Abstract
Background and purpose:
Most of the pharmaceuticals target G-protein-coupled receptors (GPCRs) which can generally activate different signalling events. The aim of this study was to achieve functional selectivity of corticotropin-releasing factor receptor type 1 (CRF1) ligands.
Experimental approach:
We systematically substituted urocortin, a natural peptide agonist of CRF1, with bulky amino acids (benzoyl-phenylalanine, naphthylalanine) and determined the effect of the analogues on coupling of CRF1 to Gs- and Gi-protein in human embryonic kidney cells, using receptor binding, [35S]-GTPγS binding stimulation, and cAMP accumulation assays.
Key results:
Native ligands stimulated Gs and Gi activation through CRF1, resulting in stimulation and then inhibition of cAMP accumulation. Single replacements in urocortin at positions 6–15 led, dependent on the position and nature of the substituent, to ligands that conserved Gs activity, but were devoid of Gi activity, only stimulating cAMP accumulation, and competitively antagonized the Gi activation by sauvagine. In contrast, analogues with substitutions outside this sequence non-selectively activated Gs and Gi, as urocortin did.
Conclusions and implications:
Modifications in a specific region, which we have called the signalling domain, in the polypeptide agonist urocortin resulted in analogues that behaved as agonists and, at the same time, antagonists for the activation of different G-proteins by CRF1. This finding implies significant differences between active conformations of the receptor when coupled to different G-proteins. A similar structural encoding of signalling information in other polypeptide hormone receptor ligands would result in a general concept for the development of signalling-selective drug candidates.
Keywords: CRF receptor, G-protein coupling, receptor binding, cAMP, functional selectivity, signalling domain, signalling-selective ligands
Introduction
Most of the pharmaceuticals today target G-protein-coupled receptors (GPCRs) (Davey, 2004). Generally, GPCRs are promiscuous (Wess, 1998), in that a single receptor can activate different intracellular signalling events. The new concept of ligand-dependent differential regulation of receptor-coupled effector pathways covers different phenomena such as agonist-selective signalling and ligand-dependent post-translational modifications of the receptor as well as its internalization (Urban et al., 2007). Functional selectivity of GPCR ligands has been often observed (Kenakin, 2003; Kristiansen, 2004; Urban et al., 2007), but the structural basis of this selectivity is far from known (Urban et al., 2007). A rational approach for the design of function-selective GPCR ligands will open an avenue for the discovery of new pharmaceuticals having potentially less side effects. In the extreme, with respect to the signalling of a single receptor, a ligand may function at the same time as agonist and antagonist for different transduction pathways. This has been shown measuring effects downstream of G-protein coupling, for instance, with the bombesin/gastrin-releasing peptide receptor and the ligand [D-Arg(1),D-Phe(5),D-Trp(7,9),Leu(11)]substance P (Jarpe et al., 1998; MacKinnon et al., 2001), and the oxytocin receptor and the ligand atosiban (Reversi et al., 2005).
Whether this phenomenon could be observed directly for the activation of different G-proteins at a single receptor has remained an open question. We have developed recently an easy method for the separate measurement of Gs and Gi coupling at HEK293 cells stably transfected with cDNA coding for rat corticotropin-releasing factor receptor type 1 (HEK-rCRF1 cells) (Wietfeld et al., 2004), which allows searching for functionally selective ligands for CRF1 receptors. Furthermore, we provided evidence (Berger et al., 2006) that different conformations of the G-protein-activating J-domain of this receptor, which consists of the transmembrane helices and intervening loops (Hillhouse and Grammatopoulos, 2006), are responsible for its coupling to Gs- and Gi-proteins. These findings should make it possible for us to find such ligands differing in their effect on stabilizing the Gs- and Gi-directed receptor conformations.
The aim of this study was to search for structural determinants of the most potent CRF1 agonist, urocortin (Ucn) I, that direct the signalling to Gs and Gi. For this purpose, the effect of single replacements by bulky amino acids, p-benzoyl-phenylalanine (Bpa) and naphthylalanine (2-Nal), on Gs and Gi signalling pathways was measured with HEK-rCRF1 cell membranes. We show here the existence of a signalling domain in the polypeptide hormone Ucn I, modifications of which yielded analogues which were, at the same time, agonists for Gs coupling and antagonists for Gi coupling of CRF1.
Methods
Preparation of HEK293 cell membranes
Membranes from HEK293 cells stably expressing rCRF1 were used as described earlier (Wietfeld et al., 2004) and designated as HEK-rCRF1 cell membranes. To obtain membranes with the receptor selectively coupled to Gi or Gs, the cells were pretreated with 100 ng ml−1 Pertussis toxin, which abolished the activation of Gi-proteins, or with 0.1 μM sauvagine, which selectively desensitized the activation of Gs-proteins (Wietfeld et al., 2004; Berger et al., 2006).
CRF1/G-protein coupling by Ucn I analogues estimated by stimulating binding of [35S]-GTPγS to HEK-rCRF1 cell membranes
The activation of G-proteins by CRF1 was measured by ligand-evoked stimulation of [35S]-GTPγS binding in HEK-rCRF1 cell membranes as described earlier (Wietfeld et al., 2004). For screening the Bpa-Ucn I peptides (at 1 μM) for the maximum G-protein activity (sum of Gs and Gi), membranes obtained from untreated cells were incubated at 25°C with 125 pM [35S]-GTPγS in a medium consisting of Tris–HCl (50 mM, pH 7.4), 100 mM NaCl, 0.1 μM GDP, 10 mM MgCl2, 0.2 mM ethylene glycol bis(β-aminoethylether)-N,N,N',N',-tetraacetic acid, 1 mg ml−1 bovine serum albumin (BSA) and 0.15 mM bacitracin for 120 min. The reaction was terminated by filtration through Whatman GF/B filters using a Brandel harvester (Gaithersburg, MD, USA). The potencies of the compounds in stimulating Gs and Gi activity were determined by fitting concentration–response curves for the stimulation of [35S]-GTPγS binding using membranes selectively exhibiting Gs or Gi activity (see above). The antagonism of the activation of Gi-protein by its selective antagonists was studied by conducting sauvagine concentration–response curves in presence of fixed concentrations of the antagonist peptides and drawing Schild plots (Berger et al., 2006).
