Abstract
Background and purpose:
Recently, there has been much attention paid to understanding the molecular mechanisms underlying apoptosis and the functional consequences of apoptotic body clearance by phagocytes. In an attempt to investigate this latter aspect, the present study evaluated the anti-inflammatory effects of in vivo administration of phosphatidylserine (PS) liposomes, a well-characterised membrane component expressed during apoptosis. The participation of peroxisome proliferator-activated receptors (PPARs) in PS-mediated effects was also investigated.
Experimental approach:
The anti-inflammatory effect of PS liposomes on the delayed phase of carrageenan mouse paw oedema was studied. PS liposomes were injected at different doses and times, after carrageenan. Hind paws were collected for evaluation of interleukin-1β (IL-1β) levels, myeloperoxidase (MPO) and N-acetyl-glucosaminidase (NAG) activities and Evans blue dye leakage. Participation of PPAR pathways was explored by using PPAR antagonists (BADGE and GW9662).
Key results:
Administration of PS, but not phosphatidylcholine (PC), liposomes (20–200 mg kg−1, i.p., 8 h after carrageenan) reduced the paw oedema in a dose-dependent manner. PS liposomes were effective even when administered 24 and 48 h after carrageenan, a time at which indomethacin (1 mg kg−1, i.p.) had no significant effects. Carrageenan-induced Evans blue leakage and IL-1β production was decreased in PS-treated paws. The PPAR antagonists (BADGE and GW9662) partially prevented the anti-inflammatory effects of PS administration.
Conclusions and implications:
PS liposomes have anti-inflammatory effects in vivo that are at least partly dependent on PPAR activation. Therapeutic strategies mimicking apoptosis may be useful for the treatment of inflammatory disorders.
Keywords: apoptosis, inflammation, resolution, PPAR, phagocytosis, phosphatidylserine, liposome
Introduction
Apoptosis is an important physiological phenomenon that participates in the cellular dynamics of all metazoans (Meier et al., 2000). Throughout the 1990s, the detailed genetic and molecular basis of apoptosis had been extensively identified and characterized (Twomey and McCarthy, 2005). However, despite the wealth of data on the molecular basis of apoptosis, the consequences of the clearance of apoptotic bodies by phagocytes and other cellular interactions in the context of the tissue dynamics remain poorly investigated (Savill and Fadok, 2000). The initial suggestion was that phagocytosis of apoptotic cells would be a quiet process that would not lead to production of inflammatory mediators, contrasting from cell death by necrosis (for example, Meagher et al., 1992). However, Voll et al. (1997) and Fadok et al. (1998) provided some solid experimental data indicating that ingestion of apoptotic cells would lead to an active ‘suppressive' process and was not simply a passive lack of proinflammatory mediator production and inflammatory stimulation. Indeed, macrophages that ingested apoptotic cells released and underwent the autocrine/paracrine action of transforming growth factor-β (TGF-β), prostaglandin E2, and platelet-activating factor (Voll et al., 1997; Fadok et al., 1998). In this context, the authors proposed that apoptosis was a ‘silencer' event, rather than simply ‘silent death'.
Among the multiple changes on the surface of apoptotic cells, the best characterized feature is the loss of phospholipid arrangement and the subsequent exposure of phosphatidylserine (PS). This appears to be a central, if not obligatory, event, although not unique, in the clearance of apoptotic bodies (Fadok et al., 2001). In this context, it has been demonstrated that PS-containing liposomes can mimic some of the effects of apoptotic cells by increasing the secretion of TGF-β (Huynh et al., 2002; Hoffmann et al., 2005) and inhibiting the expression of the inducible isoform of NO synthase (NOS2) (Otsuka et al., 2004). The latter findings suggest that the interaction of apoptotic bodies and phagocytes, and not simply what happens within the dying cells, is very relevant in vivo. This perspective becomes particularly important in the case of the inflammatory process, a situation in which apoptosis takes place extensively because of the death of migrating leukocytes. Indeed, some authors have suggested that macrophage clearance of senescent neutrophils undergoing apoptosis is an injury-limiting mechanism that favours resolution rather than persistence of the inflammatory response (for example, Meagher et al., 1992; Fadok et al., 1998; Huynh et al., 2002). Apoptosis can be considered part of the sequence of events and mediators associated with the ‘resolution of inflammation' (Serhan and Savill, 2005; Serhan et al., 2007). Resolution of inflammation is also being increasingly perceived as an active and coordinated process, rather than simply due to a passive lack of inflammatory stimulation (Serhan, 2004, 2007; Serhan and Savill, 2005). Several molecules have been described to play a role in the context of the resolution of inflammation, including lipoxins, resolvins and the peroxisome proliferator-activated receptor (PPAR), a member of the nuclear receptor superfamily and ligand-activated transcription factors implicated with the gene expression related with diverse anti-inflammatory mediators (Zingarelli and Cook, 2005).
