Abstract
Background and purpose:
In this work, a neuroactive peptide from the venom of the neotropical wasp Polybia occidentalis was isolated and its anti-nociceptive effects were characterized in well-established pain induction models.
Experimental approach:
Wasp venom was analysed by reverse-phase HPLC and fractions screened for anti-nociceptive activity. The structure of the most active fraction was identified by electron-spray mass spectrometry (ESI-MS/MS) and it was further assessed in two tests of anti-nociceptive activity in rats: the hot plate and tail flick tests.
Key Results:
The most active fraction contained a peptide whose structure was Arg-Pro-Pro-Gly-Phe-Thr-Pro-Phe-Arg-OH, which corresponds to that of Thr6-BK, a bradykinin analogue. This peptide was given by i.c.v. injection to rats. In the tail flick test, Thr6-BK induced anti-nociceptive effects, approximately twice as potent as either morphine or bradykinin also given i.c.v. The anti-nociceptive activity of Thr6-BK peaked at 30 min after injection and persisted for 2 h, longer than bradykinin. The primary mode of action of Thr6-BK involved the activation of B2 bradykinin receptors, as anti-nociceptive effects of Thr6-BK were antagonized by a selective B2 receptor antagonist.
Conclusions and implications:
Our data indicate that Thr6-BK acts through B2 bradykinin receptors in the mammalian CNS, evoking antinociceptive behaviour. This activity is remarkably different from that of bradykinin, despite the structural similarities between both peptides. In addition, due to the increased metabolic stability of Thr6-BK, relative to that of bradykinin, this peptide could provide a novel tool in the investigation of kinin pathways involved with pain.
Keywords: wasp venom, neuroactive peptides, Thr6-bradykinin, bradykinin, anti-nociception, B2 receptor
Introduction
Since the characterization of bradykinin (BK) in 1949, kinins have been implicated in a wide range of physiological processes, including vascular permeability, control of blood pressure and pain (Rocha e Silva et al., 1949; Bhoola et al., 1992; Leeb-Lundberg et al., 2005). Moreover, BK and related peptides exert potent algesic effects by stimulation of the peripheral nervous system (Calixto et al., 2000; Marceau and Regoli, 2004).
In the central nervous system (CNS), BK given into the lateral ventricles or other brain sites, may induce a variety of effects, such as sedation, catatonia, noradrenaline depletion, hyperthermia, hypertension, antidiuretic hormone release and anti-aversive effects (Graeff et al., 1971; da Silva and Rocha e Silva, 1971; Pelá et al., 1996; Couto et al., 2006). BK exerts its action through two subtypes of G protein-coupled receptors; B1 and B2. Whereas BK activates receptors of the B2 type, B1 receptors are stimulated by kinin metabolites, such as Lys-des-Arg9-BK, obtained after enzymic cleavage by a neuronal kininase (Leeb-Lundberg et al., 2005). The stimulation of B2 receptors by centrally injected BK results in potent and dose-dependent antinociceptive effects pointing to the involvement of BK in the control of painful sensations (Pelá et al., 1996; Couto et al., 2006).
Since 1954 many peptides with chemical structures and physiological activities similar to BK have been reported in the venoms of animals, such as solitary and social species of wasps (Jaques and Schachter, 1954; Griesbacher et al., 1998) The presence of BK analogues in the venoms are considered to have mainly defensive purposes, as wasp stings often provoke pain, oedema and local lesions even in higher vertebrates, such as man (Nakajima, 1986; Mortari et al., 2005). These compounds can still act as neurotoxins, as they cause paralysis in insects by the presynaptic blockade of cholinergic transmission, probably caused by a non-competitive inhibition of choline uptake (Piek et al., 1987, 1990; Hue and Piek, 1989). However, there are no previous reports on the activity of venom-derived kinins in the mammalian CNS (Piek, 1991).
Therefore, the aims of the present study were to isolate and fully sequence the low-molecular-weight (MW) antinociceptive neurokinin from the venom of the social wasp Polybia occidentalis occidentalis. Furthermore, the effects of the purified molecule in the CNS of freely moving Wistar rats were assayed in two models of pain induction.
Materials and methods
Venom extracts
Females of P. occidentalis were collected at the University of São Paulo (Campus of Ribeirão Preto City, São Paulo, Brazil), and they were killed by freezing at −20°C. After species identification by Professor Sidney Matheus (Entomology Laboratory, USP), the venom reservoirs were manually removed, crushed in Milli-Q grade water/acetonitrile (ACN) (1:1) and centrifuged at 5000 g for 10 min, at 4°C. Supernatants were carefully collected and filtered using Microcon 3 (Millipore, Billerica, MA, USA). The extract with compounds of MW lower than 3 kDa was collected, lyophilized and weighed.
Fractionation and purification
The extract was resuspended in 1:1 ACN/water H2O and 0.07% trifluoroacetic acid (TFA). The solution was chromatographed on a reverse-phase high-performance liquid chromatography (RP-HPLC) column (C18 ODS, Jupiter 15 μm, 20 × 250 mm, Phenomenex, Torrence, CA, USA) at a flow rate of 5.0 ml/min and eluted using an isocratic gradient from 2% ACN/H2O (v/v) (containing 0.07% TFA) for 20 min, followed by 2–60% for 40 min and 60% for 20 min. The active fraction was chromatographed on a RP-HPLC column (C18 ODS, Jupiter 5 μm, 4.6 × 150 mm, Phenomenex, Torrence, CA, USA) at a flow rate of 1.0 ml min−1 and it was eluted by an isocratic gradient from 2% ACN/H2O (v/v) (containing 0.07% TFA) for 10 min, followed by 2–60% for 30 min and 60% for 10 min. Effluents from both columns were monitored photometrically at 214 nm.