Characterization of CRF1 binding in HEK-rCRF1 cell membranes using I-Tyr-sauvagine/[125I]-Tyr-sauvagine
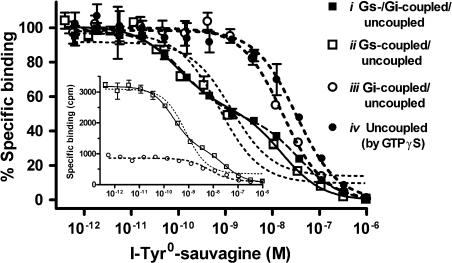
To characterize the different receptor states, homologous competition binding curves were conducted with HEK-rCRF1 cell membranes obtained from untreated cells as well as cells pretreated with Pertussis toxin or sauvagine (see above). Membranes (about 6 μg protein) were incubated with 12 pM [125I]-Tyr-sauvagine and increasing concentrations of unlabelled I-Tyr-sauvagine at exactly the same conditions optimized for the [35S]-GTPγS assay (see above). In some experiments, 30 μM GTPγS was added to the incubations to uncouple the receptor from the G-proteins. The samples were filtered through GF/C filters (Whatman) in a Brandel harvester. The Kd and Bmax values for the resulting one- or two-site binding curves were fitted by the program KELL 6 (Biosoft, Cambridge, UK). Under conditions of using tracer concentrations of as low as 12 pM, that is well below the Kd(1) of the high-affinity site (Table 1), more than 50% of the labelled sites in untreated membranes were found to be of high affinity (inset in Figure 1), which agreed well with calculations using the Kd and Bmax parameters for the high- and low-affinity sites as obtained here (Table 1) and earlier (Wietfeld et al., 2004). Therefore, if present, the high-affinity site could clearly be discriminated from the low-affinity site in the competition experiments as was shown earlier in saturation studies.
Table 1.
Kd and Bmax values for the binding of [125I]-Tyr-sauvagine/I-Tyr-sauvagine in HEK-rCRF1 cell membranes at conditions representing different states of coupling of CRF1 to G-proteins
| Coupling state of the receptora | Kd(1) (M) (±s.e.) | Kd(2) (M) (±s.e.) | % Of high-affinity site of total sites (±s.e.) | Kd (M) (1-site only) (±s.e.) | Total Bmax pmol/mg (±s.e.) | |
|---|---|---|---|---|---|---|
| i | Gs-coupled/Gi-coupled/uncoupled | 8.27 × 10−11 (±1.08 × 10−11) | 2.23 × 10−8 (±4.25 × 10−9) | 1.83 (± 0.36) | 59.80 (±2.83) | |
| ii | Gs-coupled/uncoupled | 1.20 × 10−10 | 2.19 × 10−8 | 1.42 | 57.17 | |
| (±2.26 × 10−11) | (±5.69 × 10−9) | (±0.35) | (±3.33) | |||
| iii | Gi-coupled/uncoupled | 1.23 × 10−8 | 24.12 | |||
| (±1.50 × 10−9) | (±0.15) | |||||
| iv | Uncoupled | 1.58 × 10−8 | 61.91 | |||
| (±1.75 × 10−9) | (±4.39) |
Abbreviation: HEK-rCRF1, HEK293 cells stably transfected with rCRF1.
From homologous competition binding curves as shown in Figure 1, the Kd and Bmax values (mean±s.e.) were calculated using the program Kell 6. Note that the total Bmax values do not significantly differ between the coupling states i, ii and iv, because of the low proportion of the coupling states of the receptor, that the affinity of the Gi-coupled and uncoupled state cannot be differentiated and that the desensitization of Gs leading to iii decreases the total number of receptors.
Compare legend to Figure 1.
Figure 1.
Homologous competition for receptor binding of [125I]-Tyr-sauvagine by its unlabelled form I-Tyr-sauvagine in HEK-rCRF1 cell membranes for different coupling states of the receptor. To obtain the receptor in the states (i) Gs-/Gi-coupled/uncoupled, (ii) Gs-coupled/uncoupled and (iii) Gi-coupled/uncoupled, membranes were prepared from HEK-rCRF1 cells (i) untreated, (ii) treated with 100 ng ml−1 Pertussis toxin for 24 h and (iii) treated with 0.1 μM sauvagine for 4 h. To obtain the totally uncoupled state (iv), 30 μM GTPγS was added to the incubations using membranes from untreated cells. The membranes (6 μg protein) were incubated with 12 pM [125I]-Tyr-sauvagine and increasing concentrations of unlabelled I-Tyr-sauvagine at 25°C for 2 h in the binding medium also used for the GTPγS assay. Data points were normalized with the maximum and non-specific binding taken as 100 and 0%, respectively, and represent the mean±s.d. of triplicates. Curves were fitted according to a one-site (dotted lines, valid for states iii and iv) or two-site competition model (solid lines, valid for states i and ii). The inset gives an example showing the original data for the states ii and iii, as defined above. HEK-rCRF1, HEK293 cells stably transfected with rCRF1.
CRF1 binding affinity of Ucn I analogues in HEK-rCRF1 cell membranes
For the screening of the Bpa-Ucn I peptides for CRF1 affinity, HEK-rCRF1 cell membranes obtained from untreated cells were incubated with 100 pM [125I]-Tyr-sauvagine and increasing concentrations of the peptides in 50 mM TRIS buffer (pH 7.4) at 25°C for 120 min. The reaction was terminated by filtration through Whatman GF/B filters using a Brandel harvester. At the high tracer concentration used here, the high- and low-affinity sites were not differentiated, and the competition curves were fitted according to a one-site binding model, using the program PRISM 4 (GraphPad Software, San Diego, CA, USA). The Ki values obtained, therefore, are the resultant of the receptor in its different coupling states. For some of the Bpa- and Nal-substituted compounds, the competition curves were conducted using much lower concentration of [125I]-Tyr-sauvagine (12 pM) at the conditions given above for the characterization of the high- and low-affinity receptor states. The Ki values obtained here with the program PRISM 4 are very close to their real Kd values due to the low tracer concentration used being well below its Kd value (see Table 1).
