activated protein kinase
- PI3K
phosphatidylinositol 3-kinase
Conflict of interest
The authors state no conflict of interest.
References
- Ayala JM, Goyal S, Liverton NJ, Claremon DA, O'Keefe SJ, Hanlon WA. Serum-induced monocyte differentiation and monocyte chemotaxis are regulated by the p38 MAP kinase signal transduction pathway. J Leukoc Biol. 2000;67:869–875. [PubMed] [Google Scholar]
- Badger AM, Bradbeer JN, Votta B, Lee JC, Adams JL, Griswold DE. Pharmacological profile of SB203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J Pharmacol Exp Ther. 1996;279:1453–1461. [PubMed] [Google Scholar]
- Baggiolini M, Loetscher P. Chemokines in inflammation and immunity. Immunol Today. 2002;21:418–420. doi: 10.1016/s0167-5699(00)01672-8. [DOI] [PubMed] [Google Scholar]
- Boehme SA, Sullivan SK, Crowe PD, Santos M, Conlon PJ, Sriramarao P, et al. Activation of mitogen-activated protein kinase regulates otaxin-induced eosinophils migration. J Immunol. 1999;163:1611–1681. [PubMed] [Google Scholar]
- Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, et al. Roles of Gβγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinaseγ. J Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao EH, Liu XQ, Wang JJ, Xu NF. Effect of natural antioxidant tanshinone II-A on DNA damage by lipid peroxidation in liver cells. Free Radic Biol Med. 1996;20:801–806. doi: 10.1016/0891-5849(95)02211-2. [DOI] [PubMed] [Google Scholar]
- Chiou WF, Peng CH, Chou CJ, Chen CF. Antiinflammatory properties of piperlactam S: modulates complement 5a induced chemotaxis and inflammatory cytokines production in macrophages. Planta Med. 2003a;69:9–14. doi: 10.1055/s-2003-37041. [DOI] [PubMed] [Google Scholar]
- Chiou WF, Shum AYC, Peng CH, Chou CJ, Chen CF. Piperlactam S suppresses macrophage migration by impediment of F-actin polymerization and filopodia extension. Eur J Pharmacol. 2003b;458:217–225. doi: 10.1016/s0014-2999(02)02733-4. [DOI] [PubMed] [Google Scholar]
- Czermak BJ, Sarma V, Pierson CL, Warner RL, Huber-Lang M, Bless NM, et al. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5:788–792. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, et al. New insights into the control of MAP kinase pathways. Exp Cell Res. 1999;233:255–270. doi: 10.1006/excr.1999.4687. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Cantley LC. Phosphoinositide 3-kinase in immunological systems. Immunology. 2002;14:7–18. doi: 10.1006/smim.2001.0337. [DOI] [PubMed] [Google Scholar]
- Gao JY.Inhibitory actions of tanshinone leukocyte chemotaxis Zhong Xi Yi Jie He Za Zhi 19855684–695.(Chinese) [PubMed] [Google Scholar]
- Haribabu B, Zhelev DV, Pridgen BC, Richardson RM, Ali H, Snyderman R. Chemoattractant receptors activate distinct pathways for chemotaxis and secretion. Role of G-protein usage. J Biol Chem. 1999;274:37087–37092. doi: 10.1074/jbc.274.52.37087. [DOI] [PubMed] [Google Scholar]
- Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans. 2006;34:647–662. doi: 10.1042/BST0340647. [DOI] [PubMed] [Google Scholar]
- Heller T, Hennecker M, Baumann U, Gessner JE, Zu Vilsendorf AS, Baensch M, et al. Selection of a C5a receptor antagonist from phage libraries attenuating the inflammatory response in immune complex disease and ischemia/reperfusion injury. J Immunol. 1999;163:985–994. [PubMed] [Google Scholar]
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- Jacobs AA, Huber JL, Ward RA, Klein JB, Mcleish KR. Chemoattractant receptor-specific differences in G protein activation rates regulate effector enzyme and functional responses. J Leuko Biol. 1995;57:679–686. doi: 10.1002/jlb.57.4.679. [DOI] [PubMed] [Google Scholar]
- Jin DZ, Yin LL, Ji XQ, Zhu XZ. Cryptotanshinone inhibits cyclooxygenase-2 enzyme activity but not its expression. Eur J Pharmacol. 2006;549:166–172. doi: 10.1016/j.ejphar.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- Kim SY, Moon TC, Chang HW, Son KH, Kang SS, Kim HP. Effects of tanshinone I isolated from Salvia miltiorrhiza Bunge on arachidonic acid metabolism and in vivo inflammatory responses. Phytother Res. 2002;16:616–620. doi: 10.1002/ptr.941. [DOI] [PubMed] [Google Scholar]
- Lee DS, Lee SH, Noh JG, Hong SD. Antibacterial activities of cryptotanshinone and dihydrotanshinone I from a medical herb, Salvia miltiorrhiza Bunge. Biosci Biotech Biochem. 1999;63:2236–2239. doi: 10.1271/bbb.63.2236. [DOI] [PubMed] [Google Scholar]
- Lentzsch S, Gries M, Janz M, Bargou R, Dorken B, Mapara MY. Macrophage inflammatory protein 1-alpha (MIP-1 alpha) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood. 2003;101:3568–3573. doi: 10.1182/blood-2002-08-2383. [DOI] [PubMed] [Google Scholar]
- Lin HC, Chang WL. Diterpenoids from Salvia miltiorrhiza. Phytochemistry. 2000;53:951–953. doi: 10.1016/s0031-9422(99)00433-1. [DOI] [PubMed] [Google Scholar]