The connection between apoptosis and phagocytosis has been poorly explored in vivo (Hoffmann et al., 2005; Pinho et al., 2005; Rossi et al., 2006; Sawatzky et al., 2006; Serhan et al., 2007) and little is known about the possible mechanisms integrating these events with other resolution pathways in the dynamics of inflammation. Thus, the aims of the present work were (1) to explore the ability of PS-containing liposomes to resolve the delayed phase of paw oedema triggered by carrageenan in the mouse and (2) to investigate the participation of PPAR pathway in the anti-inflammatory effects resulting from PS administration.
Methods
Animals
Female Swiss mice (weighing 30–40 g) used in this study were housed in a temperature (23±2°C) and light-controlled (12-h light/dark cycle) room with free access to water and food. All procedures were approved by our Institutional Ethics Committee and are in accordance with NIH Animal Care Guidelines.
Paw oedema
Animals were injected intradermally (maximal volume 50 μl) with carrageenan (300 μg/paw) in the right hind paw. The contralateral paw received 50 μl of saline and was used as control. Carrageenan was dissolved in sterile Dulbecco's phosphate-buffered saline (PBS, all in mM: NaCl 137, KCl 2.7, KH2PO4 1.5, NaHPO4 8.1; pH 7.4). The delayed phase of paw oedema was measured by plethysmometry, as described previously (Ferreira, 1979; Henriques et al., 1987) at the indicated time intervals. The difference in the volume between the right and the left hind paws was taken as paw oedema and was expressed in Δ paw oedema in microliters.
Liposome preparation
Phospholipid liposomes were prepared as described previously by Huynh et al. (2002) with minor modifications. Briefly, phospholipids were dissolved in chloroform/methanol (90:10), dried in vacuum, resuspended in PBS and sonicated for 20 min. Commercial PS consisted of a mixture of PS and other phospholipids 1:1 (w/w), whereas phosphatidylcholine (PC) was more than 90% pure.
Effects of liposome treatment on Evans blue dye leakage
Animals treated with PS or PC liposomes received a single intravenous injection of Evans blue (60 mg kg−1) 24 h after carrageenan. After a further 24 h (that is, 48 h after carrageenan injection), animals were killed by cervical dislocation; paws were removed and minced before being incubated with formamide/water (1:1, v/v) for 48 h at 37°C. The optical density of the supernatants was measured at 600 nm in a spectrophotometer (model U-2001, Hitachi, Japan). Dye concentration was determined using a standard curve of Evans blue in formamide as described previously (Bertrand et al., 1993). Changes in vascular permeability were expressed as the difference in the amount of dye extravasation between the hind paw injected with carrageenan and that injected with saline.
Effects of liposome treatment on IL-1β (interleukin-1β) levels
The subcutaneous tissue of the paws was homogenized in 1.0 ml PBS (pH 7.4) containing 0.05% Tween 20, followed by centrifugation at 4°C for 10 min at 10 000 g. enzyme-linked immunosorbent assay analysis was performed as described previously (Barcelos et al., 2004), according to the manufacturer's specification (R&D Systems, Minneapolis, MN, USA). All samples were assayed in duplicate.
Effects of liposome treatment on myeloperoxidase (MPO) and N-acetyl-glucosaminidase (NAG) levels
The subcutaneous tissue of the paws was homogenized in 1.5 ml cooled (4°C) phosphate buffer (80 mM, pH 5.4) containing 0.5% (w/v) HTAB (hexadecyltrimethylammonium bromide), and centrifuged at 4°C for 20 min at 10 000g. The supernatants were saved and used for the measurement of NAG (as an index of macrophage influx) and MPO (as an index of neutrophil influx) activities, as described previously (Barcelos et al., 2004). The results were expressed in optical density units (OD 450 nm for MPO and OD 405 nm for NAG) per mg protein.
