Abstract
Homeobox genes are regulators of place-dependent morphogenesis and play important roles in controlling the expression patterns of cell adhesion molecules (CAMs). To identify proteins that bind to a regulatory element common to the genes for two neural CAMs, Ng–CAM and L1, we screened a mouse cDNA expression library with a concatamer of the sequence CCATTAGPyGA and found a new homeobox gene, which we have called Barx2. The homeodomain encoded by Barx2 is 87% identical to that of Barx1, and both genes are related to genes at the Bar locus of Drosophila melanogaster. Barx1 and Barx2 also encode an identical stretch of 17 residues downstream of the homeobox; otherwise, they share no appreciable homology. In vitro, Barx2 stimulated activity of an L1 promoter construct containing the CCATTAGPyGA motif but repressed activity when this sequence was deleted. Localization studies showed that expression of Barx1 and Barx2 overlap in the nervous system, particularly in the telencephalon, spinal cord, and dorsal root ganglia. Barx2 was also prominently expressed in the floor plate and in Rathke’s pouch. During craniofacial development, Barx1 and Barx2 showed complementary patterns of expression: whereas Barx1 appeared in the mesenchyme of the mandibular and maxillary processes, Barx2 was observed in the ectodermal lining of these tissues. Intense expression of Barx2 was observed in small groups of cells undergoing tissue remodeling, such as ectodermal cells within indentations surrounding the eye and maxillo-nasal groove and in the first branchial pouch, lung buds, precartilagenous condensations, and mesenchyme of the limb. The localization data, combined with Barx2’s dual function as activator and repressor, suggest that Barx2 may differentially control the expression of L1 and other target genes during embryonic development.
Keywords: homeodomain, gene regulation, L1 promoter, cell adhesion molecules
Homeobox genes and encoded homeodomain proteins are key coordinators of gene activity during embryogenesis. Homeodomain proteins bind to ATTA-containing sequences and control the expression of particular target genes, the identities of which are largely unknown. Homeodomain binding sites (HBS) have been identified, however, within the promoters and introns of several genes for cell adhesion molecules (CAMs) (1–8). Such sequences have been shown to both activate and repress CAM gene promoter activity in vitro (2, 5). Moreover, HBSs in the N-CAM promoter have been shown to be required for the proper pattern of N-CAM gene expression during development of the spinal cord (9). CAMs are thus important targets to consider in linking homeobox gene activity to morphogenesis (10).
In recent studies of regulatory elements that restrict the expression of neural CAMs, we identified a motif designated Ng–wt that binds to the homeodomain of the Pax-3 protein (11). A sequence called L1-170, which is identical to Ng–wt, is found in the 5′ end of the gene for L1 (3), a CAM closely related to Ng–CAM in both structure and anatomical distribution (12, 13). L1-170 binds to the HoxA1 protein in vitro (6). The consensus sequence for Ng–wt and L1-170 is CCATTAGPyGA, a typical class I binding site (14–16) that is known to be recognized by homeodomain proteins of the extended Antennapedia family (17).
In an effort to identify homeodomain proteins that may regulate expression of Ng–CAM and L1, a mouse embryo expression library was screened with a DNA probe containing four copies of the CCATTAGPyGA sequence. This procedure revealed a new homeobox gene called Barx2, which is similar to Barx1 (18), and the Drosophila Bar genes (19–21). The dynamic expression pattern of Barx2 at sites of cell–cell interaction combined with its ability to both activate and repress the expression of a gene for a CAM support the suggestion that it may play a role in the differential regulation of gene expression in a variety of tissues during embryonic development.
MATERIALS AND METHODS
An embryonic day (E)11.5 mouse cDNA library in λgt11 (CL-1027b; Clontech) was screened with the Southwestern procedure (22) using a concatamer containing four copies of the Ng–wt sequence labeled by nick-translation with 32P-dCTP (DuPont/NEN). Screening yielded a single clone designated B1. Two other cDNA clones for Barx2, B2 and B3, were isolated via standard nucleic acid hybridization procedures (23) from CL-1027b and CL-3003b libraries, respectively (Clontech). The DNA sequences of both strands of B1, B2, and B3 were determined by the dideoxy chain termination method (24). Sequence comparisons were performed using the fasta (25) and blastn programs (26).
