Abstract
Background and purpose:
This study represents a novel characterisation of KCNQ-encoded potassium channels in the vasculature using a variety of pharmacological and molecular tools to determine their role in contractility.
Experimental approach:
Reverse transcriptase polymerase chain reaction (RT-PCR) experiments were undertaken on RNA isolated from mouse aorta, carotid artery, femoral artery and mesenteric artery using primers specific for all known KCNQ genes. RNA isolated from mouse heart and brain were used as positive controls. Pharmacological experiments were undertaken on segments from the same blood vessels to determine channel functionality. Immunocytochemical experiments were performed on isolated myocytes from thoracic aorta.
Key results:
All blood vessels expressed KCNQ1, 4 and 5 with hitherto ‘neuronal' KCNQ4 being, surprisingly, the most abundant. The correlated proteins Kv7.1, Kv7.4 and Kv7.5 were identified in the cell membranes of aortic myocytes by immunocytochemistry. Application of three compounds known to activate Kv7 channels, retigabine (2 –20 μM), flupirtine (20 μM) and meclofenamic acid (20 μM), relaxed vessels precontracted by phenylephrine or 1 mM 4-aminopyridine but had no effect on contractions produced by 60 mM KCl or the Kv7 channel blocker XE991 (10 μM). All vessels tested contracted upon application of the Kv7 channel blockers XE991 and linopirdine (0.1-10 μM).
Conclusions and implications:
Murine blood vessels exhibit a distinctive KCNQ expression profile with ‘neuronal' KCNQ4 dominating. The ion channels encoded by KCNQ genes have a crucial role in defining vascular reactivity as Kv7 channel blockers produced marked contractions whereas Kv7 channel activators were effective vasorelaxants.
Keywords: KCNQ, Kv7, retigabine, XE991, vascular smooth muscle, Q-PCR, immunocytochemistry, isometric tension
Introduction
The Kv7 channel family encoded by KCNQ genes (1–5) are a family of voltage-dependent ion channels that shape the cardiac action potential and stabilize neuronal membrane potential. KCNQ1 expression is relatively widespread but is most abundant in the heart where the expressed protein (Kv7.1) in association with small, single transmembrane proteins encoded by the KCNE1 gene (minK) constitute the slowly activating component of the delayed rectifier (IKs, Sanguinetti et al., 1996). Mutations in both KCNQ1 and KCNE1 underlie a large number of hereditary arrhythmias leading to long QT syndrome (Herbert et al., 2002). KCNQ1 is also expressed in the small intestine where it associates with a protein encoded by the KCNE3 gene to form a constitutively active ion channel crucial for Cl− secretion (Jentsch, 2000). Expression of KCNQ2-5 is abundant in the central nervous system (Jentsch, 2000) with KCNQ4 restricted to the auditory system (Kharkovets et al., 2000). The ion channels encoded by these genes, especially Kv7.2/7.3 heteromultimers, constitute the resting conductance inhibited by muscarinic receptor activation termed the M-current (Brown and Adams, 1980; Wang et al., 1998). As a consequence of this crucial role of Kv7 channels, loss of function mutations to KCNQ2 or KCNQ3 led to increased neuronal excitability and hereditary epilepsy (Wang et al., 1998; Jentsch, 2000) or deafness (Kharkovets et al., 2000). Conversely, activation of Kv7 channels decreases membrane excitability and recent studies have shown that the novel anti-convulsant, N-(2-amino-4-4-flurobenzylamino)-phenyl carbamic acid (retigabine), acts by selective activation of channels encoded by KCNQ2-5 with an EC50 between 1 and 5 μM (Tatulian et al., 2001). Interestingly, retigabine and a closely related analogue flupirtine have no effect on Kv7.1 at concentrations <100 μM (Schenzer et al., 2005; Wuttke et al., 2005).
Recently, Ohya et al. (2003) showed that murine portal vein myocytes also expressed KCNQ genes, with KCNQ1 being the most abundant (Ohya et al., 2003). These cells also expressed KCNE genes (Ohya et al., 2002a) whose expression products alter the biophysical and pharmacological properties of Kv7 channels (Grunnet et al., 2002, 2005; McCrossan and Abbott, 2004). Follow-up studies showed that low concentrations of 10,10-bis (4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride) (XE991) and linopirdine, agents that selectively block Kv7 channels without discriminating between individual isoforms (Wang et al., 1998), inhibited a sustained membrane conductance and depolarized the resting membrane potential of portal vein myocytes (Yeung and Greenwood, 2005). Isometric tension studies on whole portal veins showed that XE991 at concentrations specific for KCNQ channels increased spontaneous contractile activity of whole portal veins (Yeung and Greenwood, 2005). These agents also contracted segments of rat and mouse pulmonary artery with a negligible effect on rat mesenteric artery (Joshi et al., 2006). More recently, an inhibition of Kv7 channels has been proposed to underlie the increased membrane excitability of cultured A7r5 cells produced by arginine–vasopressin (Brueggemann et al., 2007).
These recent functional experiments suggest that in the portal vein and pulmonary artery myocytes, as well as in cultured cells, Kv7 channels perform a role similar to that described in neurones, that is, suppression of membrane excitability through membrane hyperpolarization. The logical corollary to these observations is that activators of Kv7 channels, such as retigabine, should have an anti-spasmogenic role in the vasculature. However, a caveat is that retigabine only activates so-called ‘neuronal' Kv7 channels encoded by KCNQ2-5 (Tatulian et al., 2001; Schenzer et al., 2005; Wuttke et al., 2005), so these gene products must be present in the vascular myocytes. Although KCNQ5 is expressed in A7r5 cells (Brueggemann et al., 2007), culture conditions are known to alter the expression of ion channels markedly. Consequently, the study by Ohya et al. (2003) represents the only investigation in the expression profile of KCNQ genes in the vasculature. We have now determined the profile of KCNQ gene expression in various murine blood vessels and investigated the effect of Kv7 channel activators and blockers in these vessels.
Materials and methods
BALB/c mice (6–8 weeks) were killed by overdose of pentobarbitone in accordance with the UK Scientific Procedures (Animals) Act (1986) and the following blood vessels were removed and placed in either cold physiological salt solution (PSS) for functional studies or RNA Later (Ambion, Huntingdon, Cambridgeshire, UK) for molecular studies: thoracic aorta (TA), carotid artery, main femoral artery and mesenteric artery (1st and 2nd order). For the molecular studies, the vessels were placed immediately in RNA Later after removal of extraneous debris and endothelium denuded by mechanical abrasion. Single aortic myocytes were prepared by incubating small pieces (∼0.5 mm2) of aortic tissue in Ca-free dissociation PSS containing 1.6 mg ml−1 collagenase type XI, 0.2 mg ml−1 protease type XIV, 1 mg ml−1 trypsin inhibitor and 2 mg ml−1 bovine serum albumin at 37°C for 15 min. Cells were liberated by gentle mechanical agitation using a wide bore Pasteur pipette.