Electrospray mass spectrometry
Molecular mass spectral analyses of peptides were performed on a high-resolution q-TOF. Electron-spray mass spectrometer (ESI-MS) spectrum was acquired on an UltrOTOF apparatus (Bruker Daltonics, Billerica, MA, USA).
Peptide sequencing by MS/MS
A Quattro-LC instrument from Micromass (Manchester, UK) was used for peptide sequencing. Solutions were infused into the ESI source at 10 μl min−1 using a Harvard Apparatus syringe pump model 1746 (Holliston, MA, USA). Deionized water (Milli-Q) was used throughout the study. The mass scan range was from m/z1000 to 2000. Experiments were performed with a cone voltage of 30 V, a capillary voltage of 3 kV and a dessolvation gas temperature of 80°C. A singly charged (protonated) molecule was submitted to collision-induced dissociation with argon gas at 10–50 eV collision energies. All MS/MS experiments were performed using continuous acquisition mode, scanning from m/z50 to 2000, with a scan time of 5 s.
Peptide sequencing was obtained using the ion products described in the mass spectra of each peptide, with the aid of AminoCalc software (Protana A/S).
Animal care
Male Wistar rats (220–250 g) from the animal house of the University of São Paulo were used in the assays. They were kept in wire-mesh cages in a room with a 12-h dark/light cycle (lights on at 0700 hour) with food and water ad libitum. Animals were maintained in accordance with the ethical statements of the Brazilian Society for Neuroscience and Behavior, which follow the guidelines for animal care prepared by the Committee on Care and Use of Laboratory Animal Resources, National Research Council, USA. Conditions of light and temperature (22°C) were kept constant in the housing and experimental rooms. Efforts were made to minimize the number and potential suffering of the animals used.
Surgical preparation of rats
All animals were anaesthetized by intraperitoneal (i.p.) administration of sodium thiopental (40 mg kg−1; Cristalia, Brazil) for stereotaxic implantation of a stainless-steel guide cannula (10 mm) in the right lateral ventricle. The coordinates used were 0.9 mm posterior to bregma, 1.6 mm lateral from midline and 3.4 mm ventral from the surface of the skull according to the Atlas of Paxinos and Watson (1986). The cannula was fixed to the skull with acrylic resin and one stainless-steel screw. At the end of the surgery, each guide cannula was sealed with a stainless-steel wire to protect it from obstruction.
The animals were allowed to recover for 5–7 days after the surgery. After that, the rats were gently wrapped in a cloth, hand-held and a thin dental needle was introduced through the guide cannula. The injection needle was linked to a polyethylene tube connected to a 10 μl Hamilton syringe, held by an infusion pump (Insight, Brazil) that injected a volume of 1 μl during 60 s.
Antinociceptive assays
Rats were tested for antinociceptive behaviour using the tail flick and hot-plate tests. In the tail flick test, the tail of each animal was laid across a nichrome wire coil, which was then heated by the passage of an electric current. The current raised the temperature of the coil at a rate of 9°C s−1. The equipment was calibrated to obtain three consecutive baseline tail flick latencies between 2.5 and 3.5 s. If at any time the animal failed to flick its tail within 6 s (cutoff time), the current was automatically turned off and the tail of the animal was removed from the wire to avoid damage to the skin.
For the hot-plate test, animals were placed on a metal plate enclosed by Plexiglas walls, maintained at 55.5±0.5°C. The behavioural end point was the time measured in seconds at which the animal jumped off the plate or licked a hind paw. Rats were removed from the hot plate if they did not respond within 30 s (cutoff time) to prevent tissue damage.
Three baseline measurements of withdrawal latencies to noxious stimulus in the tail flick and hot-plate tests were taken at 5 min intervals before the testing session. For both tests, measurements of antinociception were made at 5, 10, 20, 30, 45, 60, 90 and 120 min, after receiving in separate groups (n=5, per group), intracerebroventricular (i.c.v.) administration of physiological saline, BK (16, 8 and 4 nmol), morphine (24, 12 and 6 nmol) or peptide from the wasp venom (8, 4, 2 and 1 nmol).
Independent groups of Wistar rats were treated with concomitant i.c.v. administration of the B2-receptor antagonist, D-Arg0 (8 nmol) plus BK (8 nmol) or threonine6-bradykinin (Thr6-BK) (4 nmol).
As there were no significant statistical differences among the baseline latencies among the different experimental groups (unpaired Student's t-test; P<0.05 in all cases), all the withdrawal latencies, that is, the time taken for the motor response (LA) were normalized by an index of antinociception (IA) using the formula: The results were expressed as means±s.e.m. of IA values and plotted against time of the recordings of withdrawal reflexes. The area under the IA–time curves was calculated as area under the curve (AUC) – computer units.