Involvement of PPARs on the anti-inflammatory effects of PS liposomes
To investigate whether the anti-inflammatory effects of PS liposomes were mediated by PPARs, the non-selective PPAR antagonist bisphenol-A diglycidyl ether (BADGE, 100 mg kg−1, stock made in dimethylsulphoxide and further diluted with saline, injected intraperitoneal 2 h before liposome treatment) and the PPAR-γ-selective antagonist GW9662 (2-chloro-5-nitro-N-phenylbenzamide; 30 nmol intraplantarly, 30 min before PS treatment) were used. The PPAR-γ agonist, rosiglitazone (30 mg kg−1) was used as a positive control.
Data analysis and statistical procedures
Data are expressed as mean±s.e.m. of n animals. Statistical significance was analysed by one- and two-way analysis of variance (ANOVA) followed by t-test subjected to the Bonferroni or Dunnett correction, as indicated. P<0.05 were considered significant. Statistical analysis was performed using Graph-Pad Prism (San Diego, CA, USA) software.
Reagents
Carrageenan lambda type IV, Evans blue, 3,3-5,5-tetramethylbenzidine, p-nitrophenyl-N-acetyl-b-D-glucosaminide, BADGE, HTAB and 4-nitrophenyl-α-D-mannopyranoside were purchased from Sigma Chemical Co. (St Louis, MO, USA). Lipids were purchased from Lipoid GMBH (Ludwigshafen, Germany). GW9662 (2-chloro-5-nitro-N-phenylbenzamide) was kindly donated by Dr Fernando Cunha (Department of Pharmacology, Ribeirão Preto Medical Faculty, University of São Paulo, Brazil). Dexamethasone (Decadron, Aché) and rosiglitazone (Avandia, GlaxoSmithKline) were used from commercially available products.
Results
Dose and time-dependent effects of the administration of PS or PC liposomes on the second phase of carrageenan-induced paw oedema
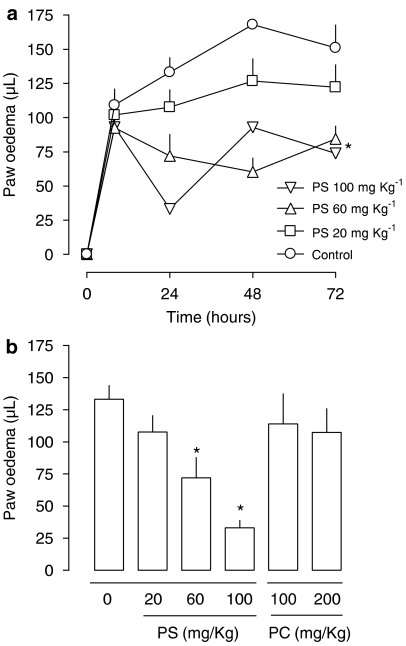
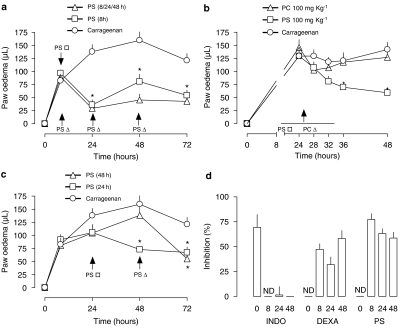
The intraperitoneal injection of PS, but not with PC, liposomes 8 h after carrageenan reduced the second phase of paw oedema measured 24 h after the inflammatory stimulus (Figure 1). The EC50 for PS was approximately 60 mg kg−1. Results from experiments exploring aspects of the kinetics of the inhibitory effect of PS liposomes are presented in Figure 2. A single injection of PS liposomes 8 h after the inflammatory stimulus caused an inhibitory effect identical to that caused by repeated administration of liposomes (Figure 2a). The time needed for the onset of the anti-inflammatory effect of PS liposome was 8 h (Figure 2b). Importantly, anti-inflammatory effects of PS liposomes could be observed even when they were administered 48 h after the stimulus (Figure 2c). The anti-inflammatory effects of PS liposomes resembled those of dexamethasone, but not of indomethacin. In this regard, indomethacin did not affect paw oedema when given after the stimulus, whereas delayed administration of dexamethasone or PS substantially reduced the delayed phase of paw oedema (Figure 2d). PC liposomes did not affect paw oedema at any time or concentration tested.