Stimulation of intracellular cAMP accumulation in HEK-rCRF1 cells
To compare the effect on the cellular cAMP of two of the Gs-selective CRF1 ligands, Bpa(7)- and Nal(9)-Ucn I, with that of the Gs- and Gi-activating peptide I-Tyr-sauvagine, each 50 000 cells/well were stimulated with the peptides in Dulbecco's modified Eagle's medium containing 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 0.5% BSA and 0.25 mM 3-isobutyl-1-methylxanthine for 30 min at 37°C. The cells were lysed at 4°C with 0.1% trifluoroacetic acid/0.005% Triton X-100. The cAMP values were determined by radioimmunoassay (Grantcharova et al., 2002).
Data analysis
All experiments were performed at least three times, each in triplicate incubations, and all response curves were fitted using the program PRISM 4 (GraphPad Software).
Materials
The peptides Ucn I, sauvagine, 3-I-Tyr0,Gln1-sauvagine (I-Tyr-sauvagine), and the benzoyl-phenylalanine (Bpa) and naphthylalanine (Nal) Ucn I analogues were synthesised in our laboratory, using standard Fmoc-chemistry and automated solid-phase synthesis technique, purified by means of preparative RP-HPLC, and their identity was proved by their correct mass. All Bpa-analogues were synthesized bearing an additional tyrosine at the N-terminus for a possible radiolabelling. [35S]-GTPγS (1250 Ci mmol−1) and 3-[125I]-Tyr0,Gln1-sauvagine ([125I]-Tyr-sauvagine) (2200 Ci mmol−1) were purchased from PerkinElmer Life Sciences (Boston, MA, USA).
Results
When CRF1 binding was characterized in HEK-rCRF1 cell membranes with the receptor in its different states, that is coupled to Gs and Gi, Gs, Gi or totally uncoupled, the Bmax values revealed that the uncoupled state predominated over all coupled states (Table 1) and confirmed the low proportion, about 1.5%, of the high-affinity site as found earlier (Wietfeld et al., 2004). The high-affinity site, which could be clearly differentiated from the low-affinity site, disappeared after de-sensitization of the Gs coupling (Figure 1). On the contrary, total uncoupling by GTPγS showed that the Gi-coupled state could not be differentiated from the uncoupled state (Figure 1 and Table 1).
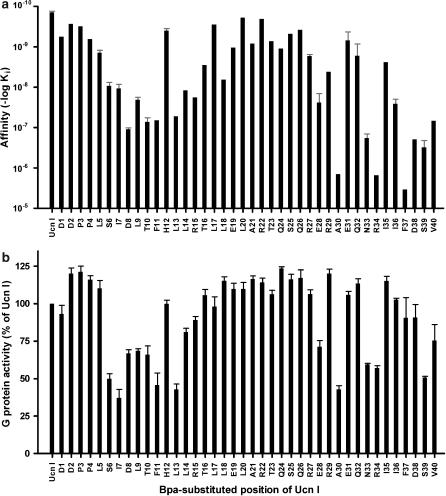
The monosubstituted Bpa-Ucn I analogues were screened for their receptor binding and total G-protein activation, that is the sum of Gs and Gi activity, in HEK-rCRF1 cell membranes to ascertain the most sensitive residues of Ucn I towards substitution (Figure 2). Single Bpa replacements at the very N-terminal positions (1–5) and within the middle part of Ucn I (17–27) led to only slight reductions in the receptor binding affinity, whereas single substitutions within the N-terminal domain (6–15) resulted in a reduced affinity and specific C-terminal replacements caused a dramatic loss of affinity (Figure 2a). The total [35S]-GTPγS binding stimulation by Bpa-Ucn I peptides was estimated at concentrations of 1 μM peptides, conditions in which natural agonists showed full intrinsic activity consisting of 28% Gs and 72% Gi activity (Wietfeld et al., 2004). In the screening (Figure 2b), analogues substituted within the N-terminal domain (6–15) and at certain C-terminal positions (28, 30, 33, 34 and 39) exhibited significantly reduced activity, whereby the low activities of the latter were explained by their extremely low affinities.
Figure 2.
Screening of monosubstituted Bpa-Ucn I analogues for receptor binding affinity and maximum stimulation of G-protein activity at HEK-rCRF1 cell membranes obtained from cells expressing Gs- as well as Gi-protein activity. (a) From competition binding curves using 100 pM [125I]-Tyr-sauvagine, fitted according to a one-site model, Ki values were obtained. These represent the resultant of the receptor in all its different states (uncoupled and coupled to Gs/Gi). When more than one screening experiment was performed, data are given as mean±s.e. (b) The sum of Gs and Gi activity was determined by measuring the stimulation of [35S]-GTPγS binding by 1 μM peptides and related to the maximum activity exhibited by 1 μM Ucn. Data are expressed as mean±s.e. of at least three independent experiments, each with triplicate incubations. HEK-rCRF1, HEK293 cells stably transfected with rCRF1.
The screening results (Figure 2) pointed to a particular role of the residues 6–15 for G-protein coupling, which was therefore investigated in more detail. For this purpose, the conditions of the receptor binding experiments, as used in the screening described above, were changed to observe high- and low-affinity sites (see Methods), and, furthermore, concentration–response curves for G-protein activation were conducted separately for Gs and Gi. Analogously to Ucn I, its analogues substituted outside of the domain 6–15 with Bpa as well as Nal exhibited a high- and low-affinity binding site, which correlated with stimulation of Gs and Gi activity, respectively (Table 2). As compared with Ucn I, we observed mostly a loss of potency and affinity that was almost parallel for the Gs- and Gi-coupled state (Table2 and shown for Bpa(5)-Ucn I in Figure 3, Figure 4).