Histology
At the end of the experiments, animals were killed by an overdose of sodium thiopental and perfused through the left ventricle first with saline (0.9%), and then with paraformaldehyde (4% phosphate-buffered saline (PBS), pH 7.4 at 4°C). After perfusion, animals were injected with toluidine blue dye in the same volume as injected in the experiments to mark the correct site of injection. The brains were immediately removed and placed into formaldehyde (4% PBS, pH 7.4) and kept at 4°C for 24 h. They were then rinsed in 10 and 20% sucrose (PBS, pH 7.3) at 4°C for 12 h each. Tissue pieces were embedded in Tissue Tek, frozen and cut (40 μm) on a cryostat (HM 505 E, Microm, Zeiss).
Statistical analysis
All normal data were submitted to repeated measures analysis of variance (ANOVA). In case of significant treatment-versus-time interaction, one-way ANOVAs followed by Duncan's test, at each time interval, were performed (SPSS for Windows, version 10.0; Chicago, IL, USA). The AUCs were analysed using one-way ANOVA, with P<0.05 or P<0.001 followed by Student–Newman–Keuls as a post hoc test. Probit methodology (Finney, 1971) was used to calculate ED50 value (with 95% confidence interval). Moreover, curves described for all treatments, were compared by means of nonlinear regression with the F-test, considering differences significant when P<0.05. The AUCs and dose–response curves of experiments were obtained with GraphPad Prism (version 4.0, GraphPad Software, San Diego, CA, USA).
Drugs
All drugs were dissolved in sterile physiological saline (NaCl, 150 mM) and injected i.c.v., as described above. The following drugs were used: BK (Sigma, St Louis, MO, USA), the competitive B2 antagonist D-Arg0-Hyp3-Thi5,8-D-Phe7-BK (D-Arg0) (Sigma) and the agonist of μ-opioid receptors, morphine (Sigma).
Results
Purification and identification of active fraction from P. occidentalis venom
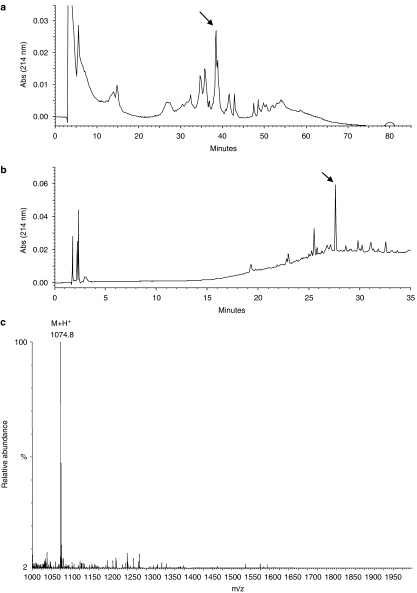
The first step of the RP-HPLC fractionation of compounds with molecular masses lower than 3 kDa (30 mg) resulted in the chromatographic profile shown in Figure 1a. The active fraction is indicated with an arrow and this peak was re-chromatographed by HPLC (Figure 1b). In this second step, the chromatogram profile revealed the presence of the major fraction that was analysed and identified by ESI-MS.
Figure 1.
Isolation and identification of the neurokinin Thr6-BK extracted from the venom of the social wasp, P. occidentalis. (a) RP-HPLC profile of the low MW extract from the venom of P. occidentalis. Chromatography was carried out with a C18 ODS column at a flow rate of 5.0 ml min−1 and eluted by a linear gradient from 2% ACN/H2O (v/v) (containing 0.07% TFA) for 20 min, followed by 2–60% for 40 min and 60% for 20 min. (b) Chromatographic profile of purification of the fraction indicated by arrow in A. RP-HPLC using a C18 column at a flow rate of 1.0 ml min−1 and eluted by a linear gradient from 2% ACN/H2O (v/v) (containing 0.07% TFA) for 10 min, followed by 2–60% for 20 min and 60% for 10 min. (c) ESI mass spectrum of the Thr6-BK, presenting a major peak at m/z1074.8.
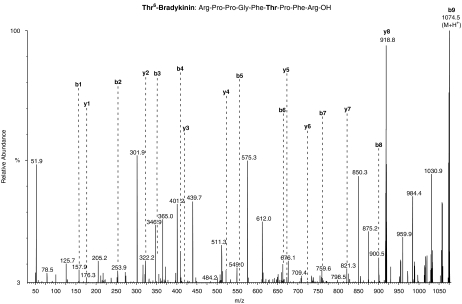
ESI-MS spectrum of the active fraction showed the high purity of the isolated peptide with molecular ion peak at m/z1074.8 Da (M+H+) (Figure 1c). The primary structure of the peptide present in the fraction was identified by MS/MS fragmentation. The complete series of b-ions starts with the ion m/z157, followed in sequence by m/z254, 351, 412, 555, 656, 753, 900 and 1075 (Figure 2). The MS/MS fragmentation profile was compared to that of peptides found in other animal venoms (Piek, 1991; Chen et al., 2002). From this analysis, the isolated peptide consisted of nine amino acids and its primary sequence was Arg-Pro-Pro-Gly-Phe-Thr-Pro-Phe-Arg-OH, which corresponded to that of the BK analogue, Thr6-BK.
Figure 2.
ESI MS/MS spectrum of the active peptide, indicating the amino-acid sequence on the top of the figure.