RNA extraction and real time quantitative PCR
Qualitative PCR was undertaken as described previously (Ohya et al., 2002a, 2003) using murine brain- and heart-derived cDNA as positive controls. RNA was extracted from each tissue, totalling 27–30 mg. Each vascular tissue was cut into smaller pieces and placed in 150 μl lysis buffer containing 4 ng μl−1 carrier RNA (Qiagen, Crawley, W. Sussex, UK). All tissues were incubated in lysis buffer at room temperature for at least 10 min before homogenization with a pellet pestle motor (Kontes, Vineland, NJ, USA). Total RNA was extracted using the RNeasy mini spin columns (Qiagen) incorporating the proteinase K incubation at 55°C for 10 min and on-column DNase I treatment. RNA was quantified using the NanoDrop ND-1000 spectrophotomer (NanoDrop Technologies, Wilmington, DE, USA) and 100 ng (brain, heart, TA and mesenteric artery) or 10 ng (carotid artery and femoral artery) total RNA was reverse transcribed to cDNA using M-MLV (Invitrogen, Paisley, UK). All PCR reactions were performed with an initial denaturation step at 94°C for 2 min, followed by 35 cycles at 94°C for 30 s, annealing temperature for 30 s and 72°C for 1 min; the reaction was completed with a 10-min extension period. All reactions were carried out using a Touchgene thermal cycler (Techne, Staffordshire, UK). All primer sets (Table 1) were designed using OligoPerfect (Invitrogen) and synthesized by Invitrogen and optimized using mouse brain and heart cDNA. All PCR products were confirmed by DNA sequencing at the ABC facility at Imperial College London. All sequencing data were checked for homology using the NCBI BLAST programme.
Table 1.
Mouse reverse transcriptase-PCR primers
| Gene | Primer (+) sense, (−) antisense | Accession number | Amplicon (bp) |
|---|---|---|---|
| mβ-actin | (+) 5′-TGTTACCAACTGGGACGACA-3′ | NM_007393 | 573 |
| (−) 5′-AAGGAAGGCTGGAAAAGAGC-3′ | |||
| mKCNQ1 | (+) 5′-TTGTGGTGTTCTTTGGGACA-3′ | NM_008434 | 647 |
| (−) 5′-TGCAGTCTGGATGAGTGAGG-3′ | |||
| mKCNQ2 | (+) 5′-TCTCCTGCCTTGTGCTTTCT-3′ | AB000504 | 490 |
| (−) 5′-GCATCTGCGTAGGTGTCAAA-3′ | |||
| mKCNQ3 | (+) 5′-ATCGGGTTCGCCTTTCTAAT-3′ | NM_152923 | 509 |
| (−) 5′CTGCTGGGATGGGTAGGTAA-3′ | |||
| mKCNQ4 | (+) 5′-GCTTACGGTGGATGATGTCA-3′ | AF249747 | 449 |
| (−) 5′-TGTGGTAGTCCGAGGTGATG-3′ | |||
| mKCNQ5 | (+) 5′-GGCTTCGCACTCCTTGGCAT-3′ | AF263836 | 535 |
| (−) 5′-CACACTGGCATCCTTTCTCA-3′ |
Quantitative analysis of mRNA expression of specific genes was determined using Syber Green chemistry with an ABI 7000 sequence detector (Applied Biosystems, Warrington, Cheshire, UK). The following PCR primers were used for real-time PCR analysis: mKCNQ1a and b (GeneBank accession no. NM_008434, 810–994), amplicon=185 bp; mKCNQ1a (NM_008434, 1450–1613), amplicon=164 bp; mKCNQ2 (AB000494, 353–551), amplicon=199 bp; KCNQ3 (BB022155, 21–164), amplicon=144 bp; KCNQ4 (AF249747, 636–804), amplicon=169 bp; KCNQ5 (AF263836, 229–427), amplicon=199 bp; mKCNE1 (NM_008424, 84–245), amplicon=162 bp; mKCNE2 (AK008619, 214–346), amplicon=133 bp; mKCNE3 (NM_020574, 280–458), amplicon=179 bp; mKCNE4 (NM_021342, 306–480), amplicon=175 bp; mKCNE5 (NM_021487, 338–438), amplicon=201 bp; GAPDH (M32599, 730–833), amplicon=104 bp. All quantitative data were accrued from five different samples. Before starting both real-time PCR and cell-based PCR experiments, the following control experiments were performed. As a negative control to identify genomic contamination, RNA from each sample was used as a template in the PCR (data not shown). Also, to show that PCR reactants were free from contamination, two other control reactions were performed; one contained only primers and no template DNA (NTC) and the other contained only template DNA and no primer. Murine brain- and heart-derived cDNA were also used as positive controls. For the quantitative PCR, the Ct values for GAPDH with a threshold value of ΔRn set to 0.20 were: 20.53±0.26, 18.78±0.55, 18.55±0.084 and 20.34±0.25, respectively, for the TA, carotid artery and femoral artery (n=5 for each).
Immunocytochemistry
Single cells were fixed and stained for confocal microscopy as described previously (Saleh et al., 2005). Protein expression was identified by immunofluorescence using an antibody against Kv7.1 (Alomone Laboratories, Tel Aviv, Israel, 1:600 dilution), two against Kv7.4 (Kv7.4SC from Santa Cruz (Santa Cruz Biotech, Santa Cruz, CA, USA) 1:200 and Kv7.4J (raised against a different epitope and kindly provided by Professor Jentsch, University of Hamburg, 1:50 dilution, Kharkovets et al., 2000) and against Kv7.5 (Santa Cruz, 1:200). The labelling was visualized with Alexa Fluor 488-conjugated chicken anti-rabbit antibodies (1:500), Alexa Fluor 633-conjugated donkey anti-goat antibodies (1:500) or Alexa Fluor 488-conjugated donkey anti-goat antibodies (1:500). PSS contained penicillin (20 U ml−1) and streptomycin (20 μg ml−1) at all times during immunocytochemical experiments. Confocal microscopy and analysis was the same as described previously (Saleh et al., 2005). The purchased antibodies were tested on crude membrane preparations from Xenopus oocytes injected with the desired KCNQ cRNA and analyzed by Western analysis. All antibodies recognized bands corresponding to the predicted molecular mass of the respective KCNQ protein (uninjected oocytes served as negative controls). These experiments demonstrate that the antibodies work in principle and together with the findings that the preincubation with the antigenic peptides led to disappearance of the signal led us to conclude that the observed staining was specific. The Kv7.4J antibody was assayed by Professor Jentsch's group as described by Kharkovets et al. (2000).
Isometric tension recordings
Isometric tension was recorded in 2 cm segments of aorta, carotid artery, femoral artery and mesenteric artery suspended on two intraluminal stainless steel wires (40 μm) in a small vessel myography apparatus (DMT, ADInstruments, Oxford, UK) containing Krebs' solution aerated with 95% O2/5% CO2. After a 30-min equilibration period, a passive length-tension curve was constructed in each segment of femoral artery, carotid artery and mesenteric artery by applying cumulative stretches to the preparation. From this curve, equivalent transmural pressures were estimated and the vessel set at a level of tension equivalent to 90% of the diameter of the vessel at a distending pressure of 100 mm Hg. After a further equilibration period, tissues were bathed in an external solution containing 60 mM KCl for 5 min, repeated twice. Continuous recordings of changes in tension were acquired by use of a PowerLab and Chart (version 5, ADInstruments).
Solutions
PSS contained (mM): NaCl (125), KCl (5.4), NaHCO3 (15.4), Na2HPO4 (0.33), KH2PO4 (0.34), glucose (10) and N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (11), adjusted to pH 7.4 with NaOH. Enzyme solutions were made up with 100 μM Ca2+ PSS. The Krebs' solution for the functional experiments contained (mM): NaCl (125), KCl (4.6), CaCl2 (2.5), NaHCO3 (15.4), Na2HPO4 (1), MgSO4 (0.6) and glucose (10) constantly aerated by 95%O2/5% CO2.