Table 2.
Receptor affinities and potencies and intrinsic activities for G-protein activation of Bpa- and Nal-Ucn I analogues in HEK-rCRF1 cell membranes
|
Receptor affinity |
G-protein activation |
|||||
|---|---|---|---|---|---|---|
|
Gs activation |
Gi activation |
|||||
| Peptide | pKi(1) | pKi(2) | pEC50 | Activity (%) | pEC50 | Activity (%) |
| Ucn I | 10.10 | 8.40 | 10.96 | 100.00 | 9.31 | 100.00 |
| ±0.03 | ±0.04 | ±0.06 | ±0.06 | |||
| Bpa(1)-Ucn I | 8.38 | 6.58 | 8.68 | 101.72 | 7.52 | 91.55 |
| ±0.02 | ±0.01 | ±0.06 | ±7.87 | ±0.15 | ±6.01 | |
| Bpa(3)-Ucn I | 9.39 | 7.67 | 9.09 | 109.01 | 8.32 | 98.93 |
| ±0.04 | ±0.12 | ±0.08 | ±2.42 | ±0.13 | ±2.91 | |
| Bpa(5)-Ucn I | 9.72 | 7.79 | 9.94 | 97.22 | 8.69 | 109.04 |
| ±0.07 | ±0.07 | ±0.07 | ±7.24 | ±0.11 | ±3.91 | |
| Bpa(6)-Ucn I | 7.76 | —a | 7.07 | 89.02 | —b | —b |
| ±0.06 | ±0.08 | ±5.23 | ||||
| Bpa(7)-Ucn I | 8.11 | —a | 8.33 | 80.56 | —b | —b |
| ±0.04 | ±0.10 | ±5.02 | ||||
| Bpa(8)-Ucn I | ND | ND | 7.82 | 101.86 | —b | —b |
| ±0.04 | ±0.58 | |||||
| Bpa(9)-Ucn I | ND | ND | 8.49 | 88.51 | —b | —b |
| ±0.12 | ±2.82 | |||||
| Bpa(10)-Ucn I | ND | ND | 7.86 | 105.81 | —b | —b |
| ±0.04 | ±1.30 | |||||
| Bpa(11)-Ucn I | ND | ND | 7.75 | 95.45 | —c | (16.64) |
| ±0.01 | ±0.73 | |||||
| Bpa(12)-Ucn I | ND | ND | 9.71 | 102.94 | 8.20 | 92.98 |
| ±0.07 | ±7.06 | ±0.07 | ±6.42 | |||
| Bpa(13)-Ucn I | ND | ND | 7.85 | 85.58 | —b | —b |
| ±0.02 | ±1.59 | |||||
| Bpa(14)-Ucn I | ND | ND | 8.64 | 99.02 | —c | (40.15) |
| ±0.09 | ±6.21 | |||||
| Bpa(15)-Ucn I | ND | ND | 8.30 | 132.03 | —c | (69.10) |
| ±0.10 | ±4.38 | |||||
| Bpa(17)-Ucn I | ND | ND | 10.30 | 109.42 | 9.11 | 93.73 |
| ±0.05 | ±13.99 | ±0.13 | ±6.38 | |||
| Bpa(21)-Ucn I | 9.19 | 7.33 | 9.76 | 113.86 | 8.25 | 116.28 |
| ±0.18 | ±0.19 | ±0.14 | ±1.78 | ±0.11 | ±8.89 | |
| Bpa(22)-Ucn I | ND | ND | 10.27 | 109.64 | 8.78 | 95.02 |
| ±0.05 | ±4.11 | ±0.09 | ±1.44 | |||
| Bpa(40)-Ucn I | ND | ND | 8.59 | 106.97 | —c | (96.69) |
| ±0.04 | ±7.13 | |||||
| Nal(1)-Ucn I | 9.63 | 7.86 | 10.07 | 99.80 | 8.69 | 94.11 |
| ±0.13 | ±0.14 | ±0.07 | ±7.19 | ±0.15 | ±3.92 | |
| Nal(2)-Ucn I | 9.74 | 7.93 | 9.97 | 87.48 | 8.36 | 102.36 |
| ±0.28 | ±0.19 | ±0.07 | ±7.56 | ±0.07 | ±11.01 | |
| Nal(3)-Ucn I | 9.50 | 7.84 | 10.03 | 92.36 | 8.56 | 95.54 |
| ±0.15 | ±0.06 | ±0.14 | ±3.69 | ±0.04 | ±3.65 | |
| Nal(4)-Ucn I | 8.93 | 7.21 | 9.57 | 98.09 | 7.98 | 90.21 |
| ±0.10 | ±0.05 | ±0.07 | ±8.82 | ±0.15 | ±3.16 | |
| Nal(5)-Ucn I | 8.84 | 7.29 | 9.69 | 89.10 | 7.91 | 96.41 |
| ±0.03 | ±0.03 | ±0.07 | ±7.70 | ±0.06 | ±2.63 | |
| Nal(6)-Ucn I | 6.83 | —a | 7.58 | 84.77 | —b | —b |
| ±0.10 | ±0.13 | ±7.18 | ||||
| Nal(7)-Ucn I | 9.08 | 7.47 | 9.25 | 125.15 | 7.58 | 59.55 |
| ±0.20 | ±0.06 | ±0.18 | ±5.11 | ±0.10 | ±5.66 | |
| Nal(8)-Ucn I | 7.63 | —a | 8.85 | 125.61 | 7.26 | 47.20 |
| ±0.05 | ±0.04 | ±5.19 | ±0.13 | ±7.85 | ||
| Nal(9)-Ucn I | 7.19 | —a | 8.37 | 88.64 | —b | —b |
| ±0.16 | ±0.12 | ±4.86 | ||||
| Nal(10)-Ucn I | 8.24 | 6.94 | 8.53 | 121.55 | 7.47 | 53.38 |
| ±0.17 | ±0.08 | ±0.19 | ±3.62 | ±0.23 | ±4.28 | |
| Nal(11)-Ucn I | 9.83 | 7.36 | 9.55 | 99.50 | 7.89 | 87.35 |
| ±0.09 | ±0.03 | ±0.10 | ±7.03 | ±0.12 | ±5.81 | |
| Nal(12)-Ucn I | 9.87 | 7.83 | 9.75 | 91.39 | 9.11 | 58.60 |
| ±0.06 | ±0.16 | ±0.01 | ±8.30 | ±0.33 | ±4.01 | |
| Nal(13)-Ucn I | 6.79 | —a | 6.78 | 112.72 | —b | —b |
| ±0.01 | ±0.02 | ±4.56 | ||||
| Nal(14)-Ucn I | 8.93 | 7.09 | 9.27 | 105.10 | 7.13 | 101.97 |
| ±0.14 | ±0.13 | ±0.20 | ±7.18 | ±0.10 | ±3.51 | |
| Nal(15)-Ucn I | 6.86 | —a | 7.56 | 99.50 | —b | —b |
| ±0.02 | ±0.39 | ±2.68 | ||||
Abbreviation: ND, not determined.