RNA blot analyses were performed as described (23). To prepare a Barx2 fusion protein, B3 was inserted into the pGex-1λT vector, and fusion protein was produced in Escherichia coli BL21 cells and purified by binding to and eluting from glutathione–Sepharose 4B beads, as described (23). A eukaryotic expression vector for Barx2 was prepared by inserting B3 into a modified pcDNA3 vector (Invitrogen) containing an amino-terminal N-myc tag. Expression of the Barx2 mRNA and protein from this vector (pcDNA3/Barx2) was confirmed using an in vitro transcription/translation system (Promega). The Barx2/N-myc tag fusion protein migrated at ≈33 kDa, a size consistent with its predicted size of 31 kDa. L1 promoter–luciferase constructs were prepared from mouse L1 genomic clones. L1-1 was prepared by fusing a 2945-bp segment of a 5′ flanking sequence and first exon of the L1 gene upstream of the luciferase gene in the pGL2 basic vector (Promega). L1-14 was prepared by inserting the luciferase gene downstream of the ATG codon in a 20-kb fragment of the L1 gene containing the first four exons. L1-14ΔHBS was prepared by site-directed deletion of the L1-170 motif (CCATTAGPyGA) within the L1-14 construct.
NIH 3T3 cells cultured in DMEM containing 10% newborn calf serum were cotransfected with 2.5 μg of DNA in 6 μl of lipofectamine and 1 ml of Opti-MEM medium (Life Technologies, Gaithersburg, MD). DNA mixtures for cotransfection experiments consisted of 1 μg of either pcDNA3 or pcDNA3/Barx2, 30–100 ng of luciferase reporter construct, and pBluescript II KS(+) as carrier. The RSV–β-galactosidase plasmid (0.5 μg) was cotransfected as an internal control to normalize transfection efficiencies. Cells were harvested after 48 h and resuspended in 150 μl of lysis buffer (0.1 M Tris acetate, pH 7.8/10 mM magnesium acetate/1 mM EDTA/1% Triton X-100/1 mM dithiothreitol). β-galactosidase activity was assayed using the Fluoreporter lacZ kit (Molecular Probes). Fluorescence was measured using the Millipore Cytofluor 2450 system. Luciferase activity was assayed by mixing 50 μl of cell lysate with 100 μl of substrate mixture containing 66 μM d-luciferin potassium, 2 mM ATP, 100 mM Tris·HCl, 10 mM magnesium acetate, and 1 mM EDTA and quantitated on an EG & G (Salem, MA) Berthold Microlumat LB96P luminometer.
For in situ hybridization, mouse embryos (E9.5–12.5) were fixed in 4% paraformaldehyde, infiltrated with 12, 18, and 24% sucrose, cryoprotected, and sectioned. DIG-labeled RNA probes for Barx1 and Barx2 were generated by in vitro transcription. For Barx1, a 267-bp segment of Barx1 cDNA outside of the homeobox (bases 505–772) (18) was generated by reverse transcriptase PCR and inserted into the pCRII vector. Sense and antisense Barx1 DIG-labeled RNAs were prepared using SP6 and T7 polymerases, respectively. For Barx2, two sets of antisense and sense RNA probes were generated using T3 and T7 RNA polymerase, respectively, from two different regions of the Barx2 cDNA. One set was derived from the 431-bp EcoRI–PstI fragment within the Barx2 coding region. The other set was derived from a 254-bp EcoRI fragment spanning from position 623 to the 3′ border of B1 (see Fig. 1). In situ hybridization was performed using sense and antisense DIG–RNA probes as described (27). Hybridized probes were detected with alkaline phosphatase-coupled anti-DIG antibody and were visualized with either nitroblue tetrazolium/5-bromo-4-chloro-3-indoylphosphate p-toluidine salt substrate or BM purple (Boehringer Mannheim).
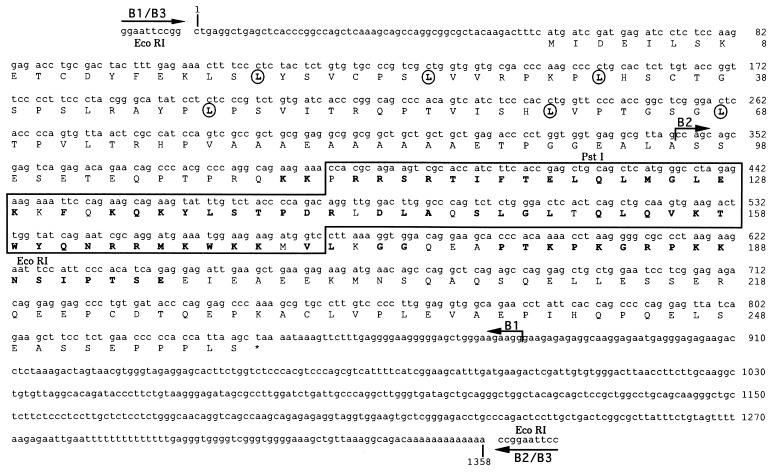
Figure 1.
