Abstract
Background and purpose:
Heparin is known to possess a range of activities, other than effects on blood coagulation, many of which are anti-inflammatory. Effects with potential anti-inflammatory applications include the inhibition of elastase release from neutrophils, as well as the adhesion of these cells to vascular endothelium. In the present study we aimed to investigate whether fractionation of heparin may yield molecules with enhanced or specific effects on human neutrophil function.
Experimental approach:
Fractions of defined molecular size were obtained from heparin by different methods and assessed for their effects on elastase release induced by formyl Met-Leu-Phe (fMLP), from neutrophils, in some cases following the priming of these cells with tumour necrosis factor-α (TNF-α). Effects of the fractions on neutrophil adhesion to interleukin-1β (IL-β)-stimulated human umbilical vein endothelial cells (HUVECs) were also examined.
Key results:
Elastase release was inhibited by very low molecular weight fractions of heparin, with an apparent minimum chain length of 10 saccharides required for full effect. In contrast, neutrophil-endothelial adhesion was unaffected by these fractionated heparins, suggesting that certain non-anticoagulant actions of heparin may be lost by such an approach.
Conclusions and implications:
These data suggest that an optimum chain length of heparin possibly exists for certain non-anticoagulant actions of heparin, which may prove to be useful in the design of novel drugs with specific anti-inflammatory actions.
Keywords: elastase, endothelium, heparin, inflammation, neutrophil
Introduction
Heparin has long been used as an anticoagulant and its properties, in this respect, are well understood. However, following early reports that heparin possessed effects apart from those on blood clotting (reviewed by Jaques, 1979), many of which involve modulation of aspects of the inflammatory process (reviewed by Lever and Page, 2002), there has been increasing interest in exploiting the non-anticoagulant pharmacology of heparin. However, the structural requirements for the majority of the anti-inflammatory effects of heparin are not well understood.
Among the anti-inflammatory effects of heparin described to date are its ability to affect leucocyte interactions with vascular endothelium, both in vitro (Bazzoni et al., 1992; Silvestro et al., 1994; Lever et al., 2000; Smailbegovic et al., 2001) and in vivo (Ley et al., 1991; Tangelder and Arfors, 1991; Xie et al., 1997; Salas et al., 2000) and to inhibit the accumulation of inflammatory cells in tissues that follows these events (Sasaki et al., 1993; Teixeira and Hellewell, 1993; Seeds et al., 1995; Yanaka et al., 1996; Vancheri et al., 2001; Seeds and Page, 2001). Furthermore, heparin can inhibit the activation of inflammatory cells, in addition to possessing the ability to bind and neutralize an array of mediators and enzymes released during an inflammatory response (see Tyrrell et al., 1999). One such cell-derived enzyme is neutrophil elastase, which is involved in the pathogenesis of a wide range of inflammatory diseases such as chronic obstructive pulmonary disease (COPD), cystic fibrosis and rheumatoid arthritis (see Doring, 1994). In addition to its ability to cleave structural proteins such as elastin and fibronectin, elastase binds the adhesion molecule Mac-1 (CD11b/CD18; Cai and Wright, 1996), important in neutrophil adhesion and transmigration and, furthermore, is able to cleave intercellular adhesion molecule-1 (ICAM-1) (Champagne et al., 1998), a ligand for Mac-1. Heparin is known to inhibit the enzymatic activity of elastase and has been found to limit the extent of airway damage in in vivo models of elastase-induced emphysema (Rao et al., 1990; Lafuma et al., 1991) and to confer benefit in patients with COPD when given in addition to standard drug therapy (Brown et al., 2006). We have demonstrated previously that heparin is able to inhibit the release of elastase from human neutrophils, in response to a range of stimuli, an effect that was found to be independent of its anticoagulant activity (Brown et al., 2003). In addition to the ability to neutralize elastase activity, inhibition of the release of this enzyme by heparin may in part underlie the positive effects seen in human disease and animal models of inflammation, following heparin administration. Importantly, an understanding of the structural basis for these properties could provide the basis for discovery of novel drugs, with application in the treatment of inflammatory diseases, which lack unwanted effects of the parent heparin molecule, such as anticoagulant activity.
A vast array of proteins is bound by heparin, many of which interact physiologically with the closely related glycosaminoglycan, heparan sulphate. Whereas the heparin binding domains of many proteins are known and, where not known, can be reliably predicted, the corresponding sequences within the heparin molecule that interact with these sites are yet to be elucidated for proteins implicated in the inflammatory response. Therefore, a rationale exists to determine structures within the parent heparin molecule that, if isolated, may possess useful properties as anti-inflammatory drugs. Such molecules might be designed around the ability of heparin to bind to specific proteins, as has been the case for the antithrombin binding pentasaccharide (fondaparinux), or around their ability to exert a particular and specific effect.