Data analysis
All data were obtained from several segments (denoted by n in the text) from more than three animals (denoted by n in the text). Results are shown as means±s.e.m., unless otherwise stated, and differences between means assessed for statistical significance with unpaired Student's t-tests. P-values <0.05 were taken as showing significant differences between means.
Materials
Flupirtine, meclofenamic acid, L-NAME, linopirdine, phenylephrine, indomethacin and nicardipine were from Sigma (Poole, UK). XE991, pinacidil and chromanol 293B (C293B) were purchased from Tocris (Bristol, UK). Stromatoxin was from Alomone Labs. Retigabine was a kind gift from Elbion AG (Germany) and L-768,673 was a kind gift from Dr J Salata, Merck Research (West Point, PA, USA). All solutions were prepared as 100 mM stock solutions in either DMSO or distilled water and frozen as small aliquots.
Results
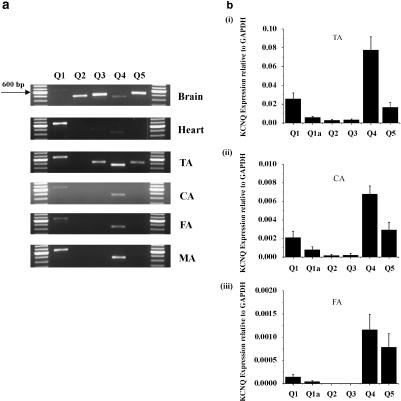
Before functional experiments were undertaken, we determined the expression profile of KCNQ genes in various murine blood vessels. Figure 1a shows examples of conventional reverse transcriptase-PCR experiments using a standard 35-cycle protocol on cDNA generated from RNA isolated from different murine blood vessels denuded of endothelium. It is clear from Figure 1a that KCNQ1 and KCNQ4 were expressed in all four blood vessels tested. KCNQ5 was also detectable in the TA. In contrast, KCNQ3 expression was low and KCNQ2 was consistently absent in all vessels tested. Consequently, the main KCNQ isoforms expressed in the blood vessels studied appeared to be KCNQ1 as well as the neuronal molecular correlates, KCNQ4 and KCNQ5.
Figure 1.
Vascular expression of KCNQ genes. (a) Reverse transcriptase-PCR analysis of five members of the KCNQ family (Q 1–5) in murine brain, heart, thoracic aorta (TA), carotid artery (CA), femoral artery (FA) and mesenteric artery (MA) after 35 cycles. (b) Summarized data for quantitative PCR of KCNQ genes in murine TA (bi), carotid artery (bii), femoral artery (biii), respectively. Values are shown for steady-state transcripts relative to GAPDH in the same preparation (means±s.e.m; n=5).
The expression of all known KCNQ genes in the TA, carotid artery and femoral artery was quantified relative to the levels of the housekeeping gene, GAPDH, in each vessel. In each case, the most abundant mRNA was KCNQ4>KCNQ5=KCNQ1 (Figure 1c). These findings differ from those obtained from the murine portal vein (Ohya et al., 2003). There is also significant expression of the novel splice variant of KCNQ1 reported by Ohya et al. (2003) but the relative expression of this isoform varied throughout, the vasculature being a greater component of the KCNQ1 mRNA in the femoral artery and carotid artery compared to the aorta. Overall, these data show that the TA, as well as conduit arteries (carotid and femoral artery) and the mesenteric artery, expressed the supposedly neurone-specific genes KCNQ4 and KCNQ5 as well as KCNQ1 that has been identified previously in the cardiovascular system (Ohya et al., 2003).
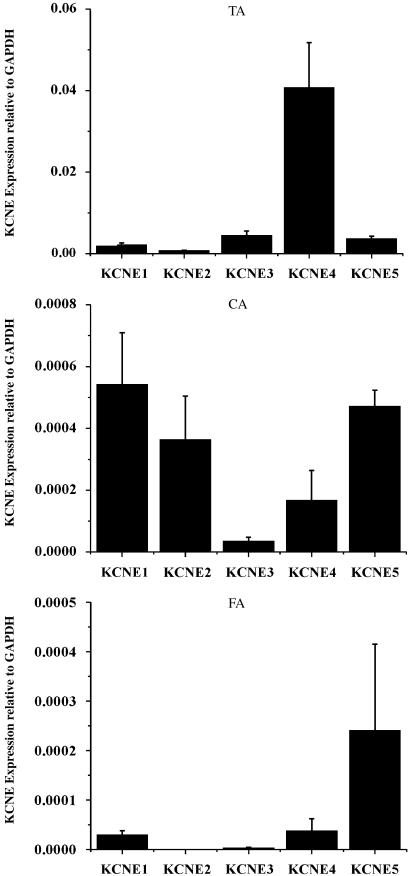
As association with KCNE gene products modulates the biophysical and pharmacological properties of Kv7 proteins, especially those encoded by KCNQ1 (Grunnet et al., 2002, 2005; McCrossan and Abbott, 2004), we also ascertained the relative abundance of KCNE genes in the aorta, carotid and femoral artery. In contrast to the expression pattern of KCNQ genes throughout the vasculature, the abundance of different KCNE mRNA was quite variable with no consistent pattern appearing (Figure 2). Certainly, KCNE4 expression was apparent in all vessels. These data raise the possibility that a number of different protein–protein interactions involving KCNQ expression products may exist in the vasculature.
Figure 2.
Vascular expression of KCNE genes. Summarized data for quantitative PCR of KCNE genes in murine thoracic aorta (TA), carotid artery (CA), femoral artery (FA). Values are shown for steady state transcripts relative to GAPDH in the same preparation (means±s.e.m; n=5).
Cellular distribution of Kv7 proteins
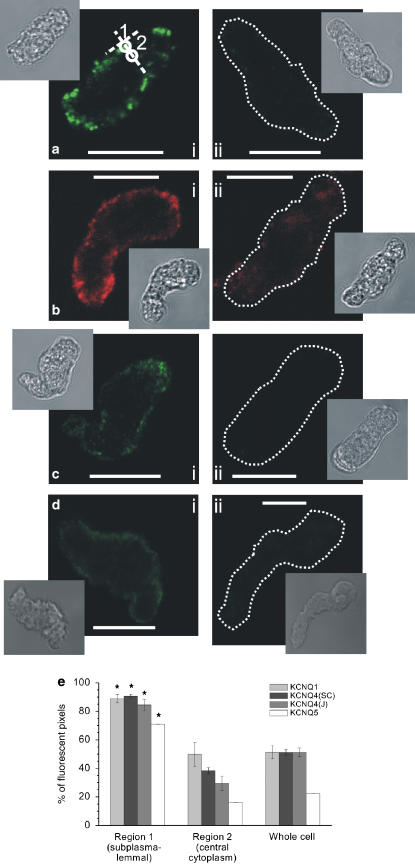
To consolidate the PCR findings, we undertook immunocytochemical imaging using different Kv7 antibodies. As Figure 3 shows smooth muscle cells isolated from mouse aorta stained positive for Kv7.1 (n=8 cells; Figure 3a) as well as Kv7.4 (n=8 cells each; Figure 3b and c) and Kv7.5 (n=6 cells; Figure 3d). The fluorescent signals for all three types of subunit were predominantly located within 1 μm of the plasma membrane, with significantly less signal originating in the cytoplasm (Figure 3e). Preincubation of the Kv7.1 antibody with its antigenic peptide (1:2 ratio of antibody to antigenic peptide) suppressed the fluorescence strongly from 29.5±2.6 (n=8) to 7.2±0.4 IU/pixel (n=8, P<0.0003; unpaired Student's t-test, Figure 3ai vs aii). Incubation of the Kv7.4SC antibody with its antigenic peptide also produced a significant reduction in the fluorescence intensity from 22.9±2.4 (n=8) to 15.9±1.5 IU/pixel (n=8, P<0.03; unpaired Student's t-test, Figure 3ci vs 3cii) although complete suppression was not observed. Similar to the Kv7.4SC antibody, the immunofluorescent signal of the Kv7.4J was located predominantly within 1 μm of plasma membrane (Figure 3c). The Kv7.5 antibody staining had a similar distribution (Figure 3d). There was virtually no fluorescent signal when the Kv7.4J or Kv7.5 antibody was omitted in control experiments (Figure 3cii (n=4) and Figure 3dii (n=6), respectively). These data show that Kv7.1, Kv7.4 and Kv7.5 channel proteins are present in the cell membranes of aortic smooth muscle cells.