DNA and encoded amino acid sequence of Barx2 cDNA clones. The positions at which B1, B2, and B3 initiate and terminate are indicated by arrows. The homeobox of Barx2 is highlighted within a box. Amino acids within the homeodomain and immediately carboxy-terminal to it that are shared with the mouse Barx1 gene product (18) are shown in boldface type. The leucine residues that define a putative leucine zipper are enclosed within circles. EcoRI and PstI restriction sites used to prepare probes for in situ hybridization analyses are indicated.
RESULTS
Isolation and Analyses of cDNA Clones, mRNAs, Genomic DNA, and Fusion Protein for Barx2.
The genes for related neural CAMs Ng–CAM and L1 share a common regulatory element with the sequence CCATTAGPyGA. To isolate homeodomain proteins that might recognize this element, we screened an E11.5 mouse cDNA library using a concatamer of CCATTAGPyGA in a Southwestern screening procedure (22). Fusion proteins from five cDNA clones selected were tested for their ability to bind either to a single copy of CCATTAGPyGA or to a variant called Ng–H containing three substitutions that disrupted the ATTA motif. Fusion protein from only one of these cDNA clones (B1) lost the ability to bind Ng–H, suggesting that the corresponding cDNA most likely encoded a protein with a homeodomain. The sequence of the B1 cDNA was determined, and database searches indicated that it was a novel homeobox gene, which we have named Barx2.
To extend the sequence of Barx2, two independent E11 mouse embryo cDNA libraries were screened with B1, and two other Barx2 cDNA clones, B2 and B3, were found (Fig. 1). B2 contained the sequence corresponding to the carboxy-terminal region of Barx2, a 516-bp segment of 3′ untranslated sequences, and a terminal poly-deoxyadenosine tail. B3, isolated from a different library, contained the sequences of both B1 and B2. The 5′ terminus of B3 matched that of B1, and the 3′ terminus of B3 matched that of B2 (Fig. 1), suggesting that B3 might represent a full length cDNA clone for Barx2. The total length of the Barx2 cDNA segment contained within B1, B2, and B3 was 1358-bp, encoding a protein segment of 258 amino acids. To confirm the reading frame derived from sequencing of cDNA clones for Barx2, a glutathione S-transferase–Barx2 fusion protein corresponding to the B3 cDNA was produced in E. coli and was analyzed by SDS/PAGE. A protein of ≈66 kDa was observed (data not shown). The size of this protein is in agreement with that predicted for a Barx2 fusion protein derived from the B3 insert.
In Northern blot analyses, Barx2 cDNA probes detected a single mRNA species of ≈1.7 kb in total RNA prepared from E12.5 embryos. Other analyses of RNA isolated from E7, E11, E15, and E17 embryos indicated that Barx2 was expressed primarily at E11 and still showed some expression at E15. In the adult, Barx2 mRNA was detected most intensely in the spleen. In addition, hybridization studies of mouse genomic DNA using two different Barx2 probes detected single bands in several genomic digests, indicating that Barx2 is encoded by a single gene in the mouse genome (data not shown).
Relationships of Barx2 with Other Homeobox Genes.
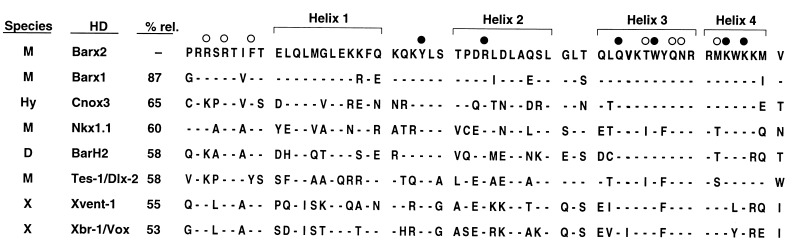
A comparison of the homeodomain encoded by Barx2 with other homeodomains (Fig. 2) revealed that it is 87% similar to the homeodomain encoded by the mouse Barx1 gene (18), 65% similar to the homeodomain encoded by the Cnox3 gene from hydra (Chlorohydra viridissima) (28), and 58% similar to homeodomains encoded by the dual Bar genes, BarH1 and BarH2 from Drosophila melanogaster (20, 21) and a homologous gene from Drosophila ananassae called Om(1D) (19). These members of the Bar family encode homeodomains that contain two atypical residues within helix 3: a threonine at position 47 and a tyrosine at position 49 (Fig. 2). In all other metazoan homeodomain proteins analyzed thus far, phenylalanine appears at position 49. Only two other homeobox genes, Xvent-1 and Xbr-1/Vox, encode a threonine at position 47; all others contain an isoleucine at this position. Comparison of the overall identity of the Barx2 homeodomain sequence to those encoded by Nkx1.1, Tes-1/Dlx-2, Xvent-1, and Xbr-1/Vox genes gave values of 60%, 58%, 55%, and 53%, respectively.