Antinociceptive assays
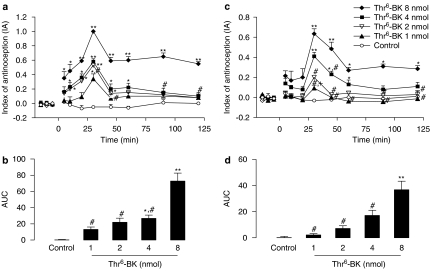
The peptide purified from the venom of P. occidentalis induced a dose-dependent antinociceptive effect in the tail flick test after i.c.v. administration. Significant differences in the effect of treatment (F(4,21)=13.36; P<0.0001), time (F(9,21)=7.27; P<0.0001), and treatment-versus-time interaction (F(36,79)=1.612; P<0.02), were observed. One-way ANOVAs showed a significant effect for the treatments in all periods (F(4,21) varying from 4.35 to 8.55; P<0.05). Data from the tail flick test submitted to the post hoc analysis indicated that all doses of Thr6-BK (8, 4, 2 and 1 nmol) produced significant antinociceptive effects when compared to control animals. The highest dose of Thr6-BK (8 nmol) induced antinociceptive effects that were expressed over the whole experimental period (2 h). Moreover, at 30 and 45 min after i.c.v. injection, a significant difference between the highest and the lowest dose was observed (Figure 3a).
Figure 3.
Antinociceptive dose-dependent effect of Thr6-BK injected i.c.v. in rats. (a) Time course of the dose-dependent increase in latencies in the tail flick test induced by i.c.v. injection of Thr6-BK. (b) AUC of the groups shown in (a). (c) Time course of the dose-dependent increase in latencies in the hot-plate test induced by i.c.v. injection of Thr6-BK. (d) AUC of the groups shown in (c). *Significantly different from control (*P<0.05 **P<0.001); #significantly different from the 8 nmol dose of Thr6-BK, and + significantly different from the 4 nmol dose of Thr6-BK. AUC, area under the curve of antinociception; IA, index of antinociception; Thr6-BK, threonine6-bradykinin.
A dose-dependent antinociceptive effect was also observed in the hot-plate test. There were significant effects of treatment (F(4,22)=7.79; P<0.0001), time (F(9,12)=12.12; P<0.0001), and treatment-versus-time interaction (F(36,198)=3.33; P<0.001). One-way ANOVAs showed a significant treatment effect from 30 to 120 min (F(4,22) varying from 3.00 to 7.68; P<0.05). Post hoc analysis indicated that doses of Thr6-BK 8 and 4 nmol produced significant increased hot-plate latencies of the rats when compared to the control group. In addition, Thr6-BK at the dose of (8 nmol) was significantly different from the doses of 2 and 1 nmol (Figure 3c).
In both tests, the greatest antinociceptive effect was observed 30 min after microinjection of Thr6-BK by i.c.v. route and the duration of the effect was also related to the dose (Figure 3a and c). The values of ED50 and confidence intervals of the antinociceptive effect of Thr6-BK in the tail flick and hot-plate tests were 2.94 nmol (1.54–3.49) and 6.82 nmol (3.17–7.57), respectively.
Similar antinociceptive effects were also observed in the AUC for both tail flick (F(4,20)=11.58; P<0.001) and hot-plate (F(4,22)=11.13; P<0.0001) tests. Post hoc analysis of data from the tail flick test showed significant antinociceptive effect for Thr6-BK 8 nmol in comparison to the lower doses and the two higher doses (8 and 4 nmol) in comparison to the control (Figure 3b). The AUCs from the hot-plate test revealed significant differences for Thr6-BK at the dose of 8 nmol in comparison to its lowest dose as well as the control animals (Figure 3d).
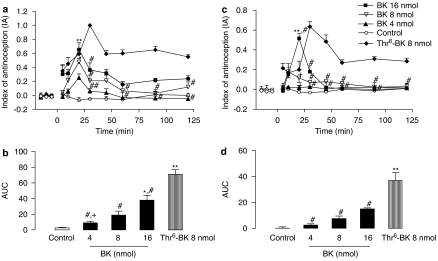
Figure 4a and c shows the comparison between the effects of i.c.v. administration of either BK (4, 8 and 16 nmol) or Thr6-BK (8 nmol) in the tail flick and hot-plate tests. In the tail flick test, significant effects of treatment (F(4,20)=18.53; P<0.01), time (F(7,14)=2.26; P<0.05), and treatment-versus-time interaction (F(28,78)=1.67; P<0.02), were observed. One-way ANOVAs showed significant treatment effects from 20 to 120 min (F(4,20) varying from 2.52 to 10.53; P<0.05). Post hoc analysis indicated that Thr6-BK produced significant increases in the tail flick latencies when compared to BK-treated groups (4 and 8 nmol) in the periods from 30 to 120 min. Moreover, the maximum effect for BK occurred at 20 min, when no differences were observed between BK and Thr6-BK treatments (Figure 4a).
Figure 4.