Figure 1.
Dose dependence and time course of phosphatidylserine (PS) or phosphatidylcholine (PC) liposomes on the delayed phase of mouse paw oedema induced by carrageenan. (a) PS liposomes were injected intraperitonealy 8 h after the intraplantar injection of carrageenan (300 μg/paw) at doses of 20, 60 and 100 mg kg−1. The control group received carrageenan only. Statistical analysis was performed using two-way ANOVA. The symbol * means that the two curves are statistically different from the control curve. Where no error bar appears, they are covered by the symbols. (b) Paw oedema was evaluated 24 h after carrageenan injection. PS or PC (phosphatidylcholine) liposomes were injected intraperitoneal at the indicated doses 8 h after the intraplantar injection of carrageenan. Each point or bar represents the mean of 6–8 animals and vertical lines are s.e.m. *P<0.05 compared to the control group. Statistical analysis was performed using one-way ANOVA test followed by Bonferroni's post hoc t-test. ANOVA, analysis of variance.
Figure 2.
Effect of the time of injection of phosphatidylserine (PS) or phosphatidylcholine (PC) liposomes on the delayed carrageenan-induced mouse paw oedema and the comparison with reference anti-inflammatory compounds. (a) PS liposomes (100 mg kg−1, intraperitonealy) were injected once 8 h or several times (8, 24 and 48 h) after the intraplantar injection of carrageenan. (b) Onset of the effect of a single injection of PS and PC liposomes (100 mg kg−1, intraperitonealy, 24 h) after carrageenan injection. (c) PS liposomes (100 mg kg−1, intraperitonealy) were injected once 24 or 48 h after carrageenan. (d) Comparative oedema inhibitory effect (as percentage of the value of carrageenan-induced oedema=100%) of indomethacin (1 mg kg−1), dexamethasone (0.5 mg kg−1) or PS liposomes (100 mg kg−1) administered intraperitoneal (bars labelled 0) at different times after carrageenan. Each point or bar represents the mean of 6–8 animals and vertical lines are s.e.m. *P<0.05 compared to the respective point in the control curve. Statistical analysis was performed using ANOVA test followed by Bonferroni's post hoc t-test. ANOVA, analysis of variance; ND, not determined.
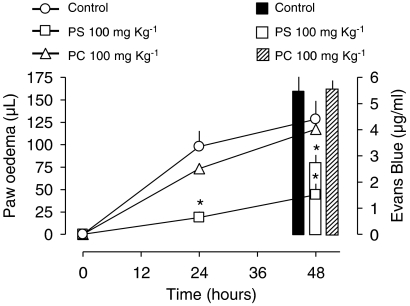
Effects of PS and PC liposome treatment on dye leakage
The second phase of mouse paw oedema induced by carrageenan was paralleled by an increase of plasma exudation, as assessed by Evans Blue leakage (Figure 3). Treatment with PS liposomes (100 mg kg−1 intraperitonealy, 8 h after carrageenan) reduced both oedema and dye leakage, whereas PC liposomes were ineffective in this regard (Figure 3).
Figure 3.
Effect of phosphatidylserine (PS) or phosphatidylcholine (PC) liposomes on the delayed carrageenan mouse paw oedema and Evans blue dye leakage. Liposomes (100 mg kg−1) of PS or PC were injected intraperitoneal 8 h and Evans blue dye (60 mg kg−1) was injected 24 h after carrageenan (300 μg/paw). Evans blue exudation (as μg ml−1) was quantified immediately after the last oedema reading. For the sake of clarity, bars were drawn apart but measurements were all made 48 h after carrageenan. Each point or bar represents the mean of 6–8 animals and vertical lines are s.e.m. *P<0.05 compared to the respective control group (carrageenan only). Statistical analysis was performed using ANOVA test followed by Bonferroni's post hoc t-test. ANOVA, analysis of variance.
