Abstract
ATP-sensitive P2X7 receptors are localized on cells of immunological origin including peripheral macrophages and glial cells in the CNS. Activation of P2X7 receptors leads to rapid changes in intracellular calcium concentrations, release of the proinflammatory cytokine interleukin-1β and following prolonged agonist exposure, the formation of cytolytic pores in plasma membranes. Both the localization and functional consequences of P2X7 receptor activation indicate a role in inflammatory processes. The phenotype of P2X7 receptor gene-disrupted mice also indicates that P2X7 receptor activation contributes to ongoing inflammation. More recently, P2X7 receptor knockout data has also suggested a specific role in inflammatory and neuropathic pain states. The recent discovery of potent and highly selective antagonists for P2X7 receptors has helped to further clarify P2X receptor pharmacology, expanded understanding of P2X7 receptor signaling, and offers new evidence that P2X7 receptors play a specific role in nociceptive signaling in chronic pain states. In this review, we incorporate the recent discoveries of novel P2X7 receptor-selective antagonists with a brief update on P2X7 receptor pharmacology and its therapeutic potential.
Keywords: purines, P2X7 receptor, calcium influx, Yo-Pro uptake, IL-1β, AZ11645373, A-438079, A-740003, allodynia, neuropathic pain, inflammatory pain
Introduction
P2X7 receptors belong to the family of ATP-sensitive ionotropic P2X receptors, which include seven homomeric receptor subtypes (P2X1–P2X7; North, 2002). Some of these receptor subunits also natively exist as functional heteromeric receptor combinations, such as P2X2/3 (North, 2002). However, P2X7 receptors are unique among the P2X receptor family as they are functional in only homomeric forms and are activated by high concentrations of ATP (>100 μM) (Jacobson et al., 2002). In addition, prolonged agonist exposure leads to formation of large cytolytic pores in the cell membrane (Surprenant et al., 1996). The P2X7 subunit, previously termed as the ‘P2Z receptor' (Di Virgilio et al., 2001), was initially cloned from rat (Surprenant et al., 1996), and then from human brain (Collo et al., 1997) and human macrophages (Rassendren et al., 1997a). P2X7 receptors are selectively expressed on cells of hematopoietic lineage including mast cells, lymphocytes, erythrocytes, fibroblasts, peripheral macrophages and epidermal Langerhans cells (Surprenant et al., 1996). Within the central nervous system, functional P2X7 receptors are localized on microglia and Schwann cells as well as on astrocytes (Ferrari et al., 1996; Collo et al., 1997; Sim et al., 2004). The existence of functional P2X7 receptors on peripheral or central neurons remains controversial owing to the poor specificity of both antibodies and ligands targeting the rat P2X7 receptor (Anderson and Nedergaard, 2006). In rat peripheral sensory ganglia (dorsal root), P2X7 receptors appear to be selectively localized on glia cells, but not neurons (Zhang et al., 2005).
Both the distribution of P2X7 receptors and the fact that high concentrations of ATP are required to activate the receptor have led investigators to consider the possibility that this P2X receptor functions as a ‘danger' sensor (Ferrari et al., 2006). However, efforts to establish a generalized function for P2X7 receptors are complicated by another distinguishing feature of this receptor subtype; at least 250 polymorphic forms of the P2X7 receptor have been identified (Ferrari et al., 2006). Among these variations, both gain-of-function (Cabrini et al., 2005) and loss-of-function (Wiley et al., 2003) single nucleotide polymorphisms (SNPs) have been reported. Although some of these SNPs may have a predictive utility as biomarkers for some forms of leukemia (Wiley et al., 2002) or extrapulmonary tuberculosis (Fernando et al., 2007), to date, no clear disease association has been linked to a specific P2X7 receptor polymorphism.
The multiplicity of polymorphic forms of the P2X7 receptor is associated with the extended length of the C-terminal region of the receptor relative to other P2X receptors (Surprenant et al., 1996). This region is essential for receptor-mediated cytolytic pore-forming activity (Surprenant et al., 1996) and also contains putative interaction sites for lipopolysaccharide (Denlinger et al., 2001), SH2 domains (Kim et al., 2001) and α-actin (Kim et al., 2001). The mechanism(s) by which P2X7 receptor activation leads to the formation of large cytolytic pores in cell membranes has not been definitively established. The available evidence indicates that the minimum stoichiometric conformation of P2X receptors is a trimer (Egan et al., 2006). However, this work has been based on an analysis of channel conductance properties of P2X receptors rather than on potential downstream signaling mechanisms. It should also be noted that investigations of P2X receptor stoichiometry have primarily studied P2X receptor subtypes other than P2X7 (Egan et al., 2006). While originally it was hypothesized that pore formation resulted from intrinsic dilation of the channel (North, 2002), the inability of some cell types that express the cation permeable receptor to show agonist-evoked pore formation in vitro has led to the generation of data showing that pore formation results from P2X7 receptor-mediated downstream signaling (Donnelly-Roberts et al., 2004; Faria et al., 2005) that ultimately may depend on the opening of nonselective hemichannels at the cell surface (Pelegrin and Surprenant, 2006).
Taken together, there is a growing body of data to indicate a pathophysiological role for P2X7 receptors in inflammatory responses (Ferrari et al., 2006). Interestingly, recent data from the P2X7 receptor knockout mouse (Chessell et al., 2005) and the development of new potent and selective P2X7 receptor antagonists (Lappin et al., 2005; Nelson et al., 2006; Honore et al., 2006b) also indicate a role for P2X7 receptors in the onset and persistence of certain types of chronic pain. This article provides an update of the current pharmacology for the P2X7 receptor, recent advances in understanding P2X7 receptor signaling pathways as well as new insights into the therapeutic potential of P2X7 receptor antagonist ligands for pain relief. Other recent reviews by Fields and Burnstock (2006), Ferrari et al. (2006), Gever et al. (2006) and Khakh and North (2006) provide extensive overviews of P2X receptor physiology and pharmacology.