Antinociceptive effect of i.c.v. injections of Thr6-BK or BK. (a) Time course of the increase in latencies in the tail flick test induced by i.c.v. injection of Thr6-BK (8 nmol) and BK (4, 8 and 16 nmol). (b) AUC of the groups shown in (a). (c) Time course of the increase in latencies in the hot-plate test induced by i.c.v. injection of Thr6-BK (8 nmol) and BK (4, 8 and 16 nmol). (d) AUC of the groups shown in C. *Significantly different from control (*P<0.05 **P<0.001); #significantly different from 8 nmol Thr6-BK, and + significantly different from 16 nmol BK. AUC, area under the curve of antinociception; IA, index of antinociception; Thr6-BK, threonine6-bradykinin.
Similar results were obtained in the hot-plate test. Significant effects of treatment (F(4,23)=8.78; P<0.001), time (F(7,28)=5.95; P<0.0001), and treatment-versus-time interaction (F(28,73)=7.74; P<0.001) were observed. One-way ANOVA indicated significant treatment effect from 20 to 120 min (F(4,23) varying from 3.27 to 6.19; P<0.05). BK presented a peak of activity at 20 min when this treatment was significantly different from Thr6-BK (P<0.001) (Figure 4c). The values of ED50 and confidence intervals of the antinociceptive effect of BK in tail flick and hot-plate tests were 7.25 nmol (1.86–12.35) and 15.90 nmol (13.7–30.50), respectively.
When comparing the AUCs calculated for BK and Thr6-BK, a significant antinociceptive effect was observed for Thr6-BK (8 nmol) in relation to all doses of BK (4, 8 and 16 nmol) in the tail flick test (F(4,22)=16.39; P<0.0001) (Figure 4b), as well as the hot-plate test (F(4,23)=21.39; P<0.0001) (Figure 4d).
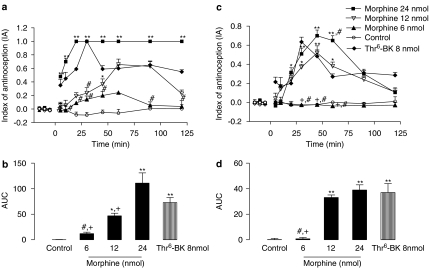
The potency of Thr6-BK was also measured in relation to morphine, both administered i.c.v. In the tail flick test, significant effects of treatment (F(4,18)=31.56; P<0.0001), time (F(9,12)=10,18; P<0.0001), and treatment-versus-time interaction (F(28,126)=3.86; P<0.0001) were observed. One-way ANOVA showed a significant effect for treatments from 5 to 120 min (F(4,18) varying from 2.01 to 8.11; P<0.05). Post hoc analysis showed that the withdrawal latencies induced by Thr6-BK at the dose of 8 nmol were significantly greater than morphine (12 nmol) at 30 min (P<0.05) and greater than morphine (6 nmol) at 5–45 min and at 90 and 120 min after treatment (Figure 5a).
Figure 5.
Antinociceptive effect of i.c.v. injections of Thr6-BK or morphine. (a) Time course of the increase in latencies in the tail flick test induced by i.c.v. injection of Thr6-BK (8 nmol) and morphine (6, 12 and 24 nmol). (b) AUC of the groups shown in A. (c) Time course of the increase in latencies in the hot-plate test induced by i.c.v. injection of Thr6-BK (8 nmol) and morphine (6, 12 and 24 nmol). (d) AUC of the groups shown in (c). *Significantly different from control (*P<0.05 **P<0.001); #significantly different from 8 nmol Thr6-BK; and + significantly different from 24 nmol morphine. AUC, area under the curve of antinociception; IA, index of antinociception; Thr6-BK, threonine6-bradykinin.
Analysis of the AUC results from the tail flick data showed statistical differences between the effects of Thr6-BK and the lowest dose of morphine. The antinociceptive effects induced by morphine measured in this way were statistically greater than those of control for all highest doses given (F(4,17)=28.41; P<0.001; Figure 5b).
Analysis of the hot-plate latencies induced by Thr6-BK and morphine showed significant effects of treatment (F(4,22)=9.94; P<0.0001), time (F(7,19)=11.79; P<0.001), and treatment-versus-time interaction (F(28,73)=4,27; P<0.0001). One-way ANOVAs showed a significant treatment effect from 20 to 60 min (F(4,22) varying from 2.84 to 5.9; P<0.05). Post hoc analysis revealed that the administration of the lowest dose of morphine did not increase latencies, compared to the saline-injected control. Moreover, there were significant differences between the latencies of animals treated with Thr6-BK and the animals treated with morphine (6 nmol) at 30, 45 and 60 min after injection. The peak of morphine activity was at 45 min after treatment and this (24nmol) drug was more effective than Thr6-BK at 60 min (Figure 5c).
Figure 5d shows the AUC values for the hot-plate test results. In this set of experiments, Thr6-BK induced a significantly greater increase in latencies than did morphine only when compared to the lowest dose of morphine (6 nmol; P<0.05). In addition, morphine treatment at all highest doses produced AUC values significantly different from those for the control group (F(4,21)=12.70; P<0.0001). The ED50 values and confidence intervals for the antinociceptive effect induced by morphine in tail flick and hot-plate tests were 7.8 nmol (5.1–12.03) and 13.35 nmol (7.05–24.5), respectively.