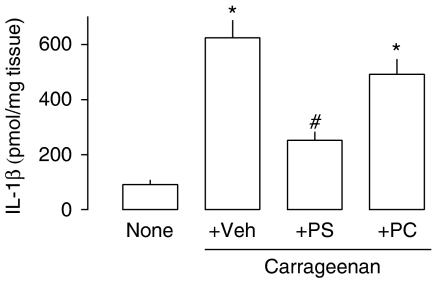
Effects of PS and PC liposomes on IL-1β levels and MPO and NAG activities
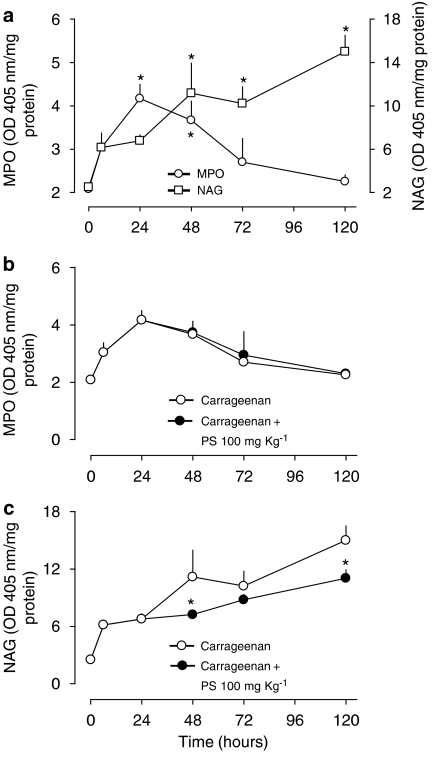
The injection of carrageenan was associated with a marked increase in the levels of IL-1β (Figure 4) at 48 h. Treatment with PS, but not with PC, liposomes halved IL-1β levels in the paw tissue (Figure 4). Carrageenan also induced increases in the activities of MPO, a marker of neutrophil infiltration, and NAG, a marker of mononuclear cell infiltration (Figure 5). Whereas the influx of neutrophils peaked at 24 h and was virtually resolved at 72 h, the influx of macrophages had a more delayed kinetics. The administration of PS liposomes 24 h after the inflammatory stimulus did not affect neutrophil influx (Figure 5b) but significantly decreased macrophage accumulation, as assessed by NAG measurement (Figure 5c).
Figure 4.
Interleukin1-β levels in mouse paw tissue treated with phosphatidylserine (PS) or phosphatidylcholine (PC) liposomes after intraplantar injection of carrageenan. PS or PC liposomes (100 mg kg−1) were injected intraperitoneal 24 h after carrageenan (300 μg/paw) injection. Forty-eight hours after carrageenan injection, IL-1β levels in the supernatant of the homogenized paw tissue were assessed by enzyme-linked immunosorbent assay. Each bar represents the mean of 6–8 animals and vertical lines are the s.e.m. *P<0.05 compared to naïve and #P<0.05 compared to carrageenan (Veh) animals. Statistical analysis was performed using ANOVA test followed by Bonferroni's post hoc t-test. ANOVA, analysis of variance; none, naïve animals; Veh, animals injected with saline.
Figure 5.
Myeloperoxidase (MPO) and N-acetyl-glucosaminidase (NAG) levels in paws of mice treated with phosphatidylserine (PS) or phosphatidylcholine liposomes after intraplantar injection of carrageenan. (a) Time course of MPO and NAG activity in the mouse paw after carrageenan (300 μg/paw) injection. *P<0.05 compared to the respective basal value (time 0). Statistical analysis was performed using ANOVA test followed by Dunnett's post hoc t-test. (b) MPO activity and (c) NAG activity. PS liposomes (100 mg kg−1) were injected intraperitoneal 24 h after carrageenan. Each point represents the mean of 6–8 animals and vertical lines are s.e.m. *P<0.05 compared to the respective point in the control (carrageenan) curve. Statistical analysis was performed using ANOVA test followed by Bonferroni's post hoc t-test. ANOVA, analysis of variance.