Affinities were determined as one- or two-site Ki values in competition binding experiments using 12 pM [125I]-Tyr-sauvagine and membranes that were obtained from untreated cells (CRF1 Gs-/Gi-coupled and uncoupled). G-protein coupling was determined by the [35S]-GTPγS binding stimulation assay using membranes obtained from cells manipulated to selectively show Gs or Gi coupling. Activity is related to that of Ucn I, and the data are given as mean±s.e. from at least 3 experiments.
Only one-site binding detected.
No significant stimulation observed, the peptide being an antagonist of Gi-coupling.
No saturation reached up to 10 μM peptide.
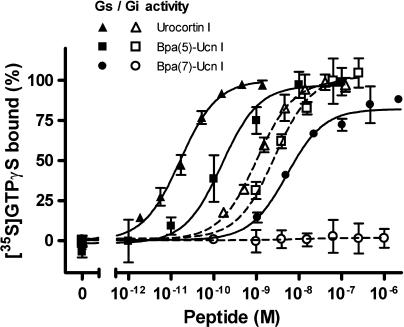
Figure 3.
Influence of Bpa(7)-Ucn I on the G-protein activity, compared with that of Ucn I and Bpa(5)-Ucn I. Using the [35S]-GTPγS binding stimulation assay, the activation of G-protein by the three peptides was determined in membranes obtained from HEK-rCRF1 cells manipulated to separately express Gs or Gi activity (see Methods). Data (mean±s.d. of triplicates) are expressed as percentage of the maximum activity exhibited by Ucn I. HEK-rCRF1, HEK293 cells stably transfected with rCRF1.
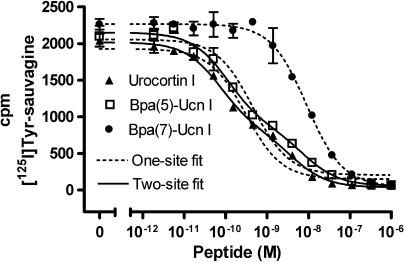
Figure 4.
Competition of Bpa(7)-Ucn I, Ucn I, and Bpa(5)-Ucn I for the binding of 12 pM [125I]-Tyr-sauvagine in membranes obtained from untreated HEK-rCRF1 cells (receptor uncoupled and coupled to Gs/Gi). Data points of membrane-bound activities represent the mean±s.d. of triplicates and were fitted according to a one-site (valid only for Bpa(7)-Ucn I) and two-site competition model (valid for Ucn I and Bpa(5)-Ucn I). HEK-rCRF1, HEK293 cells stably transfected with rCRF1.
For the Ucn I analogues substituted at positions 6–15 with the Bpa group, with the exception of position 12, the results were completely different, in that their Gi activity was much reduced or even totally abolished, whereas their intrinsic Gs activity was not or much less affected although their potency was reduced (Table 2 and shown for Bpa(7)-Ucn I in Figure 3). Furthermore, all compounds having no Gi activity at all showed only one binding site (Table 2 and Figure 4). Similar results were obtained with the Nal-analogues. However, whereas substitutions at positions 6, 9 and 13 with Bpa as well as Nal abolished totally the Gi activity, the extent of lowering the Gi activity by the two groups was not the same for the other positions 7, 8, 10–12, 14 and 15 (Table 2).
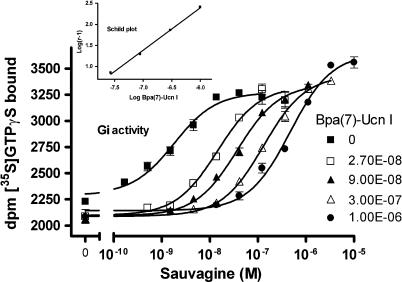
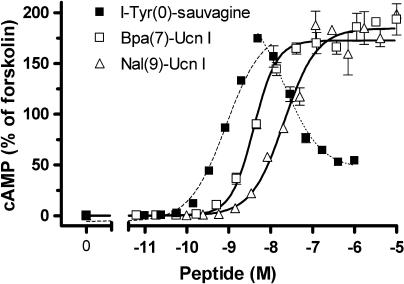
Looking at the effect of the Gs-selective agonists on concentration–response curves obtained for the natural agonists such as sauvagine, no influence on the maximum Gs and Gi activity of sauvagine was observed, however, a rightward shift was revealed only for Gi activation. An example of this effect is shown for Bpa(7)-Ucn I (Figure 5; Schild constant pKb 7.95±0.02), clearly disclosing a selective Gi antagonism. All Bpa- and Nal-Ucn I compounds that did not stimulate the Gi-protein activity (Bpa at positions 6–10 and 13, and Nal at positions 6, 9, 13 and 15, Table 2) were found to competitively antagonise the sauvagine-evoked stimulation of Gi. The absence of Gi activity of such compounds was confirmed when their effect on the accumulation of cAMP in intact HEK-rCRF1 cells was studied. It was earlier shown (Wietfeld et al., 2004) that, for the adenylyl cyclase activity in HEK-rCRF1 cell membranes, concentration–response curves of general agonists, such as sauvagine were bell-shaped, in that at subnanomolar concentrations, the cAMP production was raised with increasing concentrations of sauvagine by activating Gs, whereas at concentrations above 10 nM, the cAMP production was inhibited through the stimulation of Gi. This biphasic influence of sauvagine on cAMP was here confirmed when using intact cells (Figure 6; apparent pEC50 for the ascending part 9.04±0.09). In contrast to sauvagine, the Gs-selective agonists, such as Bpa(7)- and Nal(9)-Ucn I, exhibited exclusively stimulating curves (Figure 6), with pEC50 8.38±0.16 and 7.67±0.04, respectively.