In the present study, we have assessed, through investigation of the effects of different size fractions of heparin, whether altering the length of the glycosaminoglycan chain affects the ability of heparin to inhibit inflammatory functions of human neutrophils, with a view to identifying the optimum molecular size for this activity. We have found that fractions of heparin, within a distinct molecular weight range, inhibit the release of elastase from human neutrophils, in some cases to a greater maximum extent than unfractionated heparin (UH) but lack the effects of the unfractionated drug on neutrophil–endothelial adhesion.
Methods
Preparation of heparin fractions
Heparin fractions were prepared from low molecular weight heparin (LMWH) by nitrous acid depolymerization at pH 1.5 (Stringer et al., 2003), alkaline β-eliminative cleavage (Mayo and Carlson, 1970) or digestion with heparinase I (from Flavobacterium heparinum; Goger et al., 2002), followed by high-resolution gel chromatography on Bio Gel P10 as described previously (Goger et al., 2002; Stringer et al., 2003).
Isolation of neutrophils
Peripheral venous blood was collected from healthy volunteers (n=6–8) into citrated tubes (1:10, v/v in acid citrate dextrose (ACD)), before being transferred to Accuspin tubes (containing Histopaque 1077) for centrifugation at 1000g for 10 min. The resultant plasma layer was carefully removed and mononuclear cells, contained within a distinct opaque layer, were carefully aspirated. Following removal of mononuclear cells, the remaining blood fraction was purified further to obtain neutrophil populations. The cell mixture found below the Histopaque layer was mixed with an equal volume of hydroxyethyl starch solution (Haes-steril), containing 18% phosphate-buffered saline and 2% ACD and centrifuged for 15 min at 20g to remove erythrocytes. The supernatant was further centrifuged (7 min, 175g) to collect the neutrophils and remaining red blood cells were lysed by inverting the pellet for 10 s in sterile water, before addition of an equal volume of 2 × modified Hank's balanced salts solution (HBSS, without Ca2+ or Mg2+) to restore tonicity. Cells were pelleted by centrifugation (700g, 1 min) and then washed a further two times in modified HBSS. Neutrophil populations isolated in this manner were found to be more than 98% viable and to contain at least 95% neutrophils, contaminating cells typically being eosinophils. Blood donors were recruited and samples obtained under the approval of the Research Ethics Committees of King's College London and Camden and Islington Primary Care Trust.
Elastase release assay
Neutrophils were resuspended in complete HBSS (with Ca2+ and Mg2+) at a density calculated to yield a final concentration of 2.5 × 106 cells ml−1, once all drugs and other reagents were present in the tube. The final volume in each tube was 250 μl.
Cells were added to tubes containing cytochalasin B (50 μg ml−1, 25 μl in HBSS) and heparins (ten times final concentration required, 25 μl in normal saline, or vehicle) and were incubated at room temperature for 30 min. All tubes, including unstimulated controls, received cytochalasin B, which enhances degranulation responses of neutrophils in vitro.
In some experiments, neutrophils were primed with tumour necrosis factor-α (TNF-α; 10 ng ml−1), before stimulation. This cytokine augments the neutrophil response to subsequent stimulation, without directly causing elastase release (Brown et al., 2003; Jones et al., 2005). In these instances, all tubes treated as above, including unstimulated controls, received 25 μl TNF-α (100 ng ml−1 in HBSS) and were incubated for a further 30 min. An identical array of tubes were treated with 25 μl HBSS for 30 min. Cells were stimulated by addition of 25 μl formyl-Met-Leu-Phe (fMLP; 10−6 M) or calcium ionophore (A23187; 3 × 10−7 M), or vehicle to unstimulated control tubes. Tubes were incubated for 45 min at room temperature before centrifugation to pellet neutrophils. Duplicate 25 μl samples of supernatant were transferred to 96-well plates and 150 μl Tris-buffered saline added to each well. Finally, the chromogenic substrate for human leucocyte elastase, N-methoxysuccinyl-ala-ala-pro-val-p-nitroanilide, was introduced to each well, in a volume of 20 μl (6 mM in 25% dimethylsulphoxide). Plates were incubated at room temperature before spectrophotometric analysis at 405 nm in a plate reader. In previous experiments, it was established that the presence of heparin in neutrophil supernatants had no effect on the colorimetric assay used to detect elastase (data not shown). Results are expressed as % control elastase release, that is elastase release from neutrophils stimulated in the absence of study drugs and corrected for basal elastase release/background absorbance.
Neutrophil–endothelial adhesion assay
Human umbilical vein endothelial cells (HUVECs) at passage five or below were cultured to confluency in large vessel endothelial cell growth medium, in the central 60 wells of flat-bottomed 96-well plates (200 μl culture medium per well; 5% CO2; 37°C). Monolayers were stimulated by the addition of 22 μl per well of a solution of interleukin-1β (IL-1β; 100 U ml−1; tenfold the final concentration required in the well), or vehicle, prepared in culture medium at tenfold the final concentration required in the well, for 6 h. A 6-h HUVEC stimulation period with IL-1β was selected for use in the study, based on our previous findings that these conditions produce a significant but submaximal neutrophil adhesion.