Involvement of PPAR pathway in liposome effects
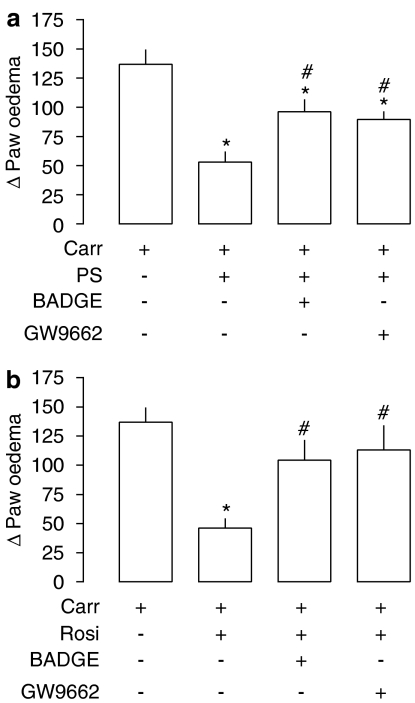
The administration of BADGE (100 mg kg−1, intraperitonealy) or GW9662 (30 nmol, intraplantarly) 2 h before PS liposomes partially reversed the anti-oedematogenic effects of PS liposomes (Figure 6a). Rosiglitazone, a PPAR-γ agonist, was used as a positive control for the antagonists used. Similarly to PS liposomes, the anti-oedematogenic effect of rosiglitazone was blocked by the PPAR antagonists tested (Figure 6b).
Figure 6.
Involvement of PPAR activation in the anti-inflammatory effect of phosphatidylserine liposomes. (a) Effects of PPAR antagonists on PS anti-inflammatory effects. The PPAR-γ non-selective antagonist, BADGE (100 mg kg−1, intraperitonealy) was injected 2 h before PS (100 mg kg−1, intraperitonealy) and the selective antagonist GW9662 (30 nmol per paw) was injected 30 min before PS. (b) Effects of PPAR antagonists on rosiglitazone (Rosi; 30 mg kg−1, intraperitonealy) anti-inflammatory effects. *P<0.05 compared to carrageenan (Carr); #P<0.05 compared to carrageenan+PS or + rosiglitazone animals. Statistical analysis was performed using ANOVA test followed by Bonferroni's post hoc t-test. ANOVA, analysis of variance; PPAR, peroxisome proliferator-activated receptors; PS, phosphatidylserine.
Discussion and conclusions
In the present work, we observed that the administration of PS, but not with PC, liposomes resulted in inhibition of the delayed phase of carrageenan-induced mouse paw oedema. These results are consistent with the notion that signalling by endogenous PS, an important feature of macrophage and apoptotic cell interaction, may participate in the resolution of inflammation. Although the ability of PS to enhance inflammation resolution has been explored in in vitro studies (see for instance Fadok et al., 1998; Aramaki, 2000), few studies have addressed this question in vivo (Huynh et al., 2002; Hoffmann et al., 2005; Pinho et al., 2005; Rossi et al., 2006; Sawatzky et al., 2006; Serhan et al., 2007). The main finding of the present report is to demonstrate that PS can modify an inflammatory response in an in vivo setting, even hours after the administration of the inflammatory stimulus.
Huynh et al. (2002) was first to propose that the structure of PS liposomes could mimic the functional effects of apoptosis in macrophages. Indeed, apoptotic cells could trigger expression of TGF-β by macrophages. However, opsonized apoptotic cells or apoptotic cells that did not express PS during apoptosis failed to induce TGF-β (Huynh et al., 2002). The relevance of PS for the effects of apoptotic cells was demonstrated by transfer experiments in which PS liposomes or PS directly transferred onto cell surface membranes restored TGF-β induction by macrophages (Huynh et al., 2002). Further studies have established that the consequences of PS interaction with macrophages resemble many of the features resulting from phagocytosis of apoptotic cells, including inhibition of tumour necrosis factor-α (TNF-α) secretion (Fadok et al., 1998; Huynh et al., 2002), NOS2 expression (Aramaki, 2000) and increases in TGF-β production (Fadok et al., 1998; Huynh et al., 2002). PS expression on the external membrane layer of dying cells is the most studied, albeit not unique, structural modification in apoptotic cells that triggers macrophage clearance behaviour (Henson and Hume, 2006). Hoffmann et al. (2001) in a study evaluating the phagocytosis of apoptotic cells demonstrated that, regardless of receptor type engaged on the phagocyte, ingestion did not occur in the absence of PS expression on apoptotic bodies.