Figure 2.
Comparison of the Barx2 homeodomain with other homeodomains. The four α helices of the homeodomain are indicated by brackets. Based on crystallographic and NMR studies of homeodomain/DNA interactions (44, 45), black circles indicate residues of the Barx2 homeodomain that are likely to contact bases. Open circles indicate residues that make contacts with the sugar–phosphate backbone of DNA. D, Drosophila; H, human; Hy, hydra; M, mouse; X, Xenopus.
Immediately downstream of the homeobox, Barx2 and Barx1 share an additional region of similarity encoding a tract of 17 amino acids (PTKPKGRPKKNSIPTS) with a number of basic residues (Fig. 1). These residues are not found in other Bar class homeodomain proteins and may comprise a functional domain that is presently unique to Barx1 and Barx2 proteins. In Barx2, this basic region is followed by a number of acidic residues (residues 195–201) that are not found in the Barx1 protein. Residues 19–38 of the Barx2 protein contain several leucines that are spaced seven residues apart. Secondary structural analyses (29) suggest that this segment may contain a leucine zipper. A strongly basic hexapeptide (RQKKPR) found at the amino terminus of the homeodomain (residues 108–114) resembles a nuclear localization signal (30), and a polyalanine tract is found further upstream at residues 78–87.
Dual Function of Barx2 as Repressor and Activator.
To determine the possible role of Barx2 in the regulation of gene expression, a Barx2 expression vector was tested in cotransfection experiments of NIH 3T3 cells for its ability to control the expression of a luciferase reporter gene driven by the promoter and other 5′ regulatory sequences of the mouse L1 gene. As shown in Table 1, L1-1, a construct containing the L1 promoter (without the CCATTAGPyGA motif) showed a high level of promoter activity in NIH 3T3 cells cotransfected with pcDNA3. L1-14, a construct containing a 20-kb segment of the L1 gene including the promoter, the first four exons, and the CCATTAGPyGA motif, was ≈8-fold less active than L1-1. L1-14/ΔHBS, a construct similar to L1-14 but lacking the CCATTAGPyGA motif, was 2-fold more active than L1-14. These data indicate that sequences downstream of the promoter in the L1 gene that include the CCATTAGPyGA motif silence activity of the promoter in NIH 3T3 cells. In NIH 3T3 cells cotransfected with the pcDNA3/Barx2 expression vector, the activity of L1-1 was reduced 3.3-fold, L1-14 was increased 2.8-fold, and L1-14ΔHBS was reduced 2.2-fold compared with the activity of these constructs in cells transfected with the control vector, pcDNA3 (Table 1). These data indicate that, in cells cotransfected with Barx2, an L1 gene construct containing the CCATTAGPyGA is activated and that those lacking this motif are repressed. Thus, the CCATTAGPyGA motif is sufficient for activation of L1 gene expression by Barx2.
Table 1.
Barx2 regulation of L1 promoter activity
| Luciferase activity*
| |||
|---|---|---|---|
| Construct | pcDNA3 | pcDNA3-Barx2 | -fold |
| LI-1 | 19500 | 5851 | 3.3 ↓ |
| LI-14 | 2345 | 6455 | 2.8 ↑ |
| LI-14ΔHBS | 4913 | 2231 | 2.2 ↓ |
Values are expressed in raw light units and are derived from duplicate samples in four separate experiments in which the activities of constructs varied no more than 5%.
↓, repression; ↑, activation.
Comparison of the Expression Patterns of Barx1 and Barx2 During Development.
In a study using RNA probes derived from the homeobox, the Barx1 gene was shown to be expressed in craniofacial mesenchyme and in the stomach (18). To compare the expression patterns of Barx2 to Barx1, we performed in situ hybridization analyses of whole mounts and sections of mouse embryos staged between E9.5 and E12.5 using DIG-labeled RNA probes. The homeobox sequences of Barx1 and Barx2 are very similar, so we excluded them from the probes to avoid the possibility of cross-hybridization, which might confound comparisons of the expression patterns of these two genes. In control experiments, the Barx1 and Barx2 sense probes produced a low level of background hybridization (data not shown).