Leucocyte–endothelial adhesion was examined using an assay described previously (Lever et al., 2000; Smailbegovic et al., 2001). Briefly, HUVEC monolayers, stimulated with IL-1β (10 U ml−1), were washed with warmed HBSS before addition of 200 μl radiolabelled neutrophil suspension per well. Neutrophils, isolated as described above, were radiolabelled by incubation with aqueous sodium [51Cr]-chromate (37 kBq per 106 cells) for 60 min at room temperature. Excess radiolabel was removed by three washes in modified HBSS before cells were resuspended at a final concentration of 106 cells ml−1 in complete HBSS. Cell viability was unaffected by this process.
Radiolabelled neutrophils were incubated on HUVEC monolayers for 30 min (37°C), following which non-adherent cells were removed by gentle aspiration and washing with warmed HBSS. Wells were examined microscopically and, upon confirmation of monolayer integrity, adherent cells were lysed by addition of 200 μl detergent solution (1% Igepal) per well. One hundred microliter samples were transferred to scintillation vials and γ-counted alongside 100 μl samples of the original radiolabelled neutrophil suspension (input). The number of adherent cells was calculated as the percentage of input counts present in sample counts, corrected for background radioactivity. Heparins were added to monolayers immediately before the addition of neutrophils. Results were corrected for basal adhesion.
Data analysis
Results are shown as means±s.e.m. Differences between means were analysed by analysis of variance, followed by Dunnett's test. IC50 values were determined using GraphPad Prism version 4. Values of P<0.05 were taken to indicate significant differences between means.
Materials and reagents
Unless stated otherwise, all reagents were obtained from Sigma-Aldrich Ltd, Poole, Dorset, UK. Haes-steril was obtained from Fresenius-Kabi Ltd, Warrington, UK; HUVECs and culture media were from TCS Cellworks Ltd, Botolph Claydon, Buckingham, UK; TNF-α and IL-1β were from R&D Sytems Ltd, Abingdon, Oxon, UK; and sodium 51[Cr]-chromate was from Amersham Life Sciences Ltd, Chalfont St Giles, UK.
Results
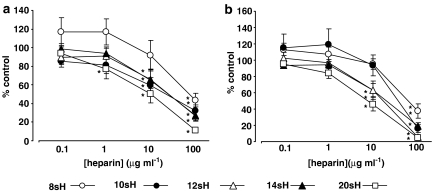
Heparin fractions of defined size (8, 10, 12, 14 and 20 saccharides; 8sH, 10sH, etc., respectively), derived from LMWH by heparinase I digestion, were found to inhibit fMLP-induced elastase release both in the absence of (Figure 1a) and following (Figure 1b) priming with TNF-α. However, inhibitory activity was lost as molecular weight decreases (Table 1); 4- 6- or 8-saccharide heparins having effect only at the highest concentration tested (100 μg ml−1), whereas larger fractions inhibited elastase release at lower concentrations. Interestingly, some of these larger fractions had a greater maximum inhibitory effect on elastase release than the LMWH from which they were prepared (Table 1). Fractions of heparin derived by nitrous acid depolymerization and β-eliminative cleavage, of 4- 6- and 14-saccharides, did not differ in their effect from those of equivalent size obtained by heparinase I cleavage (data not shown), suggesting that the method of preparation does not influence the activity of the fractions obtained.
Figure 1.
Effects of heparinase-derived LMWH fractions on fMLP-induced elastase release from (a) unprimed and (b) TNF-α-primed neutrophils. Data are expressed as % of control elastase release (fMLP; 10−7 M), without heparin in (a) saline- and (b) TNF-α (10 ng ml−1)-treated cells, respectively. Values were corrected for basal release under the relevant conditions and represent the mean±s.e.m of experiments with cells from six separate donors, each performed in duplicate. Statistical significance (P<0.05) is indicated (*) from control release (100%). fMLP, formyl-Met-Leu-Phe; LMWH, low molecular weight heparin.
Table 1.