Figure 3.
Immunocytochemical staining of murine aortic smooth muscle cells for Kv7.1, Kv7.4 and Kv7.5. (a) Fluorescence images of a single confocal plane of the cell labelled with Kv7.1 antibodies (ai) and with Kv7.1 antibodies preincubated with their antigenic peptide (aii). White circles in (i) indicate regions 1 and 2, which were used to analyze the localization of fluorescence (for details see Materials and methods). A dotted line was used, where necessary, to outline the contour of a cell, due to its low fluorescence. Insets in (a): transmitted light images of respective cells. Calibration: 10 μm. (b) Same as in a, but Kv7.4SC antibody was used (see Materials and methods). (c) Same as in a, but Kv7.4J antibody was used (see Materials and methods) and (cii) shows control with secondary antibodies alone. (d) Same as in a, but Kv7.5 antibody was used and (dii) shows control with secondary antibodies alone. (e) Summarized data on localization of Kv7.1, Kv7.4 and Kv7.5 fluorescence in the cells. There was significantly more fluorescence in the region within ∼1 μm of plasma membrane (region 1) than in the deep cytoplasm (region 2) or when compared with the average of whole confocal plane (region 3). *P<0.05 compared with regions 2 or 3; data are from 6–8 cells.
Vasorelaxant effects of Kv7 channel activators
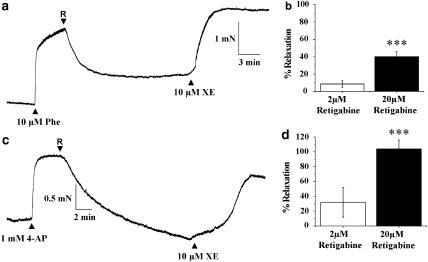
Different Kv7 channel activators were used to assess the functional impact of Kv7 in the aorta. Retigabine (2 and 20 μM) relaxed segments of aorta precontracted with 10 μM phenylephrine (Figure 4a) with a maximal relaxation of about 40%, which was not seen on application of an equivalent volume of DMSO (mean effect was +8±3%, n=8). Retigabine also fully reversed the small contraction produced by nonspecific potassium channel blockade with 1 mM 4-aminopyridine (Figure 4c). The vasorelaxant effect of 20 μM retigabine was not affected by prior incubation with 20 μM glibenclamide (mean relaxation was 40±7%, n=6, three animals) or 2 μM paxilline (mean relaxation was 78±5%, n=4, four animals), refuting an involvement of adenosine triphosphate (ATP)-sensitive or Ca2+-activated K+ channels under the conditions employed in these experiments. Retigabine had no effect on contractions produced by 60 mM KCl (mean change was 4±1%, n=8, n=4) nor on contractions produced by 10 μM XE991 (mean relaxation by 20 μM retigabine was 6±1% (n=4, three animals). XE991 also reversed the relaxant effect of retigabine in every vessel (Figure 4). These data suggest that effects of retigabine were mediated by increased K+ flux through Kv7 channels and not due to direct blockage of voltage-dependent calcium channels. An indirect relaxant effect of retigabine through generating the endothelial-derived relaxant molecules nitric oxide (NO) and prostacyclin was discounted by experiments undertaken in the presence of 100 μM L-NAME and 20 μM indomethacin, sufficient to block NO synthase and cyclooxygenase activity. Consequently, retigabine, which activates Kv7.2–7.5, is an effective relaxant of murine TA.
Figure 4.
Vasorelaxant effect of the Kv7 channel activator retigabine on precontracted aortic segments. (a) An example of the effect of 20 μM retigabine (R, applied at the arrow) on an aortic segment precontracted with 10 μM phenylephrine (Phe). Subsequent application of 10 μM XE991 (XE) reversed the relaxation produced by retigabine. (b) Mean data from 24 such experiments (11 animals) for the effect of 2 and 20 μM retigabine on Phe-induced contractions. ***P<0.01 vs. 2 μM retigabine (c) shows an example of the effect of 20 μM retigabine (R, applied at the arrow) on a contraction induced by 1 mM 4-aminopyridine (4-AP). (d) shows the mean data from 12 such experiments (five animals); ***P<0.01 vs 2 μM retigabine.
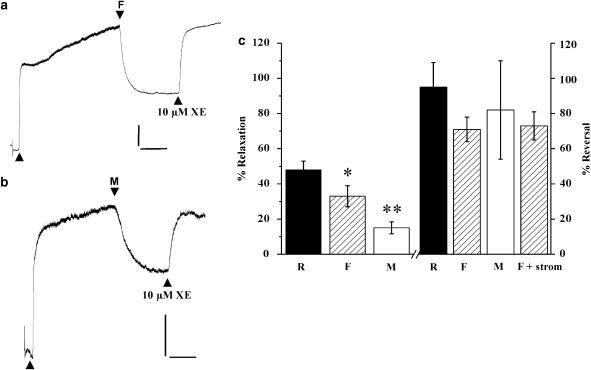
We also tested whether flupirtine, a compound structurally similar to retigabine, that activates neuronal M-currents (Wladyka and Kunze, 2006) and meclofenamic acid, a nonsteroidal anti-inflammatory agent shown to activate Kv7.2 and Kv7.3 but not Kv7.1 (Peretz et al., 2005), could also relax aortic segments precontracted with 1 μM phenylephrine (Figure 5). Application of both agents at 20 μM produced considerable relaxations that were reversed by 10 μM XE991 (Figure 5). Application of a higher concentration of meclofenamic acid (100 μM) produced considerably greater relaxation (mean was 87±9%, n=3) but these effects were poorly reversed by 10 μM XE991, suggesting an involvement of other relaxant mechanisms. As seen with retigabine, the relaxations produced by 20 μM flupirtine were not affected by preincubation with 20 μM glibenclamide (mean relaxation was 35±8%, n=6). To investigate a possible role for Kv7.1 channels, experiments were undertaken with R-L3, a benzodiazepine that augments currents generated by the overexpression of KCNQ1 in Xenopus oocytes (Seebohm et al., 2003a). R-L3 (2 and 20 μM) relaxed aortic segments precontracted with phenylephrine by 10±3 and 69±9 %, respectively (n=6 & 9). However, this agent also inhibited KCl-induced contractions markedly and relaxed contractions produced by 10 μM XE991 by 82±15 % (n=4). These effects are consistent with this agent having a direct effect on voltage-dependent calcium channels that negated any further functional studies.