Finally, the curves as well as the ED50 calculated for all highest treatments were compared. In this case, significant differences were reported for Thr6-BK in comparison to both, morphine and BK (tail flick – F(2,56)=21.61, P<0.00001; hot plate – F(2,64)=5.44, P<0.0066). However, no differences were observed between dose-effect curves described for morphine and BK. Therefore, the absolute values of ED50 indicate that Thr6-BK was 2.65- and 2.1-fold more potent than morphine and 2.5- and 3.36-fold more potent than BK in the tail flick and in the hot-plate tests, respectively.
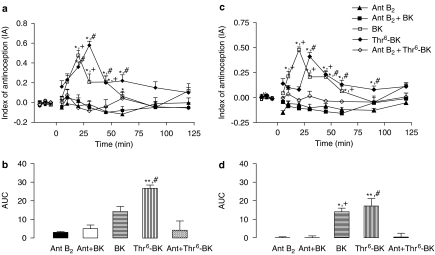
Analysis of the experiments using the selective B2-receptor antagonist in the antinociceptive effect of Thr6-BK in the tail flick test showed significant effects of treatment (F(4,18)=25.89; P<0.0001), time (F(9,10)=5.76; P<0.006), and treatment-versus-time interaction (F(36,38)=2.74; P<0.003). One-way ANOVA showed a significant treatment effect from 20 to 60 min (F(4,18) varying from 5.10 to 15.10; P<0.02). Post hoc analysis showed there was significant antagonism of the antinociceptive effects of the injection of Thr6-BK alone when the B2-receptor antagonist was added to Thr6-BK, up to 45 min. At 60 min, some loss of antagonism was seen. The B2-receptor antagonist also abolished the antinociceptive effects of BK over the same period (Figure 6a).
Figure 6.
Effect of concomitant i.c.v. injection of a selective antagonist of B2 receptors and Thr6-BK or BK on antinociceptive actions of the kinins. (a) Time course of the latencies in the tail flick test induced by concomitant i.c.v. injection of B2-receptor antagonist (Ant B2) and Thr6-BK (4 nmol) or B2-receptor antagonist and BK (8 nmol). (b) AUC of the groups shown in A. (c) Time course of the increase in latencies in the hot-plate test induced by concomitant i.c.v. injection of B2-receptor antagonist and Thr6-BK (4 nmol) or B2-receptor antagonist and BK (8 nmol). (d) AUC of the groups shown in C. *Significantly different from B2-receptor antagonist alone (*P<0.05 **P<0.001); #significantly different from the B2-receptor antagonist and Thr6-BK (4 nmol); and + significantly different from the B2-receptor antagonist and BK (8 nmol). AUC, area under the curve of antinociception; IA, index of antinociception; Thr6-BK, threonine6-bradykinin.
The simultaneous injection of the B2-selective antagonist and Thr6-BK or BK completely inhibited the antinociceptive effects of both drugs at all times of the hot-plate test. Significant effects of treatment (F(4,22)=11.13; P<0.0001), time (F(9,14)=2.41; P<0.05), and treatment-versus-time interaction (F(36,50)=2.01; P<0.01). One-way ANOVAs showed a significant treatment effect from 10 to 90 min (F(4,22) varying from 4.91 to 12.00; P<0.007). Post hoc analysis showed significant differences between the treatments of B2-receptor antagonist plus Thr6-BK and Thr6-BK; and B2-receptor antagonist plus BK and BK alone (Figure 6c).
Figure 6b and d show the AUCs after concomitant microinjections of B2-receptor antagonist and Thr6-BK or BK for both tests. In this case, i.c.v. injection of B2-receptor antagonist reduced the AUC induced by BK and Thr6-BK in hot-plate test (F(4,22)=5.11; P<0.05) and tail flick test (F(4,18)=6.21; P<0.01).
Discussion
In this study, we isolated and fully sequenced a BK analogue extracted from the venom of the social wasp P. occidentalis occidentalis, Thr6-BK. This kinin is the most active kinin isolated from the venoms of other social wasps (Watanabe et al., 1976; Nakajima et al., 1984). Our data demonstrated that Thr6-BK exerts remarkable antinociceptive effects when injected directly in the rat CNS, in both tail-flick and hot-plate tests. This is the first time that neurokinins isolated from arthropod venoms have been assayed in the mammalian CNS. However, the oedematogenic effects of Thr6-BK injected into the rat paw have been reported by Griesbacher et al (1998). These authors compared the inflammatory responses to Thr6-BK with those to BK and observed a difference in potency for the peptides (Thr6-BK>BK). Both pain and inflammation are provoked by BK injected peripherally due to the stimulation of afferent nerve terminals (Armstrong et al., 1952 in Öztürk, 2001).
The neurokinin Thr6-BK is a small linear peptide with nine amino-acid residues; Arg-Pro-Pro-Gly-Phe-Thr-Pro-Phe-Arg-OH, highly homologous to the endogenous mammalian peptide, BK, except for its secondary structure (Pellegrini et al., 1997). This structural difference is a result of one amino-acid residue substitution in position 6, where there is serine for BK and threonine for Thr6-BK. Indeed, this substitution offers a more stable molecular conformation, as it occurs in a region that defines the β-turn at the C terminus. Moreover, this modification in the region of the β-turn might also determine a high receptor affinity and thus, a greater potency for Thr6-BK than that observed for BK in in vitro investigations (Pellegrini and Mierke, 1997).