In whole mounts, at E9.5, Barx2 transcripts were restricted to the head, prominent in the region of the telencephalon and mesencephalon, and concentrated in cells along the dorsal midline (Fig. 3A). Expression of Barx1 overlapped with that of Barx2 at E9.5 but was more widespread laterally in craniofacial areas and appeared more caudally than Barx2 (Fig. 3E). At E10.5, expression of Barx2 was moderate in the most rostral region of the head but particularly prominent in a lateral band of cells in the periocular region (Fig. 3B) whereas expression of Barx1 continued to be intense throughout lateral and caudal regions of the head, particularly in the region of the diencephalon (Fig. 3F). Both genes showed diffuse expression in the limb mesenchyme. At E11.5, expression of Barx2 transcripts (Fig. 3C) became less intense but was detected in the telencephalon, frontonasal region, and limbs. In contrast, Barx1 showed persistent expression in the mesencephalon, diencephalon, telencephalon, and frontonasal regions (Fig. 3G). At E12.5, expression of Barx2 persisted in the telencephalon and hindbrain (Fig. 3D). At this stage, Barx1 was expressed to a lesser extent in the telencephalon but to a greater extent in the diencephalon (Fig. 3H).
Figure 3.
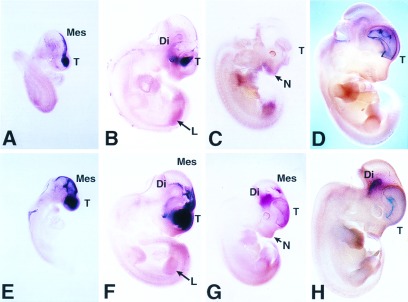
Whole mount in situ hybridization showing the distribution of Barx2 and Barx1 mRNAs during mouse development. (A-D) Pattern of Barx2 expression. (E-H) Barx1 expression. The embryos are staged as follows: E9.5 (A and E); E10.5 (B and F); E11.5 (C and G); E12.5 (D and H). T, telencephalon; Di, diencephalon; Mes, mesencephalon; N, nasal process; L, limb.
To examine the cellular distribution of Barx1 and Barx2 mRNA transcripts in more detail, we conducted in situ hybridization analyses of sections of mouse embryos between days 10.5 and 12.5 of embryonic development. Between E10.5 and E12.5, Barx2 was expressed in the ventricular zone and was more intense in the mantle layer of the telencephalon, mesencephalon, and hindbrain (Fig. 4A). Barx1 expression was also detected in the ventricular zone during development but did not show the intense expression in the mantle layer as did Barx2 (Fig. 4B). Barx2 showed intense expression in the floor plate of the midbrain (Fig. 4C), in Rathke’s pouch (Fig. 4D), and in the mantle layer of the spinal cord (Fig. 4G). Between E10.5 and E12.5, Barx2 was expressed prominently in a small group of cells forming ectodermal infoldings that surrounded the eyes (Fig. 4E, arrows). Barx1 did not show this restricted ectodermal pattern of expression in the eye (Fig. 4F). It was expressed, however, in nearby mesenchymal cells of the frontonasal region and in neural crest-derived tissues such as the trigeminal ganglion (Fig. 4F). Barx2 and Barx1 both were expressed in the spinal cord and in the dorsal root ganglia (Fig. 4 G and H). In marked contrast to the intense Barx1 expression observed in the surrounding mandibular mesenchyme (Fig. 4J), Barx2 was expressed in a complementary pattern within cells forming the ectodermal lining of the first branchial pouch (Fig. 4I). A restricted distribution of Barx2 was also observed in ectodermal cells forming the maxillo-nasal groove (data not shown). Barx2 expression also was observed in tissues undergoing epithelio–mesenchymal transformations such as the lung buds (Fig. 4K) and the precartilagenous condensations of the forelimb (Fig. 4L).
Figure 4.
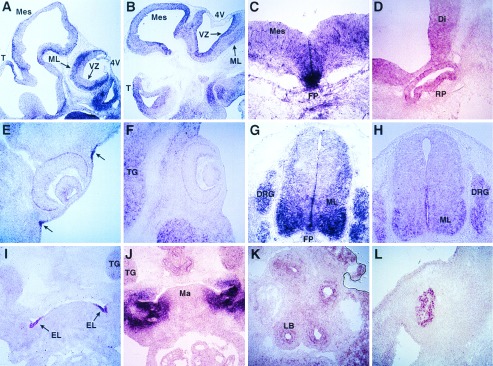
In situ hybridization showing the cellular distribution of Barx1 (B, F, H, and J) and Barx2 (A, C-E, G, I, K, and L) transcripts in tissue sections of mouse embryos staged at E10.5 (G-L), E11.5 (A, C, and D), and E12.5 (B, E, and F). T, telencephalon; Di, diencephalon; Mes, mesencephalon; 4V, fourth ventricle; FP, floor plate; VZ, ventricular zone; ML, mantle layer; TG, trigeminal ganglion; DRG, dorsal root ganglia; EL, ectodermal lining of the first branchial pouch; Ma, mandibular process.