Figure 5.
Influence of the Gs-selective Bpa(7)-Ucn I at fixed concentrations on the stimulation by sauvagine of the [35S]-GTPγS binding in HEK-rCRF1 cell membranes expressing selectively Gi activity. Data points represent the mean±s.d. of triplicates and were fitted by nonlinear regression. From the EC50 values obtained, a Schild plot for competitive antagonism was drawn (inset). HEK-rCRF1, HEK293 cells stably transfected with rCRF1.
Figure 6.
Concentration–response curves for the intracellular cAMP accumulation in HEK-rCRF1 cells evoked by I-Tyr-sauvagine and the Gs-selective ligands Bpa(7)-Ucn I and Nal(9)-Ucn I. HEK-rCRF1 cells were stimulated by the peptides for 30 min. The cellular cAMP values were determined by radioimmunoassay and are given (mean±s.d. of triplicates) as percentage of the value obtained by stimulation by 10 μM forskolin. The curves were fitted by non-linear regression, for I-Tyr-sauvagine, separately for the stimulating and inhibiting portion of the bell-shaped curve. HEK-rCRF1, HEK293 cells stably transfected with rCRF1.
Discussion
CRF1 belongs to the biologically very interesting class B GPCRs (secretin family). Natural ligands of class B GPCRs are polypeptide hormones, which have in common the finding that N-terminal truncation converts them from agonists into antagonists (growth hormone-releasing factor (Coy et al., 1985), calcitonin (Feyen et al., 1992), glucagon (Unson et al., 1989), parathyroid hormone (PTH) (Segre et al., 1979), CRF (Rivier et al., 1984)). Moreover, N-terminal modification of PTH (1–34) selectively altered activation of phospholipase C but not that of adenylyl cyclase via PTH1 (Takasu et al., 1999). This indicates a crucial role of ligand N-termini for receptor activation and allowed us to postulate that N-terminal modifications could affect signalling selectivity, if it exists. CRF1 is involved in a wide range of stress-related disorders and can couple to different G-proteins (for a review, see Dautzenberg and Hauger, 2002; Hillhouse and Grammatopoulos, 2006), at membranes obtained from HEK cells stably expressing rCRF1 (HEK-rCRF1 cells) with high, low and very low ligand potency to Gs, Gi and Gq, respectively (Wietfeld et al., 2004). The receptor is activated by several naturally occurring polypeptides such as CRF, Ucn I–III, the amphibian hormone sauvagine and the fish peptide urotensin 1, which were shown to activate Gs as well as Gi at HEK-rCRF1 cells (Wietfeld et al., 2004). This means they are not selective for the activation of Gs or Gi. On the other hand, we have provided evidence that CRF1 couples to Gs and Gi through different receptor conformations (Berger et al., 2006), which has pointed to the possibility of finding ligands selectively stabilizing one or the other conformation.
The structural characteristics that determine functional selectivity of a ligand are generally unknown (Urban et al., 2007) and the investigation of polypeptide hormones, like the ligands for CRF1, seems to be particularly attractive because earlier structure–activity relationship studies indicated the existence of activation domains within the polypeptide hormones. When the residues in Ucn I (DDPPL SIDLT FHLLR TLLEL ARTQS QRERA EQNRI IFDSV-amide), the most potent CRF1 agonist, were monosubstituted with benzoyl-phenylalanine (Bpa-Ucn I), receptor affinity and total G-protein activation were significantly lowered in the analogues substituted within the N-terminal domain 6–15 and at certain C-terminal residues (Figure 2). It should be noticed that total activity here refers to the sum of Gs and Gi activation, because the [35S]-GTPγS binding stimulation assay as used here for the determination of the G-protein activation by CRF1 estimates Gs and Gi activity and provides the possibility to selectively estimate the Gs and Gi activation after pretreatment of the cells with PTX and Gs desensitization by sauvagine, respectively, that is the small amount of Gq activation cannot be seen (Wietfeld et al., 2004).
The above results indicate that these two domains, 6–15 and the C-terminus, are the most important for receptor binding and G-protein activation. Interestingly, the substitutions with bulky residues here confirm, in principle, results of earlier structure–activity relationship studies using slight alterations, such as alanine replacements, of CRF peptides (Kornreich et al., 1992; Beyermann et al., 2000). From these investigations and ligand binding studies both on receptor chimera and soluble CRF receptor N-termini, a two-domain model was derived (for a review, see Hillhouse and Grammatopoulos, 2006). In this model, the receptor N-terminus captures the ligand C-terminus, representing the high-affinity binding site, and the ligand N-terminus then activates the receptor, presumably interacting with the juxta-membrane domain of the receptor. The activating function of the ligand N-terminus (the first 9–11 residues) was mainly deduced from the fact that corresponding N-terminal truncations of the natural agonists convert them into potent antagonists (Rivier et al., 1984).