P2X7 receptor pharmacology
Agonists
Brief agonist activation (<10 s) of the P2X7 receptor results in rapid and reversible channel opening that is permeable to Na+, K+ and Ca2+ (Surprenant et al., 1996). Acute P2X7 receptor activation also triggers a series of cellular responses, such as activation of caspases, cytokine release, cell proliferation, and apoptosis (Perregaux et al., 2000; Panenka et al., 2001; Chakfe et al., 2002; North, 2002; Verhoef et al., 2003; Kahlenberg and Dubyak, 2004). Unlike other members of the P2 receptor superfamily, homomeric P2X7 receptors are activated by high concentrations of ATP (>100 μM) and 2′,3′-O-(4-benzoylbenzoyl)-ATP (BzATP), which has significantly greater potency (EC50=20 μM) than ATP (EC50>100 μM) (Jacobson et al., 2002). The rank order agonist potency for activation of the P2X7 receptor is BzATP≫ATP, with 2MeSATP, ATPγS and ADP being essentially inactive (Jacobson et al., 2002). The chemical structures of these agonists are shown in Figure 1.
Figure 1.
Structures of prototypical adenine nucleotides modified on the phosphate moiety that have been investigated as P2 receptor agonists.
However, P2X7 receptors can also be primed and undergo agonist plasticity such that brief agonist exposure resulted in an apparent increase in potency to subsequent agonist exposure (Chakfe et al., 2002). Furthermore, agonist priming also expands the agonist pharmacology such that both ADP and AMP can now activate the receptor (Chakfe et al., 2002). Although BzATP is the most potent agonist for P2X7 receptors, it is not a selective agonist for this receptor. BzATP is 100–1000 times more potent as an agonist at both P2X1 and P2X3 receptors than at P2X7 receptors (Bianchi et al., 1999). Consequently, a rank order of potency of nonselective purine agonists (BzATP≫ATP≫UTP≫αβMeATP) has been utilized to identify P2X7 receptors in native cell lines and tissue (Bianchi et al., 1999; Anderson and Nedergaard, 2006).
Antagonists
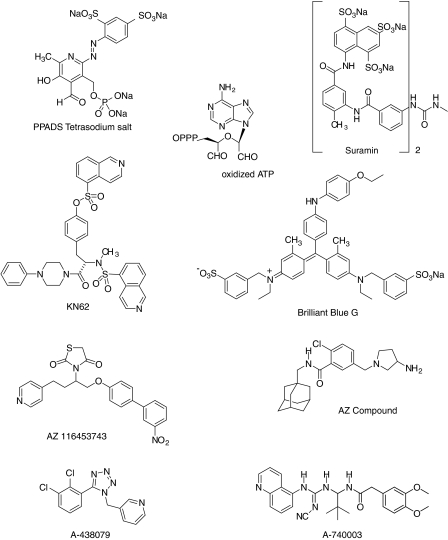
The prototypic nonselective P2X receptor antagonists, suramin and pyridoxalphosphate-6-azopheny-2′,4′-disulfonate (PPADS; Figure 2), both block P2X7 receptors with low affinity (Ki>10 μM; Table 1) and typically show noncompetitive antagonism (Jacobson et al., 2002). Brilliant Blue G (BBG; pIC50=5.1; Figure 2 and Table 1) is a more potent and selective antagonist with a 30- to 50-fold greater selectivity for rat versus human P2X7 receptors (Jiang et al., 2000). Large cationic inhibitors of calcium/calmodulin-dependent protein kinase II such as the isoquinoline, (1-[N,O-bis(5-isoquinoline-sulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine) (KN62) (Figure 2 and Table 1), potently block P2X7 receptor function in a noncompetitive fashion (Gargett and Wiley, 1997). KN62, like other putative P2X7 receptor antagonists, also shows significant species differences in in vitro assays (Humphreys et al., 1998). Analysis of the magnitude of apparent species differences for P2X7 antagonists is also complicated by the end point that is measured, cation flux and pore formation (by large dye, that is YO-Pro uptake), which requires different agonist incubation times (Gever et al., 2006) with the latter end point being host-cell dependent (North, 2002).
Figure 2.
Structures of prototypical and novel antagonists of P2X7 receptors. PPADS and suramin are nonselective antagonists, BBG has higher potency for rat versus human P2X7 receptors whereas KN62 and the AZ compounds exhibit the reverse potency of human>rat P2X7 receptors. A-740003 and A-438079 exhibited equivalent potency to both the rat and human P2X7 receptors (see Table 1). BBG, Brilliant Blue G; PPADS, pyridoxal phosphate-6-azophenyl-2-4-disulfonic acid.
Table 1.
Functional pharmacological evaluation
| Antagonist | Rat P2X7 | Human P2X7 |
|---|---|---|
| [Ca2+]I pIC50±s.e.m. | ||
| A-740003 | 7.75±0.03 | 7.36±0.04 |
| A-438079 | 6.50±0.20 | 6.90±0.20 |
| AZ11645373 | >4 | 8.15a |
| AZ compoundb | ND | ND |
| PPADS | 5.10±0.02 | 5.45±0.03 |
| KN62 | <4 | 4.88±0.18 |
| BBG | 5.08±0.07 | <4 |
| Suramin | <4 | <4 |
| Antagonist | Rat P2X7 | Human P2X7 |
| Yo-Pro activity pIC50±s.e.m. | ||
| A-740003 | 7.00±0.02 | 7.03±0.04 |
| A-438079 | 6.25±0.20 | 6.70±0.10 |
| AZ11645373 | >4 | 7.70a |
| AZ compoundb | 6.54±0.32 | 8.38±0.12 |
| PPADS | 5.92±0.09 | 5.88±0.03 |
| KN62 | <4 | 6.67±0.02 |
| BBG | 6.22±0.03 | 5.71±0.09 |
| Suramin | <4 | <4 |
| Antagonist | THP-1 cells/Human P2X7 | |
| IL-1β activity pIC50±s.e.m. | ||
| A-740003 | 7.00±0.02 | |
| A-438079 | 6.40±0.30 | |
| AZ11645373 | 7.68a | |
| AZ compoundb | 6.82±0.08 | |
| PPADS | 5.92±0.20 | |
| KN62 | 7.12±0.16 | |
| BBG | 6.22±0.03 | |
Abbreviations: BBG, Brilliant Blue G; PPADS, pyridoxal phosphate-6-azophenyl-2-4-disulfonic acid; ND, not determined; s.e.m., standard error of the mean.
pIC50 values derived from published results (Stokes et al., 2006).
Data from Fonfria et al. (2005).