Figure 5.
Vasorelaxant effect of the Kv7 channel activators flupirtine and meclofenamic acid on precontracted aortic segments. (a) An example of the effect of 20 μM flupirtine (F) on an aortic segment precontracted with 1 μM phenylephrine (first arrow on trace). Subsequent application of 10 μM XE991 (XE) reversed the relaxation produced by F. (b) An example of the effect of 20 μM meclofenamic acid (M) on an aortic segment precontracted with 1 μM phenylephrine (first arrow on trace). Subsequent application of 10 μM XE reversed the relaxation produced by M. For (a) and (b), scale bar=0.5 mN, 5 min. (c) On the left, a comparison of the effect of 20 μM retigabine (R), F and M on phenylephrine-contractions are shown. Data are the means of 29 (R), 8 (F) or 7(M) experiments (±s.e.m); *P=0.05, **P<0.01 vs results with R. On the right is shown the mean reversal of this relaxation by 10 μM XE. The last column (F+stromatoxin) showed that the ability of XE to reverse the F-induced relaxation was not affected by prior incubation with 30 nM stromatoxin. Data are the means of four to 12 experiments (±s.e.m).
Effects of Kv7 channel blockers
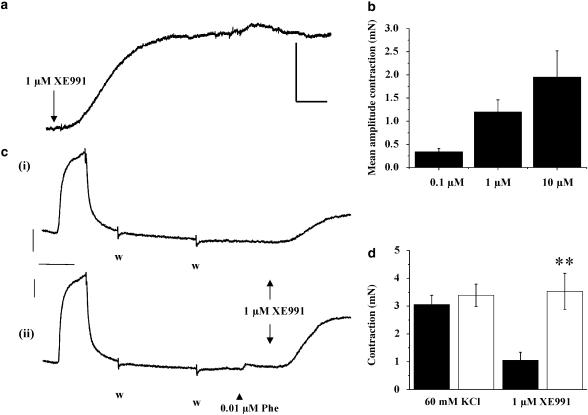
Experiments were undertaken to determine whether Kv7.x channels open at rest had an impact on vascular tone in the aorta. Application of XE991 (0.1–10 μM) to segments of TA provoked a marked increase in tension at all concentrations studied (Figure 6). In each case, the evoked contraction developed slowly although the time to peak was faster with 10 μM XE991 compared to 0.1 μM (7.4±0.9 min vs 42±7 min (n=7), paired segments). This contraction amounted to 26±8% of the contraction produced by KCl (n=13) and similar contractions were generated by 10 μM linopiridine (n=6). When a small degree of pretone was provided by the application of 0.01 μM phenylephrine (approximately EC20), considerably greater contractions were generated by 1 μM XE991 (101±10% compared to 34±7% of the KCl contraction, n=10), which also developed considerably faster than paired contractions observed in the absence of phenylephrine (Figure 6c and d). Contractions generated by the application of XE991 (0.1–10 μM) and linopiridine (10 μM) were completely abolished by blocking voltage-dependent calcium channels either directly with 1 μM nicardipine (n=11–18, Figure 7a) or indirectly by inducing membrane hyperpolarization with the ATP-sensitive K-channel opener pinacidil (10 μM, n=5–6, Figure 7b). XE991-induced contractions were not due to the depolarization of sympathetic nerves and subsequent release of noradrenaline, since 10 μM XE991 evoked a robust contraction in the presence of the α1-adrenoceptor blocker prazosin (1 μM), a concentration that prevented near-maximal contractions produced by 10 μM phenylephrine. The mean amplitude of contractions produced by 10 μM XE991 in the absence and presence of 1 μM prazosin was 2.88±1.33 and 2.43±0.58 mN (n=6 and 4, P>0.05). Application of 10 μM XE991 had no additional effect on tissues bathed in 60 mM KCl. Consequently, the mean amplitude after 15 min in KCl alone was not significantly different from the contraction produced in the same tissues bathed initially in KCl for 5 min followed by KCl and XE991 for a further 10 min (3.01±0.17 mN, compared to 2.91±0.11, n=4). These data showed that the XE991- and linopiridine-induced contractions were mediated by increased Ca2+ influx through dihydropyridinesensitive calcium channels.
Figure 6.
Vasoconstrictor effects of Kv7 blockade on tone in segments of mouse thoracic aorta. In (a), an example of the effect of 1 μM XE991 on resting tension in a segment of mouse thoracic aorta. (b). The mean amplitude of the XE991-induced contraction after 15 min application of each concentration of XE991 to different tissues. Values are the means from eight to 14 tissues (±s.e.m). (c) An example of the effect of pretone on the XE991-induced contraction. Traces show tension from aortic segments from the same animal. Washout of the chamber and replenishment with fresh bathing solution is denoted by the symbol (w). (d) shows the mean contraction produced by 1 μM XE991 in the absence (filled column) and presence (open column) of 0.01 μM phenylephrine. The mean contraction elicited by 60 mM KCl in these segments before the application of phenylephrine is shown for comparison. Data are the mean of 12 segments from six animals. **P<0.01. For (a) and (c), scale bar=1 mN, 5 min.
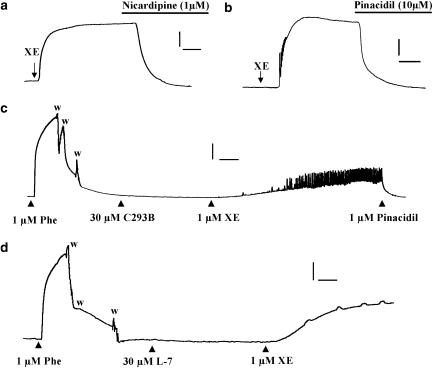
Figure 7.
Investigation of the mechanism involved with the XE991-induced contractions. The upper panels show that the contraction produced by XE991 was reversed by nicardipine (1 μM; a) or pinacidil (10 μM; b). (c and d) Lack of effect of chromanol 293B (C293B) or L-768,673 (L-7), both at 30 μM, on basal tension. Application of 1 μM XE991 (XE) in the continued presence of these agents produced robust contractions of the tissues. Phe=1 μM phenylephrine. Note in both tissues there was a tendency for the tissue to exhibit spontaneous changes in tone superimposed on the phasic increase in tension in the presence of XE991. Scale bars: (a), 2 mN, 5 min; (b), 1 mN, 3 min (c), 2 mN, 5 min; (d), 1 mN, 5 min. Washout periods in (c) and (d) shown by w.
In contrast to the effects of XE991 and linopirdine, application of two compounds that preferentially inhibit ion channels encoded by KCNQ1 had no effect on aortic tension at concentrations up to 30 μM (Figure 7c and d). Thus, neither C293B nor L-768,673 had any effect in 11 segments from six animals each although the vessel subsequently contracted on application of either 1 μM XE991 or 1 μM linopirdine (Figure 7c and d). The mean amplitude of the contractions evoked by 1 μM XE991 was 1.35±0.5 and 1.42±0.57 mN in the presence of chromanol and L-768,673, which was not different from the XE991-induced contraction evoked in the absence of these agents. C293B also failed to contract vessels precontracted by 0.01 μM phenylephrine (n=6). These data show that blockade of ion channels encoded by KCNQ by agents that do not discriminate between different isoforms had a marked spasmogenic effect in the mouse aorta, whereas specific blockers of KCNQ1-encoded channels had no apparent effect on basal tension.