Our results demonstrated that this difference in potency between Thr6-BK and BK was also observed in these two models of thermally induced nociception. Indeed Thr6-BK was even more potent than morphine, which is widely used in the clinical treatment of pain. Furthermore, the effects of this molecule on antinociception were dose- and time-dependent, although it differed in both onset and duration of activity, compared to BK and morphine.
In our assays, Thr6-BK remained active longer than BK, a finding that could be a result of a more stable conformation of its secondary structure. This in turn, probably protects Thr6-BK from hydrolysis by neuronal kininases, preserving the effects of the peptide on B2 receptors (Pellegrini and Mierke, 1997) and thus increasing its effectiveness.
Investigations focusing the neural effects of BK and analogues are particularly important in pain research, since BK is thought to play a role in central pain transmission (Millan, 1999). The antinociceptive effects of BK have been reported elsewhere (Laneuville and Couture, 1987; Laneuville et al., 1989; Pelá et al., 1996; Couto et al., 1998) and demonstrated to be independent of other physiological variables, including blood pressure (Couto et al., 1998).
BK exerts its action through two subtypes of G protein-coupled receptors. Intact BK is the characteristic agonist for the B2 receptor, whereas kinin metabolites, such as Lys-des-Arg9-BK or des-Arg9-BK, produced by neuronal endopeptidase action, activate the B1 receptor, which is far less expressed in normal tissues (Calixto et al., 2000; Ongali et al., 2003; Leeb-Lundberg et al., 2005). In this regard, Pelá et al. (1996) demonstrated that B2-receptor agonists exerted antinociceptive effects even after the administration of B1-receptor antagonists. These authors attributed the observed antinociceptive effect to the activation of descending adrenergic pathways, which are stimulated by presynaptic B2 receptors (Laneuville et al., 1989; Couto et al., 1998).
In summary, our findings indicate that i.c.v. injections of Thr6-BK exert a potent antinociceptive effect with potency approximately two-fold higher than BK and morphine. Moreover, this effect was fully inhibited by the B2-receptor selective antagonist D-Arg0, indicating that Thr6-BK acts on B2 receptors. The actions of BK, as well as its analogues, such as Thr6-BK on the CNS deserve to be better investigated to provide novel alternatives in the study and treatment of pain-related disorders.
Acknowledgments
We are grateful to Dr Ernesto Nakayasu (ICB – USP) for help in the analysis of the sequence of peptides and Mr Abílio Borghi for the critical reading of the manuscript. This work received financial support from Brazilian National Council for Scientific Research (CNPq).
Abbreviations
- ACN
acetonitrile
- AUC
area under the curve of antinociception
- BK
bradykinin
- ESI-MS
electron-spray mass spectrometer
- IA
index of antinociception
- i.c.v.
intracerebroventricular
- i.p.
intraperitoneal
- RP-HPLC
reverse phase-high performance liquid chromatography
- TFA
trifluoroacetic acid
- Thr6-BK
threonine6-bradykinin
Conflict of interest
The authors state no conflict of interest.
References
- Armstrong D, Dry RM, Keele CA, Markham JW. Pain-producing substances in blister fluid and in serum. J Physiol. 1952;117:4–5. [PubMed] [Google Scholar]
- Bhoola KD, Elson CJ, Dieppe PA. Kinins-key mediators in inflammatory arthritis. Br J Rheumatol. 1992;31:509–518. doi: 10.1093/rheumatology/31.8.509. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Cabrini DA, Ferreira J, Campos MM. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- Chen T, Orr DF, Bjourson AJ, McClean S, O'Rourke M, Hirst DG, et al. Novel bradykinins and their precursor cDNAs from European yellow-bellied toad (Bombina variegata) skin. Eur J Biochem. 2002;269:4693–4700. doi: 10.1046/j.1432-1033.2002.03174.x. [DOI] [PubMed] [Google Scholar]
- Couto LB, Corrêa FMA, Pelá IR. Brain sites involved in the antinociceptive effect of bradykinin in rats. Br J Pharmacol. 1998;125:1578–1584. doi: 10.1038/sj.bjp.0702209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto LB, Moroni CR, dos Reis Ferreira CM, Elias-Filho DH, Parada CA, Pela IR, et al. Descriptive and functional neuroanatomy of locus coeruleus-noradrenaline-containing neurons involvement in bradykinin-induced antinociception on principal sensory trigeminal nucleus. J Chem Neuroanat. 2006;32:28–45. doi: 10.1016/j.jchemneu.2006.03.003. [DOI] [PubMed] [Google Scholar]
- da Silva GR, Rocha e Silva M. Catatonia induced in the rabbit by intracerebral injection of bradykinin and morphine. Eur J Pharmacol. 1971;15:180–186. doi: 10.1016/0014-2999(71)90171-3. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Statistical logic in the monitoring of reactions to therapeutic drugs. Methods Inf Med. 1971;10:237–245. [PubMed] [Google Scholar]