ATP 2′,3′-dialdehyde (oxidized-ATP, oATP; Figure 2) is an irreversible inhibitor of P2X7 receptors that requires a 1- to 2-h incubation to inhibit functional activation of P2X7 receptors (Di Virgilio, 2003). In light of its original use as a ligand for nucleotide binding proteins (Lowe and Beechey, 1982), oATP was initially used to block P2X7 receptor-mediated responses in mouse macrophages (Murgia et al., 1993). However, it must be noted that oATP has many other pharmacological actions including blockade of P2X1 and P2X2 receptors (Evans et al., 1995) as well as inhibition of nuclear factor-κB (NF-κB) and cytokine release (Murgia et al., 1993; Beigi et al., 2003; Ferrari et al., 2006). Consequently, these newer findings raise further doubt on the utility of oATP to properly interrogate P2X7-related function in other than selective recombinant receptor expression systems (Beigi et al., 2003; Di Virgilio, 2003).
More recently, decavanadate, a polymeric form of vanadate (H2V10O28−4), was reported also to be a reversible and competitive P2X7 receptor antagonist (Michel et al., 2006a). However, decavanadate also displays nonselective activity since it blocks P2X2 and P2X4 receptors, and at nanomolar concentrations, interacts with a number of other targets including activation of 5′-nucleotidase, inhibition of inositol 1,4,5-trisphospate (IP3) binding and inhibition of ribonuclease A (Michel et al., 2006a). Thus, like oATP, the usefulness of decavanadate as a pharmacological tool for P2X7 receptors is limited.
In 2003, two novel series of P2X7 antagonists, composed of cyclic imides (Alcaraz et al., 2003) and adamantane amides (Baxter et al., 2003; Figure 2), were shown to be effective P2X7 receptor antagonists and useful starting points for directed structure–function studies. However, these compounds have not yet been evaluated for activity at P2 or other cell surface receptors. Stokes et al. (2006) recently published a more complete characterization of the cyclic imide P2X7 antagonist, AZ11645373 (3-(1-(3′-nitrobiphenyl-4-yloxy)-4-(pyridine-4-yl)butan-2-yl)thiazolidine-2,4-dione; Figure 2), which was shown to potently (IC50=10–90 nM; Table 1) block human P2X7 receptor-mediated cation influx and interleukin-1β (IL-1β) release. However, like KN62, AZ11645373 shows preferential affinity for the human versus the rat P2X7 receptor thus limiting its usefulness as an in vivo tool to examine P2X7 receptor function in rat preclinical models.
In 2006, our group at Abbott Laboratories disclosed two novel series of P2X7 antagonists: disubstituted tetrazoles and cyanoguanidines (Nelson et al., 2006; Honore et al., 2006b). Both series demonstrate enhanced potency and selectivity as antagonists at rat and human P2X7 receptors compared to previous antagonists (Nelson et al., 2006; Honore et al., 2006b). Unlike other P2X7 antagonists, these compounds are also reversible and competitive blockers. A-438079, 3-((5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl)methyl pyridine; Figure 2), from a disubstituted tetrazole series, potently blocked BzATP-stimulated changes in intracellular calcium concentrations (IC50=100 and 300 nM at rat and human P2X7 receptors, respectively; Table 1) and was essentially devoid of activity (IC50≫10 μM) at other P2 receptors (Nelson et al., 2006). Additionally, this compound showed little or no activity at a wide array of other cell-surface receptors and ion channels (Nelson et al., 2006). A-438079 and several analogs were also shown to inhibit BzATP-stimulated IL-1β release and pore formation in human THP-1 cells differentiated with LPS and IFN-γ into a macrophage-like phenotype (Nelson et al., 2006).
Another novel P2X7 antagonist pharmacophore from a cyanoguanidine series is represented by A-740003 ((N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide) (Figure 2; Honore et al., 2006b). A-740003 is a highly specific and potent antagonist for both rat and human P2X7 receptors with potencies of 18–40 nM, respectively (Honore et al., 2006b; Table 1). Similar to A-438079, A-740003 blocked P2X7 receptor-mediated changes in intracellular calcium concentrations in a competitive fashion and was shown to be highly selective for P2X7 compared to other P2 receptors as well as other cell-surface receptors and ion channels. In addition, A-740003 was more potent in blocking BzATP-evoked IL-1β release and pore formation in differentiated human THP-1 cells than A-438079 (Honore et al., 2006b). An additional feature of these compounds is that they have sufficient bioavailability following intraperitoneal administration to allow for their use in in vivo investigations on the role of P2X7 receptors in various disease models (Nelson et al., 2006; Honore et al., 2006b).
P2X7 receptor signaling
As noted above, activation of P2X7 receptors results in a rapid but reversible channel opening that is permeable to Ca2+, Na+ and K+ ions. P2X7 receptor-mediated changes in intracellular potassium concentrations lead to the activation of caspase-1 and the rapid maturation and release of the proinflammatory cytokine, IL-1β (Sanz and DiVirgilio, 2000; Kahlenberg and Dubyak, 2004; Perregaux et al., 2000; Solle et al., 2001; Ferrari et al., 2006). ATP-stimulated IL-1β release is independent of cytolysis, does not require P2X7-mediated pore formation, and is blocked by P2X7 receptor antagonists (Grahames et al., 1999; Chessell et al., 2001). Increased IL-1β concentrations, in turn, trigger the induction of nitric oxide synthase, cycloxygenase-2 and tumor necrosis factor-α (TNF-α) (Woolf et al., 1997; Samad et al., 2001; Parvathenani et al., 2003; Burnstock, 2006). Activation of caspase 3 has also been linked to P2X7 receptor activation and may underlie receptor-associated cytolytic mechanisms including pore formation (Perregaux et al., 2001). Inhibitors of caspase 1 and 3 effectively block P2X7 receptor-mediated IL-1β release and Yo-Pro uptake, respectively (Donnelly-Roberts et al., 2004).
Activation of P2X7 receptors has also been associated with other downstream signaling pathways including phospholipase D (Humphreys and Dubyak, 1996), phospholipase A2 (PLA2), NF-κB (Ferrari et al., 1997; Aga et al., 2002) and mitogen-activated protein kinases (MAPKs) (Aga et al., 2002; Armstrong et al., 2002; Bradford and Soltoff, 2002). Studies have shown that different MAPK kinases are affected by P2X7 activation that vary depending on host cell systems. For example, in human astrocytoma 1321 cells, recombinant P2X7 receptor activation mediates ERK1/2 upregulation (Gendron et al., 2003) but not in differentiated human THP-1 cells (Donnelly-Roberts et al., 2004). Recent work by Monterio da Cruz et al. (2006) supported the latter finding by demonstrating that ERK1/2 is activated by a number of extracellular nucleotides and that this activation is not dependent on ATP-induced pore formation.