DISCUSSION
We have isolated a new mouse homeobox gene called Barx2, a second member of the vertebrate Bar class of homeobox genes. Barx2 is most closely related to the Barx1 gene (18) that encodes a homeodomain that is 87% identical to that encoded by Barx2. Other genes encoding Bar class homeodomains include BarHI and BarH2 from Drosophila (19–21) and Cnox from the Cniderian Chlorohydra viridissima (28). Bar class homeodomains contain two atypical residues within helix 3: a threonine at position 47 and a tyrosine at position 49. Although the significance of these substitutions is unknown, given their location, it is tempting to speculate that they may influence the recognition of DNA target sites. These particular residues may be subject to posttranslational modifications such as phosphorylation, which may regulate the conformation of Bar class homeodomains and influence DNA binding.
Barx1 and Barx2 proteins also share a segment of 17 amino acids containing a number of basic residues not found in other homeoproteins. The basic residues and their proximity to the homeodomain suggest that this tract may engage in protein–protein interactions and regulate the function of the homeodomain, a possibility currently under investigation. The Barx2 protein also contains a putative leucine zipper and a polyalanine tract not found in Barx1. Polyalanine tracts function as repressor domains (31) and also appear in several other homeodomain proteins, including Evx-1 (32), HoxD13 (33), and Bicoid (34). The polyalanine tract of Bicoid has been shown to interact with a specific coactivator of RNA polymerase II, TATA box binding protein-associated factorII60 (35). Such interactions may allow homeodomain proteins containing polyalanine tracts to repress gene activity at the level of the basic transcription machinery.
The homeodomain encoded by Barx2 contains a glutamine residue at position 50. This residue, found in other homeodomain proteins of the extended Antennapedia family (17, 36), has been shown to be critical for determining DNA binding specificity. Antennapedia class homeodomains bind to type 1 and type 2 target sequences, both of which contain an ATTA motif (14–16). The CCATTAGPyGA motif from the Ng–CAM and L1 genes used to isolate Barx2 is a typical class I target sequence. In cellular cotransfection experiments, Barx2 activated L1 gene constructs containing the CCATTAGPyGA motif and repressed the activity of constructs lacking this sequence. These observations prompt the hypothesis that Barx2 (and possibly Barx1) may regulate expression of the L1 gene differentially. In cells that express L1, Barx2 may function as an activator, and in cells that do not normally express the L1 gene, Barx2 may act as a repressor. Pax-3, a homeodomain protein shown to bind to the CCATTAGPyGA motif in the Ng–CAM gene (11), has bifunctional properties similar to Barx2, and we have identified separate domains within Pax-3 that carry out activator and repressor functions (37). It will be of interest to define such domains within Barx2.
Our analyses of Barx1 expression in the present study were carried out with a probe that was derived from a 5′ region of the Barx1 cDNA and did not include homeobox sequences; the probe used by other workers (18) contained the homeobox. Although the expression pattern detected by our Barx1 probe was in agreement with those reported in previous studies (18), we also detected Barx1 mRNAs in the central nervous system. Our whole mount in situ hybridization studies indicated that Barx1 and Barx2 are expressed intensely in overlapping territories along both the rostral–caudal and medial–lateral axes of the head. Expression of Barx1 was found to be more widespread than Barx2 in both rostral and lateral regions of the head. Consistent with these findings, analyses of tissue sections showed that Barx1 was expressed prominently in craniofacial mesenchyme, whereas expression of Barx2 was limited to ectodermal borders of these tissues or to small groups of cells undergoing remodeling, such as the ectoderm of the periocular region, the maxillo-nasal groove, and the lining of the first branchial pouch. The extensive overlap in the expression patterns for these two genes and the extensive similarities of their homeodomains suggest that Barx1 and Barx2 may regulate similar target genes and contribute mutually to the patterning of neural and craniofacial tissues.
We conclude that Barx1 and Barx2 are expressed during development of both the central and peripheral nervous system. Both genes are expressed in the telencephalon, diencephalon, mesencephalon, hindbrain, and spinal cord and in cranial and dorsal root ganglia. Expression of Barx2 was most prominent in the mantle layer in which postmitotic neurons are located, in the floor plate, and in dorsal root ganglia. These sites of Barx2 expression overlap with those of L1 and Ng–CAM, as well as with other neural CAMs during embryogenesis (38, 39). These observations prompt the hypothesis that Barx1 and Barx2 may play a role in the regulation of genes for neural adhesion molecules such as Ng–CAM and L1.