- Graeff FG, Pelá IR, Rocha e Silva M. Behavioural and somatic effects of bradykinin injected into the cerebral ventricles of unanaesthetized rabbits. Br J Pharmacol. 1971;37:723–732. doi: 10.1111/j.1476-5381.1969.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbacher T, Althuber P, Zenz M, Rainer I, Griengl S, Lembeck F. Vespula vulgaris venom: role of kinins and release of 5-hydroxytryptamine from skin mast cells. Eur J Pharmacol. 1998;351:95–104. doi: 10.1016/s0014-2999(98)00276-3. [DOI] [PubMed] [Google Scholar]
- Hue B, Piek T. Irreversible presynaptic activation-induced block of transmission in the insect CNS by hemicholinium-3 and threonine6-bradykinin. Comp Biochem Physiol. 1989;93C:87–89. [Google Scholar]
- Jaques R, Schachter M. The presence of histamine, 5-hydroxytryptamine and a potent, slow contracting substance in wasp venom. Br J Pharmacol Chemother. 1954;9:53–58. doi: 10.1111/j.1476-5381.1954.tb00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laneuville O, Couture R. Bradykinin analogue blocks bradykinin-induced inhibition of a spinal nociceptive reflex in the rat. Eur J Pharmacol. 1987;137:281–285. doi: 10.1016/0014-2999(87)90237-8. [DOI] [PubMed] [Google Scholar]
- Laneuville O, Reader TA, Couture R. Intrathecal bradykinin acts presynaptically on spinal noradrenergic terminals to produce antinociception in the rat. Eur J Pharmacol. 1989;159:273–283. doi: 10.1016/0014-2999(89)90158-1. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg LMF, Marceau F, Müller-Esteri W, Pettibone DJ, Zuraw BL. International Union of Pharmacology. XLV. Classification of the Kinin Receptor Family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Marceau F, Regoli D. Bradykinin receptor ligands: therapeutic perspectives. Nat Rev Drug Discov. 2004;3:845–852. doi: 10.1038/nrd1522. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Mortari MR, Cunha AOS, de Oliveira L, Vieira EB, Gelfuso EA, Santos WF. Comparative toxic effects of the venosm from three wasp species of the genus Polybia (Hymenoptera, Vespidae) J Biol Sci. 2005;5:449–454. [Google Scholar]
- Nakajima T.Pharmacological biochemistry of Vespid venoms Venoms of Hymenoptera 1986Academic Press: London; 307–327.In: Piek T (ed) [Google Scholar]
- Nakajima T, Yasuhara T, Yosida H, Ueno Y, Ohtsuka C, Hamamoto M, et al. Wasp kinins in some Japanese wasps (Vespidae, hymenoptera) Jpn J Sanit Zool. 1984;35:139–149. [Google Scholar]
- Ongali B, Campos MM, Bregola G, Rodi D, Regoli D, Thibault G, et al. Autoradiographic analysis of rat brain kinin B1 and B2 receptors: normal distribution and alterations induced by epilepsy. J Comp Neurol. 2003;461:506–519. doi: 10.1002/cne.10706. [DOI] [PubMed] [Google Scholar]
- Öztürk Y. Kinin receptors and their antagonists as novel therapeutic agents. Curr Pharm Des. 2001;7:135–161. doi: 10.2174/1381612013398338. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates 1986Academic Press Inc.: Sydney; 2nd edn [Google Scholar]
- Pelá IR, Rosa AL, Silva CAA, Huidobro-Toro JP. Central B2 receptor involvement in the antinociceptive effect of bradykinin in rats. Br J Pharmacol. 1996;118:1488–1492. doi: 10.1111/j.1476-5381.1996.tb15564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Mammi S, Peggion E, Mierke DF. Threonine6-bradykinin: structural characterization in the presence of micelles by nuclear magnetic resonance and distance geometry. J Med Chem. 1997;40:92–98. doi: 10.1021/jm9605391. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Mierke DF. Threonine6-bradykinin: molecular dynamics simulations in abiphasic membrane mimetic. J Med Chem. 1997;40:99–104. doi: 10.1021/jm9605389. [DOI] [PubMed] [Google Scholar]
- Piek T. Neurotoxic kinins from wasp and ant venoms. Toxicon. 1991;29:139–149. doi: 10.1016/0041-0101(91)90098-c. [DOI] [PubMed] [Google Scholar]
- Piek T, Hue B, Le Corronc H, Mantel P, Gobbo M, Rocchi R. Presynaptic block of transmission in the insect CNS by mono- and di-Galactosyl analogues of vespulakinin 1, a wasp (Paravespula maculifrons) venom toxin. Comp Biochem Physiol. 1987;105C:189–196. doi: 10.1016/0742-8413(93)90193-o. [DOI] [PubMed] [Google Scholar]
- Piek T, Hue B, Mantel P, Nakajima T, Pelhate M, Yasuhara T. Threonine6-bradykinin in the venom of the wasp Colpa interrupta (F.) presynaptically blocks nicotinic synaptic transmission in the insect CNS. Comp Biochem Physiol. 1990;96C:157–162. doi: 10.1016/0742-8413(90)90062-e. [DOI] [PubMed] [Google Scholar]
- Rocha e Silva M, Beraldo WT, Rosenfeld G. Bradykinin, a hypotensive and smooth muscle stimulating factor released from plasma globulin by snake venoms and by trypsin. Am J Physiol. 1949;156:261–273. doi: 10.1152/ajplegacy.1949.156.2.261. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Yasuhara T, Nakajima T.Occurrence of thr6-bradykinin and its analogous peptide in the venom of Polistes rothnery iwataii V. Der Vecht Animal, Plant Microbial Toxins 1976105–112.In: Ohosaka A, Hayashi K, Sawai Y (eds)Vol 2, pp