Recent studies provide data showing that activation of P2X7 receptors leads to downstream activation of p38 MAPK (Ono and Han, 2000; Armstrong et al., 2002; Donnelly-Roberts et al., 2004). For example, BzATP-evoked increases in phosphorylated p38 expression in THP-1 cells that had been differentiated into a macrophage phenotype (Donnelly-Roberts et al., 2004). This effect was fully blocked by p38 MAPK inhibitors and by KN62 as well as a novel cyanoguanidine P2X7 receptor-selective antagonist (Donnelly-Roberts et al., 2004, unpublished observations). Like its pore-forming activity (North, 2002) and other P2X7 receptor downstream signaling mechanisms (Donnelly-Roberts et al., 2004; Faria et al., 2005), P2X7 receptor-mediated activation of p38 MAPK is likely to be cell type-dependent. While some recent reports have demonstrated this possibility in THP-1 cells (Michel et al., 2006b), a clear interpretation of the experimental data is complicated by the use of suboptimal cell differentiation conditions for p38 MAPK expression (Carter et al., 2001) and the use of oATP as a P2X7 antagonist, which has many other non-P2X7 receptor-mediated activities.
Other reports (MacKenzie et al., 2001; Verhoef et al., 2003) have also implicated that many of the functional sequelae to P2X7 receptor activation including IL-1β release, membrane blebbing and pore formation may be mediated by parallel rather than convergent intracellular signal transduction pathways (North, 2002). These data are consistent with the specific involvement of p38 MAPK in P2X7 receptor-mediated pore formation as this mechanism does not contribute to pore formation induced by maitotoxin (MTX), a marine toxin isolated from dinoflagellates (Schilling et al., 1999), or by human cathelicidin-derived peptide (LL37) (Elssner et al., 2004). Further, pore formation is not a unique property of P2X7 receptors as prolonged agonist activation of other nondesensitizing P2X receptors such as P2X2a, P2X2/3 and P2X4, also leads to the formation of cell-surface pores (Khakh et al., 1999; Virginio et al., 1999; North, 2002; Donnelly-Roberts et al., 2004), although with different time courses and pore sizes (Virginio et al., 1999).
P2X7 receptor-mediated Yo-Pro uptake does not occur in all cell types and may be dependent upon receptor density (North 2002). Some cell types such as the B-lymphocytes (Gu et al., 2000), retinal muller cells (Pannicke et al., 2000) and non-neuronal cells of the dorsal root ganglion (Zhang et al., 2005) express functional P2X7 receptors but yet do not form cytolytic pores. Thus, this disconnection has indicated the possibility that these two functional properties are not intrinsic to the P2X7 receptor itself but require the assistance of secondary molecules that are host cell-dependent (North, 2002).
The ability of P2X7 receptor activation to generate cytolytic pores has been clearly linked to interactions within the C-terminal domain of the receptor (Rassendren et al., 1997b). Removal of this portion of the channel results in pore dilating properties similar to P2X2 and P2X4 receptors based on measuring time-dependent changes in NMDG+ permeability (Khakh et al., 1999). Recent studies have shed light on differences in P2X7 receptor-mediated pore formation between Yo-Pro and NMDG+ entry. North and co-workers (Jiang et al., 2005) have shown that NMDG+ and Yo-Pro do not enter P2X7 receptor-expressing cells by the same route further supporting the hypothesis that although pore formation is triggered by P2X7 receptor activation, it is dependent on the involvement of downstream signaling interactions not involved in channel opening.
Both intrinsic P2X7 receptor channel dilation and P2X7 receptor-dependent recruitment of accessory proteins have been proposed as two potential mechanisms necessary for pore formation (North, 2002). Data demonstrating that P2X7 receptor pore formation is progressive, occurs in multiple cell types and has an identical pharmacology for the opening of the cation channel support the intrinsic receptor hypothesis. But recent data strongly suggest that pore formation involves accessory proteins such as heat shock proteins (for example, HSP90), chaperone-like proteins or hemichannels such as pannexins. Studies of the ubiquitously expressed HSP90 protein have shown that HSP90 might act as a negative regulator of P2X7 receptor function because, geldanamycin, a HSP90 inhibitor, produced a twofold increase on P2X7 receptor activation by agonists (Adinolfi et al., 2003). A more recent report has demonstrated that the recruitment of pannexin-type hemichannels that are involved in gap junction formation (Barbe et al., 2006) may contribute to pore formation following P2X7 receptor activation (Pelegrin and Surprenant, 2006; Locovei et al., 2007). Interestingly, pannexin-1 signaling is also required for the processing of caspase-1 and the subsequent P2X7 receptor-mediated release of mature IL-1β (Pelegrin and Surprenant, 2006). It was also demonstrated in these studies that pannexin siRNA selectively blocks P2X7 receptor-induced pore formation, but not ionic currents further supporting separate pathways for these two distinct P2X7 receptor-mediated functional events (Pelegrin and Surprenant, 2006). These recent data strongly suggest that the pannexin hemichannel may serve as a portal for P2X7 receptor pore formation to occur and unlock the release of IL-1β as well as to permit other downstream signaling events to proceed such as the upregulation of p38 MAPK.
Therapeutic significance
It is now well established that ATP acting at P2X7 receptors serves as an efficient secondary stimulus for the maturation and release of IL-1β from proinflammatory cells (Perregaux and Gabel, 1994; Mackenzie et al., 2001; Ferrari et al., 2006). As a consequence, P2X7 receptor activation may function as a danger signal in the context of tissue trauma (Ferrari et al., 2006). However, validation of this concept in disease models has been slow due to the previous lack of appropriate pharmacological tools. Additionally, initial phenotypic data from a P2X7 receptor null mouse revealed unremarkable changes (Sikora et al., 1999).
A detailed analysis of an independently generated P2X7 receptor knockout mouse revealed that P2X7 (−/−) mice show a disruption in cytokine signaling cascades with perturbation of ATP-induced processing of pro-IL-1β in macrophages (Solle et al., 2001). Using a monoclonal antibody-induced model of collagen arthritis, these investigators also demonstrated that P2X7 (−/−) mice show a decreased incidence and severity of arthritis in this model as compared with wild-type control mice (Labasi et al., 2002). A more recent study has demonstrated that P2X7 knockout mice also show reduced pain sensitivity following both complete Freund's adjuvant-induced inflammation and partial injury of the sciatic nerve (Chessell et al., 2005).