The low G-protein activity of certain C-terminally substituted Bpa-analogues (Figure 2b) could be clearly explained by the fact that the real intrinsic activity was not reached because of the extremely low affinity of the compounds (Figure 2a). On the other hand, concentration–response curves for the selective activation of Gs- and Gi-proteins revealed a particular role of residues 6–15 for G-protein coupling, in that the corresponding Bpa-Ucn I peptides, as well as those with another bulky group, naphthylalanine (Nal-Ucn I peptides), exhibited a range of partial Gi agonism, including no Gi agonism at all, dependent on the position substituted and the substituent, whereas the Gs activity remained nearly unchanged (Table 2 and Figure 3). Therefore, the Ucn I sequence 6–15 can be considered a signalling domain per se, modifications of which can selectively exclude Gi coupling of the receptor.
A total of 10 Gs-selective compounds (Bpa-6, -7, -8, -9, -10, -13; Nal-6, -9, -13, -15) were identified which were inactive in Gi activation, as confirmed by a normal stimulating Gs effect on the cAMP accumulation as opposed to the effect of sauvagine, which at low concentration stimulated and at higher concentration lowered, via Gi activation, the amounts of cAMP in HEK-rCRF1 cells (Figure 6). Furthermore, all these compounds also competitively antagonised the Gi activation by the non-selective ligand sauvagine, as shown for Bpa(7)-Ucn I in Figure 5. That means these compounds are, at the same time, agonists for one and antagonists for another signalling pathway, at a single receptor. When effects downstream of G-protein coupling were measured for the bombesin/gastrin-releasing peptide receptor (Jarpe et al., 1998; MacKinnon et al., 2001) and the oxytocin receptor (Reversi et al., 2005) two compounds, [D-Arg(1),D-Phe(5),D-Trp(7,9),Leu(11)]substance P and atosiban, respectively, were found which also stimulated and antagonised different effects. The effect was named ‘biased agonism' (Jarpe et al., 1998), a term which only indirectly reflects the antagonism observed. With the technologies for identifying cellular activation evolving, it has become more and more clear that many ligands can differentially activate different signalling pathways mediated by a single GPCR and that this diversity can even vary depending on the cellular characteristics (Urban et al., 2007). This functional selectivity of ligands has challenged the traditional definition of intrinsic efficacy, which presently can often only be related to a specified system. To functionally classify ligands, the Gs-selective Bpa- and Nal-analogues of Ucn I may be defined by the term Gs-ago-Gi-antagonists for the HEK-rCRF1 cells investigated here.
As was earlier (Wietfeld et al., 2004) shown for the natural peptide ligands and here verified in membranes with the receptor in different coupling states (Figure 1 and Table 1), a high- and a low-affinity binding site were also responsible for the activation of Gs and Gi, respectively, by the non-selective Ucn analogues, with the corresponding affinities and potencies generally being within the same order of magnitude (Table 2; also shown for Bpa(5)-Ucn I in Figure 4). However, total uncoupling of the receptor by GTPγS revealed that the main part of the receptor remained in the uncoupled state under all conditions, which could not be differentiated from the Gi-coupled state (Figure 1 and Table 1), and which can be explained by the fact that the number of receptors was found to be much higher than that of the GTPγS binding sites in these cells (Wietfeld et al., 2004).
The competitive Gi antagonism of the Gs-selective Ucn analogues is in line with their competitive character towards labelled sauvagine at the receptor (as shown for Bpa(7)-Ucn I in Figure 4). But, remarkably, they exhibited only one binding site. (Table 2 and Figure 4). This suggests that, in contrast to general agonists, which bind more strongly to Gs-coupled than to uncoupled receptor, their affinity for the Gs-coupled receptor state is similar to that of the uncoupled one, which may indicate the existence of different Gs-coupled receptor conformations to which general and Gs-selective agonists bind. This is further complicated by the fact that peptides with similar affinities differed much more in their Gs-activating potencies (Bpa(6)-, Bpa(7)-, and Nal(8)-Ucn I, Table 2). Nevertheless, all the monosubstituted Ucn analogues used here attain, independently of whether being general or Gs-selective agonists and despite their very different potencies, almost the same efficacy for Gs activation. This could mean that, even if different active receptor states for Gs coupling exist dependent on different ligands bound, only the active conformation of the receptor domain that binds to Gs plays a crucial role for activation, which should then be the same for all these ligands. The active conformation of the receptor domain that binds to Gi should be rather different from that binding to Gs as anticipated from the selective signalling behaviour of the Gs-ago–Gi-antagonists and from the finding that a non-peptide antagonist inhibited Gs and Gi activation in HEK-rCRF1 cell membranes differently, by a competitive and an allosteric mechanism, respectively (Berger et al., 2006).
In summary, our findings show, for the first time, on the level of G-protein coupling of a class B GPCR, the phenomenon of ligand-selective signalling. This selectivity was accomplished by specific modifications of a separate signalling domain in a polypeptide hormone, yielding analogues that are, at the same time, agonists and antagonists of the activation of different G-proteins by a single receptor. This different signalling cannot be explained by a single active receptor state, but implies significant differences between active conformations of the receptor when coupled to different G-proteins. A similar structural encoding of signalling information in other peptide receptor ligands would result in a general concept for the development of signalling-selective drug candidates based on polypeptides.
Acknowledgments
We thank Monika Georgi, Gabriela Vogelreiter, Annerose Klose and Dagmar Krause for excellent technical assistance.
Abbreviations
- Bpa
p-benzoyl-phenylalanine
- CRF
corticotropin-releasing factor
- CRF1
CRF receptor type 1
- GPCR
G-protein-coupled receptor
- HEK
human embryonic kidney
- HEK-rCRF1 cells
HEK293 cells stably transfected with rCRF1
- I-Tyr-sauvagine
3-I-Tyr0,Gln1-sauvagine
- 2-Nal
naphthylalanine
- PTH
parathyroid hormone
- PTH1
PTH receptor type 1
- PTX
Pertussis toxin
- rCRF1
rat CRF1
- Ucn
urocortin
Conflict of interest
The authors state no conflict of interest.