Inhibition of elastase release by a range of heparinase-derived fractions of heparin, with respect to molecular size, the LMWH from which they were derived and UH
|
fMLP-treated cells |
TNFα+fMLP-treated cells |
|||
|---|---|---|---|---|
| Number of saccharides | Inhibition at 100 μg ml−1 (%) | IC50 (μg ml−1) | Inhibition at 100 μg ml−1 (%) | IC50 (μg ml−1) |
| 4 | 39.2±0.5* | — | 32.5±4.4* | — |
| 6 | 44.4±4.1* | — | 41.7±3.1* | — |
| 8 | 56.3±7.6* | 81.8±1.5 | 62.6±8.8* | 71.9±1.3 |
| 10 | 68.1±6.4* | 20.6±1.6 | 84.2±4.9* | 42.0±1.9 |
| 12 | 73.4±5.8* | 23.9±1.5 | 94.7±2.9* | 14.4±1.3 |
| 14 | 72.5±5.7* | 25.4±1.4 | 81.1±4.7* | 18.7±1.2 |
| 20 | 88.5±3.0* | 7.9±1.5 | 95.3±3.1* | 7.5±1.3 |
| LMWH | 65.6±1.3* | 16.2±1.4 | 63.8±13.5* | 11.7±2.2 |
| UH | 63.3±4.4* | 18.3±3.6 | 66.4±5.5* | 5.6±1.7 |
Abbreviations: fMLP, formyl-Met-Leu-Phe; LMWH, low molecular weight heparin; TNF-α, tumour necrosis factor-α; UH, unfractionated heparin.
Data are shown for inhibition of neutrophil elastase release, when induced by fMLP alone (10−7 M; first columns) and fMLP (10−7 M) following priming with TNF-α (10 ng ml−1; second column).
P<0.05.
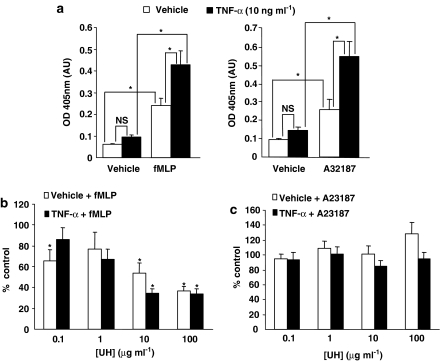
Given that the elastase release observed, following TNF-α priming, was approximately twice that seen without this pretreatment of neutrophils (Figure 2a), yet the degree of inhibition by each heparin species tested was similar, irrespective of whether or not the cells were primed, we were interested to see whether the TNF-α-induced component of elastase release in our assays is inhibited directly by heparin. UH was found to have no effect on elastase release, regardless of assay conditions, when the calcium ionophore, A23187, was used in place of fMLP (Figure 2c), despite the fact that TNF-α pretreatment had similar effect on the A23187 response as on that to fMLP (Figure 2a). In contrast, however, UH inhibited fMLP-induced elastase release, again, to a similar extent both with and without TNF-α priming (Figure 2b). Furthermore, the lack of effect of heparin on A23187-induced elastase release further confirms that the inhibitory effects seen when fMLP is used as the stimulus are not due to heparin interfering in any way with the detection of elastase.
Figure 2.
Effects of UH on fMLP and A23187-induced neutrophil elastase release. (a) A23187 (3 × 10−8 M) and fMLP (10−7 M) elicited a similar level of elastase release from neutrophils, both with and without prior priming with TNF-α (10 ng ml−1). UH inhibits this release of elastase (b) in response to fMLP (10−7 M) but (c) not in response to A23187 (3 × 10−8 M). Data are expressed as % of control elastase release (fMLP or A23187 without heparin, primed or unprimed cells as appropriate), corrected for basal release under the relevant conditions and represent the mean±s.e.m of experiments with cells from 6 to 8 separate donors, each performed in duplicate. Statistical significance (P<0.05) is indicated (*) from control release (100%). fMLP, formyl-Met-Leu-Phe; UH, unfractionated heparin.
Interestingly, over the same concentration range found to inhibit elastase release, heparin fractions of 4- to 14-saccharides had no effect on the adhesion of neutrophils to endothelial cells that had been stimulated with IL-1β. This is in contrast to the effects of UH, which inhibits both neutrophil adhesion (Lever et al., 2000; Smailbegovic et al., 2001) and degranulation (Brown et al., 2003). The LMWH, from which the defined-length fractions were obtained, inhibited elastase release (Table 1) and also had a weak inhibitory effect on neutrophil adhesion (maximum inhibition 30.0±6.3%; 100 μg ml−1; P<0.05: basal adhesion was 6.7±1.7% input, increasing to 17±0.9% input after IL-β stimulation). Although not significant, there was a trend among all of the defined-length heparin fractions examined to increase neutrophil adhesion at lower concentrations. It is unclear whether this reflects a real effect, which could possibly relate to inhibition of elastase released by adhering neutrophils and further investigation may be warranted. However, in the present study, the fact that fractionation differentially affects the inhibitory actions of heparin on neutrophil function is, we believe, of interest.