The finding that disruption of P2X7 receptors not only altered inflammatory pain but also reduces pain associated with frank nerve injury (Chessell et al., 2005), provided new insights into the potential physiological role of P2X7 receptors in sensory functions. Additionally, these data are also consistent with the mechanistic role of P2X7 receptors in modulating IL-1β release and the ability of IL-1β to alter pain sensitivity in experimental models. Previous data have shown that endogenous IL-1 levels are increased in the nervous system in response to trauma associated with mechanical damage, ischemia, seizures and hyperexcitability (Touzani et al., 2002). Increased IL-1 levels are also associated with enhanced nociceptive signaling in a concentration-related fashion (Bianchi et al., 1998; Horai et al., 2000). At the level of the spinal cord, blockade of IL-1 receptors with the IL-1 receptor antagonist (IL-1ra), results in reduced nociception in animal models of inflammation and nerve injury-induced pain (Maier et al., 1993; Safieh-Garabedian et al., 1995; Sommer et al., 1999). Other genetic manipulations of IL-1 signaling including targeted gene disruption of the IL-1 type I receptor or the IL-1 accessory protein (IL-1acp) as well as transgenic overexpression of the IL-1ra (Wolf et al., 2004) or IL-1αβ double knockout (Honore et al., 2006a) have generated mice that show reduced nociceptive responses relative to wild-type animals.
Complementing these genetic data are recent studies using receptor-selective antagonists. Collectively, these studies indicate a specific role for P2X7 receptor activation in pain signaling. Early work by Dell'Antonio et al. (2002a, 2002b) showed that local administration of oATP reduced inflammation-induced mechanical hyperalgesia in rats. These results were attributed to pharmacological blockade of P2X7 receptors. However, as noted above, oATP has weak affinity for P2X7 receptors, slow kinetics and many other pharmacological actions that limit interpretations about P2X7 receptor specificity. More direct support for a role of P2X7 receptors in pain modulation are provided by the recent P2X7 knockout study (Chessell et al., 2005) and studies using selective antagonists (Nelson et al., 2006; Honore et al., 2006b). Similar to the nociceptive phenotype of mice lacking P2X7 receptors (Chessell et al., 2005) or lacking both isoforms of IL-1 (Honore et al., 2006a), systemic administration of P2X7 receptor-selective antagonists (for example, A-438079 and A-740003) produced dose-dependent antinociceptive effects in models of neuropathic (Nelson et al., 2006; Honore et al., 2006b) and inflammatory pain (Honore et al., 2006a). Consistent with their in vitro potencies, A-740003 was more potent than A-438079 at reducing mechanical allodynia observed 2 weeks after spinal L5/L6 nerve ligation. These data are also consistent with an independent study of one of the adamantane P2X7 antagonists that showed dose-dependent antinociception in an inflammatory pain model (Lappin et al., 2005). These data illustrate the potential role of P2X7 receptor modulation of IL-1β in reducing nociception in neuropathic pain models.
The robust antinociceptive effects of P2X7 antagonists in inflammatory pain models does not appear to be secondary to an anti-inflammatory effect as A-740003 was more efficacious in reducing nociception compared with paw edema in inflammation models (Honore et al., 2006b). It should be noted, however, that the anti-inflammatory activity of P2X7 antagonists may be more pronounced in arthritis models as compared with acute (carrageenan) and subacute complete Freund's adjuvant (CFA) inflammatory models, where the contribution of IL-1β to ongoing inflammatory processes may be more prominent than in chronic arthritis (Labasi et al., 2002).
Summary and perspective
The discovery of P2X7 receptor-selective antagonists has provided data demonstrating that the acute in vivo blockade of P2X7 receptors significantly reduced nociception in animal models of persistent neuropathic and inflammatory pain. While there is growing appreciation for the role of P2X7 receptor modulation of proinflammatory IL-1 processing (Ferrari et al., 2006), the analgesic activity of P2X7 receptor antagonists (Lappin et al., 2005; Honore et al., 2006b; Nelson et al., 2006), indicates a specific role for P2X7 receptors in neural–glial cell interactions associated with ongoing pain (Zhang et al., 2005).
In addition to the analgesic effects of P2X7 receptor antagonists, there exist multiple P2 receptor-based mechanisms by which ATP can alter nociceptive sensitivity following tissue injury (Fields and Burnstock, 2006). Evidence from a variety of experimental strategies including genetic disruption and the development of other selective antagonists has indicated that the activation of several P2X receptors, including P2X3, P2X2/3, P2X4 and P2X7, and P2Y (P2Y2) receptors can modulate pain. For example, A-317491, a selective P2X3 antagonist (Jarvis et al., 2002) effectively blocks both CFA-induced inflammatory thermal hyperalgesia as well as mechanical and thermal hyperalgesia resulting from chronic constriction of the sciatic nerve. Intrathecally delivered antisense oligonucleotides specifically targeting P2X4 receptors have been shown to decrease tactile allodynia following nerve injury (Tsuda et al., 2003). In addition, activation of P2Y2 receptors leads to sensitization of transient receptor potential vanilloid-1 (TRPV1) receptors (Tominaga et al., 2001; Lakshmi and Joshi, 2005). Thus, ATP acting at multiple purinergic receptors either directly on neurons (for example, P2X3, P2X2/3 and P2Y receptors) or through indirect neural–gial cell interactions (P2X4 and P2X7) appear to alter nociceptive sensitivity (Tsuda et al., 2003; Inoue et al., 2004; Zhang et al., 2005).
As this article illustrates, the discovery of receptor-selective antagonists for individual P2X receptor subtypes has led to important insights into the roles of individual P2X receptors in chronic pain states. From a pharmacological perspective, there is presently a wider structural diversity of non-nucleotide P2X7 receptor antagonist pharmacophores than have been described for P2X3 receptors (Jarvis, 2003; Gever et al., 2006) or for P2X4 receptors, for which, no potent antagonists have yet been identified (Gever et al., 2006). While both P2X4 and P2X7 receptors have been implicated in glial-based modulatory mechanisms involved in sensory neurotransmission (Tsuda et al., 2003; Zhang et al., 2005), the discovery of selective P2X4 antagonists are needed to differentiate the respective roles of these receptors in pain signaling. The identification of novel P2X7 receptor-selective antagonists has provided new research tools for both in vitro and in vivo studies of P2X7 receptor pharmacology and has led to the generation of new data that indicates an expanded role for this receptor in pain signaling associated with nerve injury and inflammation.
Acknowledgments
We are grateful to Derek Nelson for his assistance with the figures and comments on earlier versions of this manuscript.