References
- Berger H, Heinrich N, Wietfeld D, Bienert M, Beyermann M. Evidence that corticotropin-releasing factor receptor type 1 couples to Gs- and Gi-proteins through different conformations of its J-domain. Br J Pharmacol. 2006;149:942–947. doi: 10.1038/sj.bjp.0706926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyermann M, Rothemund S, Heinrich N, Fechner K, Furkert J, Dathe M, et al. A role for a helical connector between two receptor binding sites of a long-chain peptide hormone. J Biol Chem. 2000;275:5702–5709. doi: 10.1074/jbc.275.8.5702. [DOI] [PubMed] [Google Scholar]
- Coy DH, Murphy WA, Sueirasdiaz J, Coy EJ, Lance VA. Structure activity studies on the N-terminal region of growth-hormone releasing-factor. J Med Chem. 1985;28:181–185. doi: 10.1021/jm00380a006. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Davey J. G-protein-coupled receptors: new approaches to maximise the impact of GPCRs in drug discovery – 19–20 January 2004, London, UK. Expert Opin Ther Targets. 2004;8:165–170. doi: 10.1517/14728222.8.2.165. [DOI] [PubMed] [Google Scholar]
- Feyen JH, Cardinaux F, Gamse R, Bruns C, Azria M, Trechsel U. N-terminal truncation of salmon-calcitonin leads to calcitonin antagonists - structure activity relationship of n-terminally truncated salmon-calcitonin fragments in vitro and in vivo. Biochem Biophys Res Commun. 1992;187:8–13. doi: 10.1016/s0006-291x(05)81450-0. [DOI] [PubMed] [Google Scholar]
- Grantcharova E, Furkert J, Reusch HP, Krell H-W, Papsdorf G, Beyermann M, et al. The extracellular N terminus of the endothelin B (ETb) receptor is cleaved by a metalloprotease in an agonist-dependent process. J Biol Chem. 2002;277:43933–43941. doi: 10.1074/jbc.M208407200. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Jarpe MB, Knall C, Mitchell FM, Buhl AM, Duzic E, Johnson GL. -Arg(1),D-Phe(5),D-Trp(7,9),Leu(11)]substance P acts as a biased agonist toward neuropeptide and chemokine receptors. J Biol Chem. 1998;273:3097–3104. doi: 10.1074/jbc.273.5.3097. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Ligand-selective receptor conformations revisited: the promise and the problem. Trends Pharmacol Sci. 2003;24:346–354. doi: 10.1016/S0165-6147(03)00167-6. [DOI] [PubMed] [Google Scholar]
- Kornreich WD, Galyean R, Hernandez JF, Craig AG, Donaldson CJ, Yamamoto G, et al. Alanine series of ovine corticotropin releasing factor-(oCRF) – a structure activity relationship study. J Med Chem. 1992;35:1870–1876. doi: 10.1021/jm00088a024. [DOI] [PubMed] [Google Scholar]
- Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004;103:21–80. doi: 10.1016/j.pharmthera.2004.05.002. [DOI] [PubMed] [Google Scholar]
- MacKinnon AC, Waters C, Jodrell D, Haslett C, Sethi T. Bombesin and substance P analogues differentially regulate G-protein coupling to the bombesin receptor – direct evidence for biased agonism. J Biol Chem. 2001;276:28083–28091. doi: 10.1074/jbc.M009772200. [DOI] [PubMed] [Google Scholar]
- Reversi A, Rimoldi V, Marrocco T, Cassoni P, Bussolati G, Parenti M, et al. The oxytocin receptor antagonist atosiban inhibits cell growth via a ‘biased agonist' mechanism. J Biol Chem. 2005;280:16311–16318. doi: 10.1074/jbc.M409945200. [DOI] [PubMed] [Google Scholar]
- Rivier J, Rivier C, Vale W. Synthetic competitive antagonists of corticotropin-releasing factor: effect on ACTH secretion in the rat. Science. 1984;224:889–891. doi: 10.1126/science.6326264. [DOI] [PubMed] [Google Scholar]
- Segre GV, Rosenblatt M, Reiner BL, Mahaffey JE, Potts JT. Characterization of parathyroid-hormone receptors in canine renal cortical plasma-membranes using a radioiodinated sulfur-free hormone analog – correlation of binding with adenylate–cyclase activity. J Biol Chem. 1979;254:6980–6986. [PubMed] [Google Scholar]
- Takasu H, Gardella TJ, Luck MD, Potts JT, Bringhurst FR. Amino-terminal modifications of human parathyroid hormone (PTH) selectively alter phospholipase C signaling via the type 1 PTH receptor: implications for design of signal-specific PTH ligands. Biochemistry. 1999;38:13453–13460. doi: 10.1021/bi990437n. [DOI] [PubMed] [Google Scholar]
- Unson CG, Gurzenda EM, Iwasa K, Merrifield RB. Glucagon antagonists - contribution to binding and activity of the amino-terminal sequence 1–5, position-12, and the putative alpha-helical segment 19–27. J Biol Chem. 1989;264:789–794. [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Wess J. Molecular basis of receptor/G-protein-coupling selectivity. Pharmacol Ther. 1998;80:231–264. doi: 10.1016/s0163-7258(98)00030-8. [DOI] [PubMed] [Google Scholar]
- Wietfeld D, Heinrich N, Furkert J, Fechner K, Beyermann M, Bienert M, et al. Regulation of the coupling to different G-proteins of rat corticotropin-releasing factor receptor type 1 in human embryonic kidney 293 cells. J Biol Chem. 2004;279:38386–38394. doi: 10.1074/jbc.M405335200. [DOI] [PubMed] [Google Scholar]