Discussion and conclusions
The results of this study demonstrate that our previous observations, that commercially available, unfractionated and LMW heparins inhibit neutrophil elastase release, extend to very LMWH preparations. Furthermore, it would appear that some loss of this activity occurs as the length of the heparin chain is reduced. A number of physiological ligands for heparin and its chemical analogue, heparan sulphate, display optimal binding to sequences of 12–14 sugar units. For example, the heparin binding site in the hepII domain of fibronectin accommodates a 14-saccharide sequence (Walker and Gallagher, 1996; Lyon et al., 2000) and the high affinity binding sites in heparan sulphate for transforming growth factor-β (Lyon et al., 1997) and basic fibroblast growth factors (FGF) are 12–14 sugars in length (Turnbull et al., 1992; Gallagher, 1998). In the case of the FGF family, the long sequences bind two growth factor molecules, which then engage and dimerize cognate signalling receptors (Walker et al., 1994; Pellegrini, 2001). Thus, the finding in this study, that heparin structures in this range are required for inhibition of neutrophil elastase release, is compatible with the effect being mediated by a distinct protein target, such as a cytokine or membrane receptor.
Furthermore, these results suggest, firstly, a possible physiological role for heparin/heparan sulphate in modulation of neutrophil activity and, secondly, that design of structures holding potential therapeutic applications in the treatment of inflammation could be based on templates in this size range. In order to examine further the possibility that heparin may inhibit elastase release in our assays by interacting with the cytokine TNF-α, used as a priming agent in the study described, we carried out further experiments to investigate the effects of heparin (unfractionated in this case), on A23187-induced elastase release. Pretreatment of neutrophils with TNF-α leads to an increase in the elastase release that is seen in response to subsequent stimulation with agents such as fMLP or platelet activating factor (PAF; Brown et al., 2003). Heparin inhibits the elastase release induced by these agents, to a similar extent, whether or not the cells have been primed with TNF-α, demonstrating firstly that heparin inhibits the response to fMLP/PAF but suggesting also that the effects of TNF-α on neutrophils may be inhibited. However, while the heparin binding sites of numerous proteins have been successfully modelled, TNF-α, to date, has neither been found nor predicted to contain a heparin binding site.
The calcium ionophore, A23187, induces degranulation of neutrophils in a manner that is not inhibited by heparin (Laghi-Pasini et al., 1983). Therefore, we chose to use this agonist in the present study in lieu of fMLP, both with and without prior TNF-α priming, in an attempt to dissect out the TNF-α-induced element of the elastase release observed in our assays. TNF-α priming increased the elastase release obtained in response to treatment with A23187 but heparin had no effect on this release nor, in accordance with earlier reports (Laghi-Pasini et al., 1983), on that seen in response to treatment with A23187 alone. These data would suggest that heparin inhibits neutrophil degranulation and, hence, elastase release through a mechanism downstream of TNF-α priming, an important pro-inflammatory mechanism of clear relevance to neutrophil-driven inflammatory diseases (Oudijk et al., 2005). One potential site for contact between TNF-α and the neutrophil is in the microvasculature, given that activated endothelial cells produce TNF-α (Ranta et al., 1999; Albaugh et al., 2001) and that neutrophils must interact with this cell layer in order to subsequently migrate to the inflamed tissue. The fact that heparin, in vitro at least, inhibits the augmented release of elastase from TNF-α-primed neutrophils, without direct effect on the priming event itself, suggests that heparin may be useful in limiting the release of tissue-destructive granule components at sites of their release, such as the lung. Coupled with our data showing that very low molecular weight fractions of heparin have no effect on neutrophil–endothelial adhesion but retain the ability to inhibit elastase release, this observation suggests that fractions of heparin may possess useful anti-inflammatory effects when administered locally to tissues, thus avoiding problems potentially associated with systemic delivery. Furthermore, unfractionated and LMW heparin preparations are comprised of a heterogenous mixture of heparin molecules with a range of molecular weights. If a specific molecular weight, or weight range, is required for a given activity it would follow that this activity would be reduced by the presence of molecules outside that range. The defined length heparins used in this study, although each containing only molecules of the size specified, are not necessarily identical with respect to molecular composition. In the heparins used in the present study, the average sulphation was 2.4 sulphate groups per disaccharide and the principal disaccharide unit (75% of total) was IdoA,2S – GlcNS,6S. Thus, although any sequence present is likely to be dominated by this component, it remains possible that a specific saccharide sequence in the heparin molecule might be responsible for the activity we have observed. This said, the three different methods of preparation of the heparin fractions did not yield products that differed from one another with respect to the activities we tested. However, whereas treatment of heparin with nitrous acid at pH 1.5 leads to selective deaminative cleavage at N-sulphated glucosamine residues, digestion of heparin by the enzyme heparinase I leads to breakage of (1 → 4) glucosaminido–iduronic linkages, involved in the region responsible for binding of basic FGF (Turnbull et al., 1992; Whitelock et al., 1996), demonstrating that certain biological functions of heparin can indeed be removed by specific scission and subsequent fractionation.