Abbreviations
- AMP
adenosine 5′-monophosphate sodium salt
- ATP
adenosine triphosphate
- ATP-γs
adenosine 5′-[γ-thio]triphosphate tetralithium salt
- 2-MeS-ATP
2-methlthioadenosine 5′-triphosphate
- αβ-me-ATP
α,β-methylene-adenosine 5′-triphosphate
- A-438079
3-(5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl)methyl pyridine
- A-740003
N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide
- AZ11645373
3-(1-(3′-nitrobiphenyl-4-yloxy)-4-(pyridine-4-yl)butan-2-yl)thiazolidine-2,4-dione
- ADP
adenosine 5′-diphosphate monopotassium salt dihydrate
- BBG
Brilliant Blue G
- BzATP
2,3-O-(4-benzoylbenzoyl)-ATP
- CFA
complete Freund's adjuvant
- FLIPR
fluorometric imaging plate reader
- IL-1β
interleukin 1β
- KN62
(1-[N,O-bis(5-isoquinoline-sulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine)
- LL37
human cathelicidin-derived peptide
- MAPK
mitogen-activated protein kinase
- MTX
maitotoxin
- NF-kappa B
nuclear factor-κB
- oATP
ATP 2′,3′-dialdehyde
- pp38 MAPK
phosphorylated p38 MAPK
- PLA2
phospholipase A2
- PPADS
pyridoxal phosphate-6-azophenyl-2-4-disulfonic acid
- TNF-α
tumor necrosis factor-α
- UTP
uridine 5′-triphosphate
Conflict of interest
These authors are employees of Abbott Laboratories.
References
- Adinolfi E, Kim M, Young MT, Di Virgillio F, Surprenant A. Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors. J Biol Chem. 2003;278:37344–37351. doi: 10.1074/jbc.M301508200. [DOI] [PubMed] [Google Scholar]
- Aga M, Johnson CJ, Hart AP, Guadarrama AG, Suresh M, Svaren J, et al. Modulation of monocyte signaling and pore formation in response to agonists of the nucleotide receptor P2X7. J Leukocyte Biol. 2002;72:222–232. [PubMed] [Google Scholar]
- Alcaraz L, Baxtrer A, Bent J, Bowers K, Braddock M, Cladingboel D, et al. Novel P2X7 receptor antagonists. Bioorg Med Chem Lett. 2003;13:4043–4046. doi: 10.1016/j.bmcl.2003.08.033. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Nedergaard M. Emerging challenges of assigning P2X(7) receptor function and immunoreactivity in neurons. Trends Neurosci. 2006;29:257–262. doi: 10.1016/j.tins.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Armstrong JN, Brust TB, Lewis RG, MacViar BA. Activation of presynaptic P2X7-like receptors depresses mossy fiber-CA3 synaptic transmission through p38 mitogen-activated protein kinase. J Neurosci. 2002;22:5938–5945. doi: 10.1523/JNEUROSCI.22-14-05938.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe MT, Monyer H, Bruzzone R. Cell–cell communication beyond connexins: the pannexin channels. Physiol. 2006;21:103–114. doi: 10.1152/physiol.00048.2005. [DOI] [PubMed] [Google Scholar]
- Baxter A, Bent J, Bowers K, Braddock M, Brough S, Fagura M, et al. Hit-to-lead studies: the discovery of potent adamantane amide P2X7 receptor antagonists. Bioorg Med Chem Lett. 2003;13:4047–4050. doi: 10.1016/j.bmcl.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Beigi RD, Kertesy SB, Aquilina G, Dubyak GR. Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor-independent mechanisms. Br J Pharmacol. 2003;140:507–519. doi: 10.1038/sj.bjp.0705470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, et al. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Dib B, Panerai AE. Interleukin-1 and nociception in the rat. J Neurosci Res. 1998;53:645–650. doi: 10.1002/(SICI)1097-4547(19980915)53:6<645::AID-JNR2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bradford MD, Soltoff SP. P2X7 receptors activate protein kinase D and p42/p44 mitogen-activated protein kinase (MAPK) downstream of protein kinase C. Biochem J. 2002;366:745–755. doi: 10.1042/BJ20020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–78. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- Cabrini G, Flazonni S, Forchap SL, Pellegatti P, Balboni A, Agostini P, et al. A His-155 to tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J. Immunol. 2005;175:82–89. doi: 10.4049/jimmunol.175.1.82. [DOI] [PubMed] [Google Scholar]
- Carter AB, Tephly LA, Hunnunghake GW. The absence of activator protein1-dependent gene expression in THP-1 macrophages stimulated with phorbol esters is due to lack of p38 mitogen-activate protein kinase activation. J Biol Chem. 2001;276:33826–33832. doi: 10.1074/jbc.M100209200. [DOI] [PubMed] [Google Scholar]
- Chakfe Y, Seguin R, Antel JP, Morissette C, Malo D, Henderson D, et al. ADP and AMP induce interleukin-1 beta release from microglia cells through activation of ATP-primed P2X7 receptor channels. J Neurosci. 2002;22:3061–3069. doi: 10.1523/JNEUROSCI.22-08-03061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell IP, Grahames CBA, Michel AD, Humphrey PPA. Dynamics of P2X7 receptor pore dilation: pharmacological and functional consequences. Drug Dev Res. 2001;53:60–65. [Google Scholar]
- Chessell IP, Hatcher J, Bountra C, Michel AD, Hughes JP, Green P, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Collo G, Neidhart S, Kawashima E, Kosco-Vibois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- Dell'Antonio A, Quattrini A, Dal Cin E, Fulgenzi A, Ferrero ME. Antinociceptive effect of a new P2Z/P2X7 antagonist, oxidized ATP, in arthritic rats. Neurosci Lett. 2002a;327:87–90. doi: 10.1016/s0304-3940(02)00385-3. [DOI] [PubMed] [Google Scholar]
- Dell'Antonio A, Quattrini A, Dal Cin E, Fulgenzi A, Ferrero ME. Relief of inflammatory pain in rats by local use of the selective P2X7 ATP receptor inhibitor, oxidized ATP. Arthritis Rheum. 2002b;46:3378–3385. doi: 10.1002/art.10678. [DOI] [PubMed] [Google Scholar]
- Denlinger LC, Fisette PL, Sommer JA, Watters JJ, Prabhu U, Dubyak GR, et al. The nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J Immunol. 2001;167:1871–1876. doi: 10.4049/jimmunol.167.4.1871. [DOI] [PubMed] [Google Scholar]
- Di Virgilio FD, Vishawanath V, Ferrari D.On the role of the P2X7 receptor in the immune system Purinergic and Pyrimidinergic Signalling 2001Springer Verlag: Heidelberg, GR; 355–373.In: Abbracchio MP and Williams M (eds). [Google Scholar]
- Di Virgilio F. Novel data point to a broader mechanism of action of oxidized ATP: the P2X7 receptor is not the only target. Br J Pharmacol. 2003;140:441–443. doi: 10.1038/sj.bjp.0705469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Namovic M, Faltynek CR, Jarvis MF. Mitogen-activated protein kinase and caspase signaling pathways are required for P2X7 receptor (P2X7R)-induced pore formation in human THP-1 cells. J Pharmacol Exp Ther. 2004;308:1053–1061. doi: 10.1124/jpet.103.059600. [DOI] [PubMed] [Google Scholar]