Effect of Kv7 channel modulators in other blood vessels
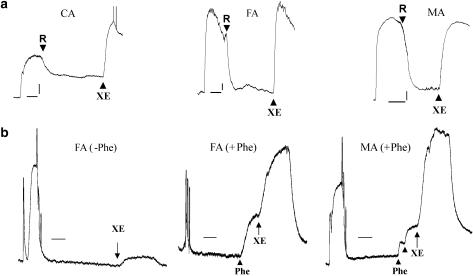
As quantitative PCR results showed KCNQ1, 4 and 5 were the abundant isoforms in murine carotid artery, femoral artery and mesenteric artery, we investigated whether modulators of Kv7 channels affected vascular reactivity in these preparations. Figure 8 shows retigabine-relaxed segments of carotid, femoral and mesenteric artery precontracted with phenylephrine with mean relaxations of 61±7, 94±8 and 73±9 %, respectively (n=5 from five animals). The relaxation produced by retigabine was reversed completely by 10 μM XE991 in all vessels (Figure 8, upper panels) consistent with this effect being mediated by Kv7 channels. As already observed in the aorta, segments of carotid and femoral artery were contracted by the KCNQ channel blocker XE991. The amplitude of contraction generated by XE991 was considerably larger if this agent was applied in the continued presence of a low concentration of phenylephrine (0.01–0.1 μM; Figure 8, first two lower panels). Interestingly, XE991 (1 μM) was able to contract segments of mesenteric artery when applied in the presence of phenylephrine (Figure 8, last lower panel), whereas XE991 was totally ineffective at this concentration in the absence of any pretone. These data show that XE991 was spasmogenic in a variety of blood vessels and that a precontraction of the tissues markedly enhanced the contractile effects of this agent.
Figure 8.
Effect of retigabine in different blood vessels. (a) The upper panels show examples of the effect of 20 μM retigabine (R) on segments of the carotid artery (CA), femoral artery (FA) and mesenteric artery (MA) precontracted by 1 μM phenylephrine. XE991 (10 μM; XE) was applied in the continued presence of R. Scale bar=0.5 mN, 5 min. (b) The lower panels show examples of the spasmogenic effect of XE991 in FA in the absence (−Phe) and presence (+Phe) of phenylephrine. The final panel is a trace of the effects of XE991 on MA in the presence (+Phe) of phenylephrine. Scale bar=0.5 mN, 4 min.
Discussion
The present study represents the first rigorous investigation of KCNQ and KCNE gene expression in different blood vessels, following on our initial study in murine portal vein (Ohya et al., 2003) and provides considerable pharmacological and molecular evidence that Kv7 channels influence reactivity across the vascular tree. It has revealed that all the blood vessels studied expressed KCNQ1 and KCNQ4 abundantly but KCNQ5 mRNA was also present. Expression of KCNE genes whose expression products modify the pharmacological and biophysical properties of Kv7 channels were also detected in every blood vessel type, although the nature of the isoforms expressed was not consistent across the vasculature. Pharmacological experiments on isolated arterial preparations showed that the Kv7.2-5-selective activators retigabine, flupirtine and meclofenamic acid relaxed precontracted segments of the aorta. Retigabine also relaxed precontracted segments of the carotid, femoral and mesenteric artery. These vessels also contracted in response to application of XE991 and linopirdine, which block all isoforms of Kv7 channels. Thus, our data suggest that these channels, hitherto considered unique to neuronal cells, play a significant role in controlling arterial tone.
Novel role for Kv7.2-5 channels in the vasculature
Previous studies on ion channels encoded by KCNQ genes have focused primarily on neurones and cardiomyocytes and have established that KCNQ1 expression is predominantly in cardiac cells as well as colonic epithelia, whereas KCNQ2-5 expression is restricted to the central nervous system, some structures in the ear and sympathetic neurones (Jentsch, 2000; Kharkovets et al., 2000; Lerche et al., 2000a; Schroeder et al., 2000). The only studies so far on native smooth muscle cells have not dispelled this concept as both rat stomach and murine portal vein cells express KCNQ1 abundantly (Ohya et al., 2002b, 2003), whereas KCNQ2-5 expression was considerably lower. The present study challenges this dogma by showing that murine aorta, carotid, femoral and mesenteric artery have a very similar KCNQ expression pattern, with KCNQ4 and KCNQ5 at levels equal to or higher than KCNQ1. Furthermore, immunocytochemistry showed that these associated proteins were distributed predominantly in the plasmalemma. These observations establish KCNQ4 and KCNQ5 as having a far wider expression profile than previously considered. It is worth stressing that although KCNQ1 is by far the most abundant gene in murine portal vein, this vessel also expressed KCNQ4 and KCNQ5 at levels higher than KCNQ2 and KCNQ3 (Ohya et al., 2003).
Functional evidence for role of KCNQ-encoded channels novel to the vasculature
The pharmacological data presented here suggest that the KCNQ expression products controlling tone in arterial preparations are unlikely to be the expected Kv7.1 channels. Thus, from the combined effects of several inhibitors and activators of KCNQ-derived potassium channels, we would argue, rather, that it is the ‘neuronal' members of Kv7.2-5 that are most likely to contribute a negative effect on depolarizing influences such as vasoconstrictors. Furthermore, the contractile effect of blocking KCNQ-derived channels in many preparations at rest indicates that these channels are likely to be open under these conditions and make an important physiological contribution to resting membrane potential. XE991 blocks all K+ channels generated by the heterologous expression of KCNQ genes with variable selectivity and IC50 values that range from 0.6 μM (KCNQ1, 2 and 3, Wang et al., 1998, 2000) to 6 μM (KCNQ4, Søgaard et al., 2001) and 50 μM (KCNQ5, Schroeder et al., 2000; Jensen et al., 2005). Currents generated by the coexpression of KCNQ1 and KCNE1 are less sensitive to XE991 (IC50 ∼11 μM, Wang et al., 2000). There are no reports of XE991 affecting other voltage-dependent or -independent K+ channels at concentrations up to 100 μM (Wang et al., 1998) except for Kv2.1, which is inhibited by 6.5 and 20% on application of 10 μM and 100 μM XE991, respectively (Wladyka and Kunze, 2006). In the present study, application of XE991 (0.1–10 μM) or linopirdine (10 μM) (which similarly blocks all KCNQ channels) evoked robust contractions in segments of TA, carotid and femoral artery similar to those reported in rodent pulmonary artery (Joshi et al., 2006). These contractions were reversed by decreasing the activity of voltage-dependent calcium channels either directly (by blockade with nicardipine or cadmium) or indirectly (by opening KATP channels with pinacidil) consistent with XE991 blocking a conductance that maintains a negative membrane potential leading to membrane depolarization. Importantly, similar pharmacological effects were observed for low concentrations of linopirdine, consistent with a role for KCNQ channels. The similar effects of XE991 and linopirdine were almost certainly not due to the depolarization of sympathetic nerves and subsequent release of noradrenaline as XE991-induced contractions were unaffected when tissues were incubated with the α1-adrenoceptor-antagonist prazosin. It is worth stressing that stromatoxin, a potent inhibitor of Kv2.1 channels did not affect basal tension and did not affect the ability of XE991 to reverse retigabine- or flupirtine-induced relaxations.