Over the same range of concentrations that the defined length heparins inhibited neutrophil elastase release, none of the materials inhibited the adhesion of neutrophils to cytokine-stimulated endothelial cells. It is of interest that whereas UH inhibits both neutrophil adhesion and neutrophil elastase release, upon fractionation to small, defined-length polymers, only one of these activities is lost. While it should not be overlooked that the static adhesion assay used in our study does not take into account any potential modulation of selectin-mediated neutrophil rolling and any effects would most likely reflect binding of CD11b/CD18 (Mac-1) by heparin (Diamond et al., 1995), our observation does nonetheless support the notion that different actions of heparin might be separable from one another through structural manipulation. Such effects of UH on neutrophil–endothelial adhesion appear to be diminished or removed by fractionation although, in contrast, inhibition of neutrophil degranulation is retained by smaller fractions of the parent molecule. However, it would appear that an optimum chain length of heparin exists for this latter effect. Our data suggest that to retain the potency of standard UH, with regard to inhibition of elastase release, a minimum number of saccharides per molecule is required. Interestingly, the binding of platelet factor 4 (PF4), a property of heparin that underlies one of the most serious side effects associated with this drug (heparin-induced thrombocytopaenia), has been reported to correlate with chain length, with eight saccharides being the minimum length required for binding (Maccarana and Lindahl, 1993). However, high affinity binding to PF4 requires a saccharide of approximately 30 sugar units, this long structure being needed to engage binding sites on opposite faces of the PF4 tetramer (Stringer and Gallagher, 1997). Whereas the heparins found to be most active in inhibiting elastase release in our experiments contain more than eight saccharides, it is likely that interactions with PF4, or other proteins, will be significantly reduced in the case of these preparations, compared to those seen with standard unfractionated or LMW heparins. This clearly requires further investigation, along with the exact mechanism by which elastase release is inhibited by heparin fractions. A greater understanding of the latter may ultimately allow the protein binding characteristics of heparin preparations to be manipulated in order to maximize those that may be therapeutically useful while reducing those that are unwanted, as has been described with respect to the anticoagulant actions of this drug (Petitou et al., 1999). The results of these studies may prove to be important in the design of novel drugs, based on the heparin template, with specific anti-inflammatory actions.
Abbreviations
- ACD
acid citrate dextrose
- COPD
chronic obstructive pulmonary disease
- FGF
fibroblast growth factor
- fMLP
formyl-Met-Leu-Phe
- HBSS
Hank's balanced salts solution
- HUVEC
human umbilical vein endothelial cell
- ICAM-1
intercellular adhesion molecule-1
- IL-1β
interleukin-1β
- LMWH
low molecular weight heparin
- PAF
platelet activating factor
- PF4
platelet factor 4
- TNF-α
tumour necrosis factor-α
- UH
unfractionated heparin
Conflict of interest
The authors state no conflict of interest.
References
- Albaugh G, Kann B, Strande L, Vemulapalli P, Hewitt C, Alexander JB. Nicotine induces endothelial TNF-α expression, which mediates growth retardation in vitro. J Surg Res. 2001;99:381–384. doi: 10.1006/jsre.2001.6215. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Nuñez AB, Mascellani G, Bianchini P, Dejana E, Del Maschio A. Effect of heparin, dermatan sulfate, and related oligo-derivatives on human polymorphonuclear leukocyte functions. J Lab Clin Med. 1992;121:268–275. [PubMed] [Google Scholar]
- Brown RA, Allegra L, Matera MG, Page CP, Cazzola M. Additional clinical benefit of enoxaparin in COPD patients receiving salmeterol and fluticasone propionate in combination. Pulm Pharmacol Ther. 2006;19:419–424. doi: 10.1016/j.pupt.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lever R, Jones NA, Page CP. Effects of heparin and related molecules upon neutrophil aggregation and elastase release in vitro. Br J Pharmacol. 2003;139:845–853. doi: 10.1038/sj.bjp.0705291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai TQ, Wright SD. Human leukocyte elastase is an endogenous ligand for the integrin CR3 (CD11b/CD18, Mac-1, alpha M beta 2) and modulates polymorphonuclear leukocyte adhesion. J Exp Med. 1996;184:1213–1223. doi: 10.1084/jem.184.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne B, Tremblay P, Cantin A, St Pierre Y. Proteolytic cleavage of ICAM-1 by human neutrophil elastase. J Immunol. 1998;161:6398–6405. [PubMed] [Google Scholar]