- Lu Y, Foo LY. Polyphenolics of Salvia – a review. Phytochemistry. 2002;59:117–140. doi: 10.1016/s0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Dinarello CA, Ghezzi P. Pharmacology of Cytokines. Oxford University Press: Oxford; 2000. pp. 1–8. [Google Scholar]
- Matsui T, Nagoshi T, Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle. 2003;2:220–223. [PubMed] [Google Scholar]
- Monsinjon T, Gasque P, Chan P, Ischenko A, Brady JJ, Fontaine MC. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003;17:1003–1014. doi: 10.1096/fj.02-0737com. [DOI] [PubMed] [Google Scholar]
- Mosaddik MA. In vitro cytotoxicity of tanshinones isolated from Salvia miltiorrhiza Bunge against P388 lymphocytic leukemia cells. Phytomed. 2003;10:682–685. doi: 10.1078/0944-7113-00321. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Pasinetti GM. Complement anaphylatoxin C5a neuroprotects through mitogen-activated ptotein kinase-dependent inhibition of caspase 3. J Neurochem. 2001;77:43–49. doi: 10.1046/j.1471-4159.2001.00167.x. [DOI] [PubMed] [Google Scholar]
- Naccache PH, Levasseur S, Lachance G, Chakravarti S, Bourgoin SG, McColl SR. Stimulation of human neutrophils by chemotactic factors is associated with the activation of phosphatidylinositol 3-kinase γ. J Biol Chem. 2000;275:23636–23641. doi: 10.1074/jbc.M001780200. [DOI] [PubMed] [Google Scholar]
- Nick JA, Young SK, Brown KK, Avdi NJ, Arndt PG, Suratt BT, et al. Role of p38 mitogen-activated protein kinase in a murine model of pulmonary inflammation. J Immunol. 2000;164:2151–2159. doi: 10.4049/jimmunol.164.4.2151. [DOI] [PubMed] [Google Scholar]
- Okada T, Sakuma L, Fukui Y, Hazeki O, Ui M. Blockage of chemotactic peptide-induced stimulation of neutrophils by wortmannin as a result of selective inhibition of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:3563–3567. [PubMed] [Google Scholar]
- Ryu SY, Lee CO, Choi SU. In vitro cytotoxicity of tanshinones from Salvia miltiorrhiza. Planta Med. 1997;63:339–342. doi: 10.1055/s-2006-957696. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- Sato E, Koyama S, Camhi SL, Nelson DK, Robbins RA. Ractive oxygen and nitrogen metabolites modulate fibronectin-induced fibroblast migration in vitro. Free Radic Biol Med. 2001;30:22–29. doi: 10.1016/s0891-5849(00)00436-6. [DOI] [PubMed] [Google Scholar]
- Siddiqui RA, English D. Phosphatidylinositol 3′-kinase-mediated calcium mobilization regulates chemotaxis in phosphatidic acid-stimulated human neutrophils. Biochim Biophy Acta. 2000;1483:161–173. doi: 10.1016/s1388-1981(99)00172-9. [DOI] [PubMed] [Google Scholar]
- Stephens L, Ellson C, Hawkins P. Roles of PI3K in leukocyte chemotaxis and phagocytosis. Curr Opin Cell Biol. 2002;14:203–213. doi: 10.1016/s0955-0674(02)00311-3. [DOI] [PubMed] [Google Scholar]
- Suh SJ, Jin UH, Choi HJ, Chang HW, Son JK, Lee SH, et al. Cryptotanshinone from Salvia miltiorrhiza BUNGE has an inhibitory effect on TNF-α-induced matrix metalloproteinase-9 production and HASMC migration via down-regulated NF-κB and AP-1. Biochem Pharmacol. 2006;72:1680–1689. doi: 10.1016/j.bcp.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Sun CM, Chin TM, Lin YL, Chen CJ, Chen WC, Wu TS, et al. Isolation, structure elucidation, and syntheses of isoneocryptotanshinone II and tanshinlactone A from Salvia miltiorrhiza. Heterocycles. 2006;68:247–255. [Google Scholar]
- Taub DD, Oppenheim JJ. Chemokines, inflammation and the immune system. Ther Immunol. 1994;1:229–246. [PubMed] [Google Scholar]
- Tsai HR, Yang LM, Chen CF, Chiou WF. Andrographolide acts through inhibition of ERK1/2 and Akt phosphorylation to suppress chemotactic migration. Eur J Pharmacol. 2004;498:45–52. doi: 10.1016/j.ejphar.2004.07.077. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Brown RF, Traynor-Kaplan AE, Heyworth PG, Prossnitz ER, et al. Investigation of neutrophil signal transduction using a specific inhibitor of phosphatidylinositol 3-kinase. J Immunol. 1995;154:2413–2422. [PubMed] [Google Scholar]
- Wang N, Luo HW, Niwa M, Ji J. A new platelet aggregation inhibitor from Salvia miltiorrhiza. Plant Med. 1989;55:390–391. doi: 10.1055/s-2006-962037. [DOI] [PubMed] [Google Scholar]
- Wenzel-Seifert K, Seifert R.Chemoattractant receptor-G-protein coupling Physiology of Inflammation 2001Oxford University Press: Oxford; 146–188.In: Ley K (eds). [Google Scholar]
- Widmann C, Gibson S, Japre MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Yuan SL, Wang XJ, Wei YQ.Anticancer effect of tanshinone and its mechanisms Ai Zheng 2003221363–1366.(Chinese) [PubMed] [Google Scholar]
- Zhou Z, Zheng J, Xu W.Study on the effect of ofloxacin and tanshinone II A on human leukocyte chemotactic migration in vitro Zhongguo Yi Xue Ke Xue Yuan Xue Bao 199719232–235.(Chinese) [PubMed] [Google Scholar]