In every vessel studied, the ability of XE991 to cause contractions at rest was augmented by applying a small degree of pretone through a low concentration of phenylephrine. This phenomenon was more obvious in segments of mesenteric artery that were refractory to concentration of XE991 up to 10 μM, as reported for rat mesenteric artery by Joshi et al. (2006). However, marked contractions were generated even by low concentrations (1 μM) of XE991 if the mesenteric artery was slightly precontracted with phenylephrine. As blood vessels in vivo have a degree of tone due to myogenic and circulating influences, these experiments reveal that XE991-sensitive channels are probably important determinants of vascular reactivity throughout the body but their influence is determined by predisposing factors such as degree of channel activation or the effect of counteracting depolarizing conductances.
Having identified KCNQ channels as having an important role in arterial smooth muscle function, we aimed to use the available pharmacological tools to determine which of the gene products identified in our comprehensive screen were responsible for the observed effects of the nonspecific channel blockers XE991 and linopirdine. We were surprised to find that the blockers of Kv7.1 channels, C293B and L-768,673, did not mimic the effect of XE991 and linopirdine, suggesting that ‘neuronal' Kv7.2-7.5 mediate resting potassium currents in arterial preparations. C293B is a well-established inhibitor of Iks, the delayed rectifier current generated by the association of Kv7.1 with minK subunit encoded by KCNE1 (Lerche et al., 2000b) and L-768,673 is a recently developed benzodiazepines that blocks Kv7.1 channels in the absence and presence of KCNE1-encoded proteins (Seebohm et al., 2003b) but does not affect KCNQ2-derived channels at concentrations up to 30 μM (Seebohm et al., 2003b). However, in contrast to the effect of XE991 and linopiridine, which block all KCNQ-encoded channels, C293B and L-768,673 had no effect on basal tone in tissues that subsequently contracted to low concentrations of XE991 or linopiridine. Moreover, C293B had no effect on basal tone in aortic segments precontracted with 0.01 μM phenylephrine. At the concentrations used in the present study, C293B reduced voltage-dependent K+ currents in portal vein myocytes (Yeung and Greenwood, 2005) and 10 μM L-768,673 inhibited K+ currents generated by the expression of KCNQ1 in Xenopus oocytes (M Schwake, unpublished data). These data therefore suggest that of all the Kv7 channels present in the cell membrane, it is unlikely to be Kv7.1 channels that influence aortic tone.
KCNQ channel activators are potential vasodilator therapeutics
Consistent with the postulate that KCNQ-encoded K+ channels other than Kv7.1 influence blood flow, retigabine, which selectively activates Kv7.2-5 but not Kv7.1 channels at concentrations ⩽100 μM (Tatulian et al., 2001; Schenzer et al., 2005; Wuttke et al., 2005), relaxed precontracted aortic, carotid, femoral and mesenteric artery segments. This effect was not dependent on endothelial-derived relaxant factors and was not inhibited by glibenclamide or paxilline. Interestingly, one of the main side effects of retigabine, in clinical trials as an anti-convulsant, is the development of hypotension (Wickenden et al., 2004), which suggests that the so-called ‘neuronal' KCNQ channels have a real impact at the level of resistance vessels in vivo. The structurally related compound flupirtine and meclofenamic acid also relaxed precontracted aortic segments. The latter compound enhances Kv7.2/3 channel currents without an effect on Kv7.1 channel currents (Peretz et al., 2005) and this effect was additive to the retigabine-induced enhancement. The vasorelaxant effects of these agents that enhance Kv7.2-7.5 but do not affect Kv7.1, reinforce the hypothesis that the Kv7 ion channels involved with vascular tone are likely to be those encoded by KCNQ4 or KCNQ5, as these genes are the most abundantly expressed. The caveat of these conclusions is that we must rely on the pharmacological selectivity of the compounds used. There are no reports of effects of XE991 and linopirdine on any ion channels except those encoded by KCNQ genes at the low concentrations used in the present study. Moreover, retigabine is a very selective agent that only activates specific Kv7 isoforms (Tatulian et al., 2001; Schenzer et al., 2005; Wuttke et al., 2005) and the relaxant effects of this agent were reversed by the application of XE991 in the present studies. Together, these various pharmacological manipulations support our postulate that K+ channels encoded by KCNQ genes other than KCNQ1 influence arterial tone. Further studies with gene-silencing technology might help to confirm these observations.
In conclusion, we report that KCNQ genes previously regarded to be exclusively expressed by neurones and cardiomyocytes, have an equally important role in vascular myocytes. We have shown previously (Yeung and Greenwood, 2005) that XE991-sensitive currents in the murine portal vein were well sustained, consistent with a conductance that stabilizes the resting membrane potential. Although it was not within the scope of the present study to undertake a thorough electrophysiological study of Kv7 channel currents in the different vessels studied, we have seen XE991-sensitive currents similar to those reported in the portal vein in murine aortic and carotid artery myocytes (Yeung and Greenwood, unpublished data) and it will be the goal of future studies to characterize completely the electrophysiological aspects of the Kv7 channels in these arteries. Mutations in KCNQ genes underlie a number of disorders including hereditary arrhythmias, benign epilepsy and deafness, so that a logical corollary to our finding that KCNQ gene products constitute an important mechanism in the vasculature is that the development of essential hypertension or a predisposition to respond inappropriately to certain vascular stressors may also be congenital. It is worth stressing that our findings do not negate those of other research groups who have determined the expression of various other K+ channels in vascular myocytes (Fountain et al., 2004; Platoshyn et al., 2004; Plane et al., 2005). Certainly, the impact of Kv7 channels in dictating vascular reactivity will be coupled to a contribution from other Kv channels. The precise interplay between different voltage-dependent K+ channels will differ between individual beds and different ion channels may have a greater role in certain vascular scenarios (Plane et al., 2005).
Acknowledgments
Research in Dr Greenwood's laboratory was funded by the British Heart Foundation (PG/O3/085/15747) and Wellcome Trust (VS/05/STG/A2). This research was also supported by a Grant-in-Aid for Young Scientists from Japanese Society for the Promotion of Science (JSPS 14771290, SO), a grant (SCHW866/3) from the Deutsche Forschungsgemeinschaft (MS) and a British Heart Foundation Intermediate Research Fellowship FS/04/052 (VP). We thank Christiane Kronbach and Elbion AG (Germany) for the gift of retigabine and Dr Bernhard Westermann, Leibniz-Institut für Pflanzenbiochemie and Halle who synthesized the R-L3. The input of the following is gratefully acknowledged: Steve Johnston, Prasanth Sathiagnanam and Edward Amankwatia (SGUL).
Abbreviations
- C293B
chromanol 293B
- Retigabine
N-(2-amino-4-4-flurobenzylamino)-phenyl carbamic acid
- XE991
10,10-bis (4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride)
Conflict of interest
The authors state no conflict of interest.