- Diamond MS, Alon R, Parkos CA, Quinn MT, Springer TA. Heparin is an adhesive ligand for the leukocyte integrin Mac-1 (CD11b/CD18) J Cell Biol. 1995;130:1473–1482. doi: 10.1083/jcb.130.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doring G. The role of neutrophil elastase in chronic inflammation. Am J Respir Crit Care Med. 1994;150:S114–S117. doi: 10.1164/ajrccm/150.6_Pt_2.S114. [DOI] [PubMed] [Google Scholar]
- Gallagher JT. The interaction and regulation of basic and acidic fibroblast growth factors by heparan sulphate. Trends Glycosci Glycotechnol. 1998;10:137–144. [Google Scholar]
- Goger B, Halden Y, Rek A, Mosl R, Pye D, Gallagher J, et al. Different affinities of glycosaminoglycan oligosaccharides for monomeric and dimeric interleukin-8: a model for chemokine regulation at inflammatory sites. Biochemistry. 2002;41:1640–1646. doi: 10.1021/bi011944j. [DOI] [PubMed] [Google Scholar]
- Jaques LB. Heparins – anionic polyelectrolyte drugs. Pharmacol Rev. 1979;31:99–167. [PubMed] [Google Scholar]
- Jones NA, Boswell-Smith V, Lever R, Page CP. The effect of selective phosphodiesterase isoenzyme inhibition on neutrophil function in vitro. Pulm Pharmacol Ther. 2005;18:93–101. doi: 10.1016/j.pupt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Lafuma C, Frisdal E, Harf A, Robert L, Hornebeck W. Prevention of leucocyte elastase-induced emphysema in mice by heparin fragments. Eur Respir J. 1991;8:1004–1009. [PubMed] [Google Scholar]
- Laghi-Pasini F, Pasqui AL, Ceccatelli L, Di Perri T. Heparin inhibits FMLP and Con-A dependent activation of human polymorphonuclear leucocytes in vitro. Int J Tissue React. 1983;5:145–151. [PubMed] [Google Scholar]
- Lever R, Hoult JRS, Page CP. The effects of heparin and related molecules upon the adhesion of polymorphonuclear leucocytes to vascular endothelium in vitro. Br J Pharmacol. 2000;129:533–540. doi: 10.1038/sj.bjp.0703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever R, Page CP. Novel drug development opportunities for heparin. Nat Rev Drug Discov. 2002;1:140–148. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- Ley K, Cerrito M, Arfors K-E. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol. 1991;260:H1667–H1673. doi: 10.1152/ajpheart.1991.260.5.H1667. [DOI] [PubMed] [Google Scholar]
- Lyon M, Rushton G, Askari JA, Humphries MJ, Gallagher JT. Elucidation of the structural features of heparan sulfate important for interaction with the Hep-2 domain of fibronectin. J Biol Chem. 2000;275:4599–4606. doi: 10.1074/jbc.275.7.4599. [DOI] [PubMed] [Google Scholar]
- Lyon M, Rushton G, Gallagher JT. The interaction of the transforming growth factor-βs with heparin/heparan sulphate is isoform specific. J Biol Chem. 1997;272:18000–18006. doi: 10.1074/jbc.272.29.18000. [DOI] [PubMed] [Google Scholar]
- Maccarana M, Lindahl U. Mode of interaction between platelet factor 4 and heparin. Glycobiology. 1993;3:271–277. doi: 10.1093/glycob/3.3.271. [DOI] [PubMed] [Google Scholar]
- Mayo JW, Carlson DM. Effect of alkali and sodium borohydride at alkaline pH on N-acetylchondrosine: reduction versus cleavage. Carbohydr Res. 1970;15:300–303. [Google Scholar]
- Oudijk EJ, Nijhuis EH, Zwank MD, van de Graaf EA, Mager HJ, Coffer PJ, et al. Systemic inflammation in COPD visualised by gene profiling in peripheral blood neutrophils. Thorax. 2005;60:538–544. doi: 10.1136/thx.2004.034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L. Role of heparan sulphate in fibroblast growth factor signalling: a structural view. Curr Opin Struct Biol. 2001;11:629–634. doi: 10.1016/s0959-440x(00)00258-x. [DOI] [PubMed] [Google Scholar]
- Petitou M, Herault J-P, Bernat A, Driguez P-A, Duchaussoy P, Lormeau J-C, et al. Synthesis of thrombin-inhibiting heparin mimetics without side effects. Nature. 1999;398:417–422. doi: 10.1038/18877. [DOI] [PubMed] [Google Scholar]
- Ranta V, Orpana A, Carpen O, Turpeinen U, Ylikorkala O, Viinikka L. Human vascular endothelial cells produce tumor necrosis factor-alpha in response to proinflammatory cytokine stimulation. Crit Care Med. 1999;27:2184–2187. doi: 10.1097/00003246-199910000-00019. [DOI] [PubMed] [Google Scholar]