- Egan TM, Samways DSK, Li Z. Biophysics of P2X Receptors. Eur J Physiol. 2006;452:501–512. doi: 10.1007/s00424-006-0078-1. [DOI] [PubMed] [Google Scholar]
- Elssner A, Duncan M, Gavrillin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1β processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Buell G, Vlaera S, North RA, Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2X purinoceptors) Mol Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- Faria RX, DeFarias FP, Alves LA. Are second messengers crucial for opening the pore associated with P2X7 receptor. Am J Physiol Cell Physiol. 2005;288:C260–C271. doi: 10.1152/ajpcell.00215.2004. [DOI] [PubMed] [Google Scholar]
- Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Golbberg H, Marks GB, et al. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med. 2007;175:360–366. doi: 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, DiVirgilio F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J. Immunol. 1996;156:1531–1539. [PubMed] [Google Scholar]
- Ferrari D, Wesselborg S, Bauer M, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-[kappa]B through the P2Z purinoreceptor by selectively targeting NF-[kappa]B. J Cell Biol. 1997;139:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signaling in neuron–glia interactions. Nature Review. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfria E, Chambers LJ, Demont EH, Roman SA, Skaper SD, Michel AD.Species and temperature dependent effects of a novel P2X7 receptor antagonist on recombinant and native P2X7 receptors Soc Neurosci 2005. Abstr no. 958.1
- Gargett CE, Wiley JS. The isoquinoline derivative KN-62 is a potent antagonist of the P2XZ-receptor of human lymphocytes. Br J Pharmacol. 1997;120:1483–1490. doi: 10.1038/sj.bjp.0701081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron F-P, Neary JT, Theiss PM, Sun GY, Gonzalez FA, Weismann GA. Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am J Physiol Cell Physiol. 2003;284:C571–C581. doi: 10.1152/ajpcell.00286.2002. [DOI] [PubMed] [Google Scholar]
- Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford APDW. Pharmacology of P2X channels. Eur J Physiol. 2006;452:513–537. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- Grahames GBA, Michel AD, Chessell IP, Humpreys PPA. Pharmacological characerization of ATP-and LPS-induced IL-1β release in human monoctyes. Br J Pharmacol. 1999;127:1915–1921. doi: 10.1038/sj.bjp.0702732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu BJ, Zhang WY, Bendall LJ, Chessell IP, Buell GN, Wiley JS. Expression of P2X7 purinoceptors on human lymphocytes and monoctyes: evidence for nonfunctional P2X7 receptors. Am J Physiol. 2000;279:C1189–C1197. doi: 10.1152/ajpcell.2000.279.4.C1189. [DOI] [PubMed] [Google Scholar]
- Honore P, Wade CL, Zhong C, Harris RR, Wu C, Ghayur T, et al. Interleukin-1αβ gene-deficient mice show reduced nociceptive sensitivity in models of inflammatory and neuropathic pain but not post-operative pain. Behav Brain Res. 2006a;167:355–364. doi: 10.1016/j.bbr.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Honore PM, Donnelly-Roberts D, Namovic M, Hsieh G, Zhu C, Mikusa J, et al. A-740003 (N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide, a novel and selective P2X7 receptor antagonist dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006b;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Dubyak GR. Induction of the P2z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-γ in the human THP-1 monocytic cell line. J Immunol. 1996;157:5627–5637. [PubMed] [Google Scholar]
- Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoquinolines as antagonists of the P2X7 nucelotide receptor: high selectivity for the human versus rat receptor. Mol Pharmacol. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M, Koizumi S. ATP- and adenosine-mediated signaling in the central nervous system: chronic pain and microglia: involvement of the ATP receptor P2X4. J Pharmacol Sci. 2004;94:112–114. doi: 10.1254/jphs.94.112. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Jarvis MF, Williams M. Perspective: purine and pyrimidine. (P2) receptors as drug targets. J Med Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, et al. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in rats. Proc Natl Acad Sci USA. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF. Contributions of P2X3 homomeric and heteromeric channels to acute and chronic pain. Expert Opin Ther Targets. 2003;7:513–522. doi: 10.1517/14728222.7.4.513. [DOI] [PubMed] [Google Scholar]
- Jiang LH, MacKenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X7 receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- Jiang LH, Rassendren F, MacKenzie AB, Zhang YH, Surprenant A, North RA. N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X7 receptors. Am J Physiol Cell Physiol. 2005;289:C1295–C1302. doi: 10.1152/ajpcell.00253.2005. [DOI] [PubMed] [Google Scholar]
- Kahlenberg J, Dubyak GW. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Cell Physiol. 2004;286:C1100–C1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Bao XR, Labarca C, Lester HA. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nature Neurosci. 1999;2:322–330. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Kim M, Jiang LH, Wilson HL, North RA, Suprenant A. Proteomic and functional evidence for a P2X7 receptor signaling complex. EMBO J. 2001;20:6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- Lakshmi S, Joshi PG. Co-activation of P2Y2 receptor and TRPV channel by ATP: implications for ATP induced pain. Cell Mol Neurobiol. 2005;25:819–832. doi: 10.1007/s10571-005-4936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin SC, Winyard LA, Clayton N, Chambers LJ, Demont EH, Chessell IP, et al. Reversal of mechanical hyperalgesia in a rat model of inflammatory pain by a potent and selective P2X7 antagonist Soc Neurosci 2005. Abstr no. 958.2
- Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe PN, Beechey RB. Interactions between the mitochondrial adenosinetriphosphatase and periodate-oxidized adenosine 5′-triphosphate, an affinity label for adenosine 5′-triphosphate binding sites. Biochemistry. 1982;21:4073–4082. doi: 10.1021/bi00260a025. [DOI] [PubMed] [Google Scholar]