It is interesting to note that Bar class and other homeodomain proteins most related to Barx1 and Barx2 (see Fig. 2) all are expressed during the development of anterior embryonic structures. For example, in Drosophila, BarH1, BarH2, and Om(1D) are expressed in photoreceptor cells R1 and R6 and in the maxilla (21). Cnox, a Bar class gene from hydra, is induced during head regeneration (28). Nkx1.1 and Tes-1/Dlx-2 are expressed in the rostral central nervous system (40, 41), Xvent-1 is expressed during gastrulation and plays a role in mesodermal cell fate (42), and Xbr-1/Vox participates in the establishment of dorso–ventral polarity in the retina (43). The correlation between the relatedness of the homeodomains in these proteins and the sites of their expression suggests that they may all regulate similar target genes. Further studies will be required to address this possibility.
Acknowledgments
We thank Brett Fenson and Madhu Katragadda for excellent technical assistance and Drs. K. Crossin, L. Krushel, and G. Miklos for critical reading of the manuscript. This work was supported by U.S. Public Health Service Grants NS34493 (F.S.J.) and HD33576 (G.M.E.) and a by grant from the G. Harold and Leila Y. Mathers Charitable Foundation (G.M.E). G.M.E. is a consultant to Becton Dickinson and Company.
ABBREVIATIONS
- CAM
cell adhesion molecule
- HBS
homeodomain binding sites
- DIG
digoxygenin
- E
embryonic day
Footnotes
References
- 1.Hirsch M-R, Gaugler L, Deagostini-Bazin H, Bally-Cuif L, Goridis C. Mol Cell Biol. 1990;10:1959–1968. doi: 10.1128/mcb.10.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones F S, Prediger E A, Bittner D A, De Robertis E M, Edelman G M. Proc Natl Acad Sci USA. 1992;89:2086–2090. doi: 10.1073/pnas.89.6.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohl A, Giese K P, Mohajeri M H, Montag D, Moos M, Schachner M. J Neurosci Res. 1992;32:167–177. doi: 10.1002/jnr.490320206. [DOI] [PubMed] [Google Scholar]
- 4.Jones F S, Holst B D, Minowa O, De Robertis E M, Edelman G M. Proc Natl Acad Sci USA. 1993;90:6557–6561. doi: 10.1073/pnas.90.14.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valarché I, Tissier-Seta J-P, Hirsch M-R, Martinez S, Goridis C, Brunet J-F. Development. 1993;119:881–896. doi: 10.1242/dev.119.3.881. [DOI] [PubMed] [Google Scholar]
- 6.Chalepakis G, Wijnholds J, Giese P, Schachner M, Gruss P. DNA Cell Biol. 1994;13:891–900. doi: 10.1089/dna.1994.13.891. [DOI] [PubMed] [Google Scholar]
- 7.Goomer R S, Holst B D, Wood I C, Jones F S, Edelman G M. Proc Natl Acad Sci USA. 1994;91:7985–7989. doi: 10.1073/pnas.91.17.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buttiglione M, Cangiano G, Goridis C, Gennarini G. Mol Brain Res. 1995;29:297–309. doi: 10.1016/0169-328x(94)00262-d. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Jones F S, Krushel L A, Edelman G M. Proc Natl Acad Sci USA. 1996;93:1892–1896. doi: 10.1073/pnas.93.5.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelman G M, Jones F S. J Biol Chem. 1993;268:20683–20686. [PubMed] [Google Scholar]
- 11.Kallunki P, Jenkinson S, Edelman G M, Jones F S. J Biol Chem. 1995;270:21291–21298. doi: 10.1074/jbc.270.36.21291. [DOI] [PubMed] [Google Scholar]
- 12.Moos M, Tacke R, Scherer H, Teplow D, Fruh K, Schachner M. Nature (London) 1988;334:701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- 13.Burgoon M P, Grumet M, Mauro V, Edelman G M, Cunningham B A. J Cell Biol. 1991;112:1017–1029. doi: 10.1083/jcb.112.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beachy P A, Krasnow M A, Gavis E R, Hogness D S. Cell. 1988;55:1069–1081. doi: 10.1016/0092-8674(88)90251-6. [DOI] [PubMed] [Google Scholar]
- 15.Hoey T, Levine M. Nature (London) 1988;332:858–864. doi: 10.1038/332858a0. [DOI] [PubMed] [Google Scholar]
- 16.Treisman J, Gonczy P, Vshishtha M, Harris E, Desplan C. Cell. 1989;59:553–562. doi: 10.1016/0092-8674(89)90038-x. [DOI] [PubMed] [Google Scholar]