- Rao NV, Kennedy TP, Rao G, Ky N, Hoidal JR. Sulfated polysaccharides prevent human leukocyte elastase-induced acute lung injury and emphysema in hamsters. Am Rev Respir Dis. 1990;2:407–412. doi: 10.1164/ajrccm/142.2.407. [DOI] [PubMed] [Google Scholar]
- Salas A, Sans M, Soriano A, Reverter JC, Anderson DC, Pique JM, et al. Heparin attenuates TNF-alpha induced inflammatory response through a CD11b dependent mechanism. Gut. 2000;47:88–96. doi: 10.1136/gut.47.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Herd CM, Page CP. Effect of heparin and a low-molecular weight heparinoid on PAF-induced airway responses in neonatally immunized rabbits. Br J Pharmacol. 1993;110:107–112. doi: 10.1111/j.1476-5381.1993.tb13778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds EAM, Horne AP, Tyrrell DJ, Page CP. The effect of inhaled heparin and related glycosaminoglycans on allergen-induced eosinophil infiltration in guinea-pigs. Pulm Pharmacol. 1995;8:97–105. doi: 10.1006/pulp.1995.1012. [DOI] [PubMed] [Google Scholar]
- Seeds EAM, Page CP. Heparin inhibits allergen-induced eosinophil infiltration into guinea-pig lung via a mechanism unrelated to its anticoagulant activity. Pulm Pharmacol Ther. 2001;14:111–119. doi: 10.1006/pupt.2000.0277. [DOI] [PubMed] [Google Scholar]
- Silvestro L, Viano I, Macario M, Colangelo D, Montrucchio G, Panico S, et al. Effects of heparin and its desulfated derivatives on leukocyte–endothelial adhesion. Sem Thromb Haemost. 1994;20:254–258. doi: 10.1055/s-2007-1001910. [DOI] [PubMed] [Google Scholar]
- Smailbegovic A, Lever R, Page CP. The effects of heparin on the adhesion of human peripheral blood mononuclear cells to human stimulated umbilical vein endothelial cells. Br J Pharmacol. 2001;134:827–836. doi: 10.1038/sj.bjp.0704321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer SE, Gallagher JT. Specific binding of the chemokine platelet factor 4 to heparan sulphate. J Biol Chem. 1997;272:20508–20514. doi: 10.1074/jbc.272.33.20508. [DOI] [PubMed] [Google Scholar]
- Stringer SE, Kandola BS, Pye DA, Gallagher JT. Heparin sequencing. Glycobiology. 2003;13:97–107. doi: 10.1093/glycob/cwg006. [DOI] [PubMed] [Google Scholar]
- Tangelder GJ, Arfors K-E. Inhibition of leukocyte rolling in venules by protamine and sulfated polysaccharides. Blood. 1991;7:1565–1571. [PubMed] [Google Scholar]
- Teixeira MM, Hellewell PG. Suppression by intradermal administration of heparin of eosinophil accumulation but not oedema formation in inflammatory reactions in guinea-pig skin. Br J Pharmacol. 1993;110:1496–1500. doi: 10.1111/j.1476-5381.1993.tb13991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull JE, Fernig DE, Ke Y, Wilkinson MC, Gallagher JT. Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulphate. J Biol Chem. 1992;267:10337–10341. [PubMed] [Google Scholar]
- Tyrrell DJ, Horne AP, Holme KR, Preuss JM, Page CP. Heparin in inflammation: potential therapeutic applications beyond anticoagulation. Adv Pharmacol. 1999;46:151–208. doi: 10.1016/s1054-3589(08)60471-8. [DOI] [PubMed] [Google Scholar]
- Vancheri C, Mastruzzo C, Armato F, Tomaselli V, Magri S, Pistorio MP, et al. Intranasal heparin reduces eosinophil recruitment after nasal allergen challenge in patients with allergic rhinitis. J Allergy Clin Immunol. 2001;108:703–708. doi: 10.1067/mai.2001.118785. [DOI] [PubMed] [Google Scholar]
- Walker A, Gallagher JT. Stuctural domains of heparan sulphate for specific recognition of the C-terminal heparin-binding domain of human plasma fibronectin (HepII) Biochem J. 1996;317:871–877. doi: 10.1042/bj3170871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A, Turnbull JE, Gallagher JT. Specific heparan sulphate saccharides mediate the activity of basic fibroblast growth factor. J Biol Chem. 1994;269:931–935. [PubMed] [Google Scholar]
- Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin and heparanases. J Biol Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- Xie X, Thorlacius H, Raud J, Hedqvist P, Lindbom L. Inhibitory effect of locally administered heparin on leukocyte rolling and chemoattractant-induced firm adhesion in rat mesenteric venules in vivo. Br J Pharmacol. 1997;122:906–910. doi: 10.1038/sj.bjp.0701454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaka K, Nose T, Hindman BJ. Heparin ameliorates brain injury by inhibiting leukocyte accumulation. Stroke. 1996;27:2146–2147. [PubMed] [Google Scholar]