- MacKenzie A, Wilson HL, Kiss-Tosh E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity. 2001;8:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- Maier SF, Wietelak EP, Martin D, Watkins LR. Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin. Brain Res. 1993;623:321–324. doi: 10.1016/0006-8993(93)91446-y. [DOI] [PubMed] [Google Scholar]
- Michel AD, Xing M, Thompson KM, Jones CA, Humphrey PPA. Decavanadate, a P2X receptor antagonist, and its use to study ligand interactions with P2X7 receptors. Br J Pharmacol. 2006a;534:19–26. doi: 10.1016/j.ejphar.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Michel AD, Thompson KM, Simon J, Boyfield I, Fonfria E, Humphrey PPA. Species and response dependent differences in the effects of MAPK inhibitors on P2X7 receptor function. Br J Pharmacol. 2006b;149:948–957. doi: 10.1038/sj.bjp.0706938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterio da Cruz C, Ventura ALM, Schachter J, Costa-Junior HM, da Silva Souza HA, Gomes FR, et al. Activation of ERK1/2 by extracellular nucleotides in macrophages is mediated by multiple P2 receptors independently of P2X7-associated pore or channel formation. Br J Pharmacol. 2006;147:324–334. doi: 10.1038/sj.bjp.0706559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268:8199–81203. [PubMed] [Google Scholar]
- Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, et al. Structure–activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem. 2006;49:3659–3666. doi: 10.1021/jm051202e. [DOI] [PubMed] [Google Scholar]
- North AR. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Panenka W, Jijon H, Herx LM, Armstrong N, Feighan D, Wei T, et al. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci. 2001;21:7135–7142. doi: 10.1523/JNEUROSCI.21-18-07135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannicke T, Fisher W, Biefermann B, Schadlich H, Grosche J, Faude F, et al. P2X7-receptors activation in Mueller glial cells from the human retina. J Neurosci. 2000;20:5965–5972. doi: 10.1523/JNEUROSCI.20-16-05965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvathenani LK, Svetlana T, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1b release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perregaux DG, Gabel CA. Interleukin-1β maturation and release in response to ATP and nigericin. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- Perregaux DG, McNiff P, Laliberte R, Conklyn M, Gabel CA. ATP acts as an agonist to promote stimulus-induced secretion of IL-1β and IL-18 in Human Blood. J Immunol. 2000;165:4615–4623. doi: 10.4049/jimmunol.165.8.4615. [DOI] [PubMed] [Google Scholar]
- Perregaux DG, Labasi J, Laliberte R, Stam E, Solle M, Koller B, et al. Interleukin-1β posttranslational processing-exploration of P2X7 receptor involvement. Drug Dev Res. 2001;53:83–90. [Google Scholar]
- Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7: cloning and expression of a human cDNA. J Biol Chem. 1997a;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- Rassendren F, Buell GN, Newbolt A, North RA, Surprenant A. Identification of amino acid residues contributing to the pore of a P2X receptor. EMBO J. 1997b;16:3446–3454. doi: 10.1093/emboj/16.12.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Sanz JM, DiVirgilio F. Kinetics and mechanism of ATP-dependent IL-1β release from microglia cells. J. Immunol. 2000;164:4893–4898. doi: 10.4049/jimmunol.164.9.4893. [DOI] [PubMed] [Google Scholar]
- Schilling WP, Sinkins WG, Estacion M. Maitotoxin activates a nonselective cation channel and a P2Z/P2X7-like cytolytic pore in human skin fibroblasts. Am J Physiol Cell Physiol. 1999;277:C755–C765. doi: 10.1152/ajpcell.1999.277.4.C755. [DOI] [PubMed] [Google Scholar]
- Sikora A, Li J, Brosnan C, Buell G, Chessel I, Bloom BR. Cutting edge: purinergic signaling regulates radical-mediated bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J Immunol. 1999;163:558–561. [PubMed] [Google Scholar]
- Sim JA, Young MT, Sung HY, North RA, Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004;24:6307–6314. doi: 10.1523/JNEUROSCI.1469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, et al. Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Sommer C, Petrausch S, Lindenlaub T, Toyka K. Neutralizing antibodies to interleukin1-receptor reduce pain-associated behavior in mice with experimental neuropathy. Neurosci Lett. 1999;270:25–28. doi: 10.1016/s0304-3940(99)00450-4. [DOI] [PubMed] [Google Scholar]
- Stokes L, Jiang L-H, Alcaraz L, Bent J, Bowers K, Fagura M, et al. Characterization of a selective and potent antagonist of human P2X7 receptors, AZ11645373. Br J Pharmacol. 2006;149:880–887. doi: 10.1038/sj.bjp.0706933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The Cytolytic P2z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani O, Boutin H, LeFeurve R, Parker L, Miller A, Luheshi G, et al. Interleukin-1 influences ischemic brain damage in the mouse independently of the interleukin-1 type 1 receptor. J Neurosci. 2002;22:38–43. doi: 10.1523/JNEUROSCI.22-01-00038.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter M, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Verhoef PA, Estacion M, Schilling W, Dubyak GR. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 release. J Immunol. 2003;170:5728–5738. doi: 10.4049/jimmunol.170.11.5728. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, Rassendren FA, North RA, Suprenant A. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. Nature Neurosci. 1999;2:315–321. doi: 10.1111/j.1469-7793.1999.0335m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JS, Dao-Ung LP, Gu BJ, Sluyter R, Shemon AN, Li C, et al. A loss of function polymorphic mutation in the cytolytic P2X7 receptor gene and chronic lymphocytic leukemia: a molecular study. Lancet. 2002;359:1114–1119. doi: 10.1016/S0140-6736(02)08156-4. [DOI] [PubMed] [Google Scholar]
- Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, Smart ML, et al. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem. 2003;278:17108–17113. doi: 10.1074/jbc.M212759200. [DOI] [PubMed] [Google Scholar]
- Wolf G, Yirmiya R, Goshen I, Iverfeldt K, Holmlund L, Takeda K, et al. Impairment of interleukin-1 (IL-1) signaling reduces basal pain sensitivity in mice: genetic, pharmacological and developmental aspects. Pain. 2004;104:471–480. doi: 10.1016/S0304-3959(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-F, Han P, Faltynek CR, Jarvis MF, Shieh C-C. Functional expression of P2X7 receptors in non-neuronal cells of rat dorsal root ganglion. Brain Res. 2005;1052:63–70. doi: 10.1016/j.brainres.2005.06.022. [DOI] [PubMed] [Google Scholar]