- 17.Scott M P, Tamkun J W, Hartzell G W. Biochem Biophys Acta. 1989;989:25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- 18.Tissier-Seta J-P, Mucchielli M-L, Mark M, Mattei M-G, Goridis C, Brunet J-F. Mech Dev. 1995;51:3–15. doi: 10.1016/0925-4773(94)00343-l. [DOI] [PubMed] [Google Scholar]
- 19.Tanda S, Corces V G. EMBO J. 1991;10:407–417. doi: 10.1002/j.1460-2075.1991.tb07962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higashijima S-I, Kojima T, Michiue T, Ishimaru S, Emori Y, Saigo K. Genes Dev. 1992;6:50–60. doi: 10.1101/gad.6.1.50. [DOI] [PubMed] [Google Scholar]
- 21.Higashijima S-I, Michiue T, Emori Y, Saigo K. Genes Dev. 1992;6:1005–1018. doi: 10.1101/gad.6.6.1005. [DOI] [PubMed] [Google Scholar]
- 22.Vinson C R, LaMarco K L, Johnson P F, Landschulz W H, McKnight S L. Genes Dev. 1988;2:801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- 23.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1989. [Google Scholar]
- 24.Tabor S, Richardson C C. Proc Natl Acad Sci USA. 1987;84:4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson W R, Lipman D J. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 27.Bober E, Franz T, Arnold H-H, Gruss P, Tremblay P. Development. 1994;120:603–612. doi: 10.1242/dev.120.3.603. [DOI] [PubMed] [Google Scholar]
- 28.Schummer M, Scheurlen I, Schaller C, Galliot B. EMBO J. 1992;11:1815–1823. doi: 10.1002/j.1460-2075.1992.tb05233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou P Y, Fasman G D. Adv Enzymol Relat Areas Mol Biol. 1978;47:145–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- 30.Boulikas T. J Cell Biochem. 1994;55:32–58. doi: 10.1002/jcb.240550106. [DOI] [PubMed] [Google Scholar]
- 31.Hanna-Rose W, Hansen U. Trends Gen. 1996;12:229–234. doi: 10.1016/0168-9525(96)10022-6. [DOI] [PubMed] [Google Scholar]
- 32.Bastian H, Gruss P. EMBO J. 1990;9:1839–1852. doi: 10.1002/j.1460-2075.1990.tb08309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muragaki Y, Mundlos S, Upton J, Olsen B R. Science. 1996;272:548–551. doi: 10.1126/science.272.5261.548. [DOI] [PubMed] [Google Scholar]
- 34.Driever W, Nusslein-Volhard C. Nature (London) 1989;337:138–143. doi: 10.1038/337138a0. [DOI] [PubMed] [Google Scholar]
- 35.Sauer F, Hansen S K, Tjian R. Science. 1995;270:1825–1828. doi: 10.1126/science.270.5243.1825. [DOI] [PubMed] [Google Scholar]
- 36.Kappen C, Schughart K, Ruddle F H. Genomics. 1993;18:54–70. doi: 10.1006/geno.1993.1426. [DOI] [PubMed] [Google Scholar]
- 37.Chalepakis G, Jones F S, Edelman G M, Gruss P. Proc Natl Acad Sci USA. 1994;91:12745–12749. doi: 10.1073/pnas.91.26.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krushel L A, Prieto A L, Cunningham B A, Edelman G M. Neuroscience. 1993;53:797–812. doi: 10.1016/0306-4522(93)90625-p. [DOI] [PubMed] [Google Scholar]
- 39.Moscoso L M, Sanes J R. J Comp Neurol. 1995;352:321–334. doi: 10.1002/cne.903520302. [DOI] [PubMed] [Google Scholar]
- 40.Porteus M H, Brice A E J, Bulfone A, Usdin T B, Ciaranello R D, Rubenstein J L R. Mol Brain Res. 1992;12:7–22. doi: 10.1016/0169-328x(92)90063-h. [DOI] [PubMed] [Google Scholar]
- 41.Bulfone A, Puelles L, Porteus M H, Frohman M A, Martin G R, Rubenstein J L R. J Neurosci. 1993;13(7):3155–3172. doi: 10.1523/JNEUROSCI.13-07-03155.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gawantka V, Delius H, Hirschfeld K, Blumenstock C, Niehrs C. EMBO J. 1995;14:6268–6279. doi: 10.1002/j.1460-2075.1995.tb00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papalopulu N, Kintner C. Dev Biol. 1996;174:104–114. doi: 10.1006/dbio.1996.0055. [DOI] [PubMed] [Google Scholar]
- 44.Kissinger C R, Liu B, Martin-Blanco E, Kornberg T B, Pabo C O. Cell. 1990;63:579–590. doi: 10.1016/0092-8674(90)90453-l. [DOI] [PubMed] [Google Scholar]
- 45.Wolberger C, Vershon A K, Liu B, Johnson A D, Pabo C O. Cell. 1991;67:517–528. doi: 10.1016/0092-8674(91)90526-5. [DOI] [PubMed] [Google Scholar]