References
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brueggemann LI, Moran CJ, Barakat JA, Yehy JZ, Cribbs LL, Byron KL. Vasopressin stimulates action potential firing by protein kinase C dependent inhibition of KCNQ5 in A7r5 rat aortic smooth muscle cells. Am J Physiol-Heart. 2007;292:1352–1363. doi: 10.1152/ajpheart.00065.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SJ, Cheong A, Flemming R, Mair L, Sivaprasadarao A, Beech DJ. Functional up-regulation of KCNA gene family expression in murine mesenteric resistance artery smooth muscle. J Physiol. 2004;556:29–42. doi: 10.1113/jphysiol.2003.058594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, Rasmussen HB, Ljungstøm T, Jorgensen NK, Olesen SP, et al. KCNE4 is an inhibitory subunit to the KCNQ1 channel. J Physiol. 2002;542:119–130. doi: 10.1113/jphysiol.2002.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, Olesen SP, Klaerke DA, Jespersen T. hKCNE4 inhibits the hKCNQ1 potassium current without affecting the activation kinetics. Biochem Biophys Res Commun. 2005;328:1146–1153. doi: 10.1016/j.bbrc.2005.01.071. [DOI] [PubMed] [Google Scholar]
- Herbert E, Trusz-Gluza M, Moric E, milowska-Dzielicka E, Mazurek U, Wilczok T. KCNQ1 gene mutations and the respective genotype-phenotype correlations in the long QT syndrome. Med Sci Mon. 2002;8:RA240–RA248. [PubMed] [Google Scholar]
- Jensen HS, Callø K, Jespersen T, Jensen BS, Olesen SP. The KCNQ5 potassium channel from mouse: a broadly expressed M-current like potassium channel modulated by zinc, pH and volume changes. Mol Br Res. 2005;139:52–62. doi: 10.1016/j.molbrainres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Joshi S, Balan P, Gurney AM. Pulmonary vasoconstrictor action of KCNQ potassium channel blockers. Respir Res. 2006;7:31. doi: 10.1186/1465-9921-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, et al. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci USA. 2000;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche C, Scherer CR, Seebohm G, Derst C, Wei AD, Busch AE, et al. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J Biol Chem. 2000a;275:22395–224000. doi: 10.1074/jbc.M002378200. [DOI] [PubMed] [Google Scholar]
- Lerche C, Seebohm G, Wagner CI, Scherer CR, Dehmelt L, Abitbol I, et al. Molecular Impact of MinK on the enantiospecific block of IKs by chromanols. Br J Pharmacol. 2000b;131:1503–1506. doi: 10.1038/sj.bjp.0703734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacol. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Ohya S, Horowitz B, Greenwood IA. Functional and molecular identification of ERG channels in murine portal vein myocytes. Am J Physiol Cell Physiol. 2002a;283:C866–C877. doi: 10.1152/ajpcell.00099.2002. [DOI] [PubMed] [Google Scholar]
- Ohya S, Asakura K, Muraki K, Watanabe M, Imaizumi Y. Molecular and functional expression of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am J Physiol (Gastro Liv Physiol) 2002b;282:277–287. doi: 10.1152/ajpgi.00200.2001. [DOI] [PubMed] [Google Scholar]
- Ohya S, Sergeant G, Greenwood IA, Horowitz B. Molecular variants of KCNQ channels expressed in murine portal vein myocytes: a role in delayed rectifier current. Circ Res. 2003;92:1016–1023. doi: 10.1161/01.RES.0000070880.20955.F4. [DOI] [PubMed] [Google Scholar]
- Peretz A, Degani N, Nachman R, Uziyel Y, Gibor G, Shabat D, et al. Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties. Mol Pharm. 2005;67:1053–1066. doi: 10.1124/mol.104.007112. [DOI] [PubMed] [Google Scholar]
- Plane F, Johnson R, Kerr P, Wiehler W, Thorneloe K, Ishii K, et al. Heteromultimeric Kv1 channels contribute to myogenic control of arterial diameter. Circ Res. 2005;96:216–224. doi: 10.1161/01.RES.0000154070.06421.25. [DOI] [PubMed] [Google Scholar]
- Platoshyn O, Remillard CV, Fantozzi I, Mandegar M, Sison TT, Zhang S, et al. Diversity of voltage-dependent K+ channels in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L226–L238. doi: 10.1152/ajplung.00438.2003. [DOI] [PubMed] [Google Scholar]
- Saleh S, Yeung SY, Prestwich S, Pucovsky V, Greenwood IA. Electrophysiological and molecular identification of voltage-gated sodium channels in murine vascular myocytes. J Physiol. 2005;568:155–169. doi: 10.1113/jphysiol.2005.090951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zhou A, Shen J, Spector PS, Atkinson DL, et al. Coassembly of KvLQT1 and minK (IsK) proteins to form the cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Schenzer A, Friedrich T, Pusch M, Saftig P, Jentsch TJ, Grötzinger J, et al. Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine. J Neurosci. 2005;25:5051–5060. doi: 10.1523/JNEUROSCI.0128-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Hechenberger M, Weinrich F, Kubisch C, Jentsch TJ. KCNQ5, a novel channel broadly expressed in brain, mediates M-current. J Biol Chem. 2000;275:24089–24095. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- Seebohm G, Pusch M, Chen J, Sanguinetti MC. Pharmacological activation of normal and arrhythmia-associated mutant KCNQ1 channels. Circ Res. 2003a;93:941–947. doi: 10.1161/01.RES.0000102866.67863.2B. [DOI] [PubMed] [Google Scholar]
- Seebohm G, Chen J, Strutz N, Culberson C, Lerche C, Sanguinetti MC. Molecular determinants of KCNQ1 channel block by a benzodiazepine. Mol Pharm. 2003b;64:70–77. doi: 10.1124/mol.64.1.70. [DOI] [PubMed] [Google Scholar]
- Søgaard R, Ljungstrom T, Pedersen KA, Oleson S-P, Jensen BS. KCNQ4 channels expressed in mammalian cells: functional characteristics and pharmacology. Am J Physiol (Cell Physiol) 2001;280:C859–C866. doi: 10.1152/ajpcell.2001.280.4.C859. [DOI] [PubMed] [Google Scholar]
- Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci. 2001;21:5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-S, Pan Z, Shi W, Barry BS, Wymore RS, Cohen IR, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Wang H-S, Brown BS, McKinnon D, Cohen IR. Molecular basis for differential sensitivity of KCNQ and IKs channels to the cognitive enhancer XE991. Mol Pharmacol. 2000;57:1218–1223. [PubMed] [Google Scholar]
- Wickenden AD, Roeloffs R, McNaughton-Smith G, Rigdon GC. KCNQ potassium channels: drug targets for the treatment of epilepsy and pain. Expert Opinion Ther Patents. 2004;14:1–13. [Google Scholar]
- Wladyka CL, Kunze DL. KCNQ/ M-currents contribute to the resting membrane potential in rat visceral sensory neurones. J Physiol. 2006;575:175–189. doi: 10.1113/jphysiol.2006.113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H. The new anticonvulsant retigabine favors voltage-dependent opening of the Kv7.2 (KCNQ2) channel by binding to its activation gate. Mol Pharmacol. 2005;67:1009–1017. doi: 10.1124/mol.104.010793. [DOI] [PubMed] [Google Scholar]
- Yeung SY, Greenwood IA. Electrophysiological and functional effects of the KCNQ channel blocker XE991 on murine portal vein smooth muscle cells. Br J Pharmacol. 2005;146:585–595. doi: 10.1038/sj.bjp.0706342. [DOI] [PMC free article] [PubMed] [Google Scholar]