In the present work, the kinetics of the inhibition of oedema formation by PS liposome treatment was investigated. A single administration of PS liposomes 8 h after the inflammatory stimulus resulted in the same degree of anti-inflammatory effect as observed with repeated administrations. Additionally, we found that the anti-oedematogenic effect of PS liposomes was first noticeable only 8 h after treatment. Altogether, these findings indicate that the consequences of PS administration are long lasting and have a lag phase of few hours. The latter results are consistent with the need for protein synthesis. It is well documented that after interaction with apoptotic cells or PS liposomes, macrophages switch their cytokine production pattern and start producing large amounts of TGF-β (Fadok et al., 1998; Huynh et al., 2002), a cytokine shown to decrease NOS2 expression (Aramaki, 2000) and promote matrix synthesis (Freire-de-Lima et al., 2000). It will be important to investigate in future studies whether cytokines such as TGF-β play a role in our system.
Treatment with dexamethasone after the administration of carrageenan produced anti-oedematogenic effects that were similar to those of PS-containing liposomes. In contrast, indomethacin, a conventional non-steroidal anti-inflammatory drug, did not inhibit oedema formation when given hours after the administration of carrageenan. Our findings are consistent with recent studies that indicated that inhibition of COX in the late phases of inflammation was without effect or could even worsen the inflammatory process (Gilroy et al., 1999; Gilroy and Colville-Nash, 2000).
It was also observed that concomitantly with oedema formation, there was an increase of vascular permeability and of the local production of IL-1β, events reported to occur in the delayed phase of mouse paw oedema (Zhang et al., 2002; Fernandes and Assreuy, 2004). Treatment with PS, but not with PC, liposomes inhibited the vascular permeability and decreased the local concentrations of IL-1β along with the anti-oedematogenic effect. IL-1β may facilitate the inflammatory process by promoting expression of adhesion molecules, leukocyte migration and increase of vascular permeability (Dinarello, 1993; Hallegua and Weisman, 2002). Thus, the ability of PS liposomes to reduce IL-1β production in the paw represents an important anti-inflammatory event. Consistent with our findings, Fadok et al. (1998) demonstrated that macrophages fed with apoptotic cells had reduced secretion of proinflammatory cytokines, including IL-1β and TNF-α.
The measurement of MPO and NAG activities in tissues is thought to be good indicators of granulocyte and mononuclear cell infiltration, respectively (Werner and Szelenyi, 1992; Barcelos et al., 2004). The decrease in the delayed phase of paw oedema (48–120 h) coincided with a decrease in MPO activity and an increase in NAG activity. PS liposomes reduced the influx of mononuclear cells but, curiously, had no effect on polymorphonuclear cell influx. The effects of PS liposomes on mononuclear cells are consistent with the partial inhibition of IL-1β production and a role for IL-1β in the recruitment of these leukocytes (Frode et al., 2001). It is of note that PS liposomes were given at 24 h after carrageenan at the peak of polymorphonuclear influx. It is, thus, possible that polymorphonuclear influx was already resolving before the full effect of PS liposomes occurred. Further studies are under way to evaluate this issue.
PPARs are members of the nuclear hormone receptor superfamily of ligand-activated transcription factors. Particularly, the PPAR-γ receptor appears to play a pivotal participation in cellular proliferation and inflammation (Nencioni et al., 2003). In the context of inflammation resolution, PPAR-γ activation has been linked to the expression of many anti-inflammatory cytokines, to downregulation of NOS2 and to reduction in proinflammatory cytokine expression (Strandiford et al., 2005). In addition, Cuzzocrea et al. (2004) demonstrated that rosiglitazone, a PPAR-γ agonist, diminished the delayed phase of carrageenan-induced paw oedema in the mouse, an effect blocked by PPAR antagonists. In our experiments, both PPAR antagonists, BADGE and GW9662, partially blocked the anti-oedematogenic effect of PS liposomes, suggesting the participation of PPAR during PS signalling. Thus, the present report provides strong pharmacological evidence that PPAR activation is triggered by and underlies the ability of PS liposome treatment to reduce the late phase of inflammation induced by carrageenan injection in the mouse.
The resolution of inflammation is an active process that is not simply due to the passive cessation of the inflammatory stimulus. In the present report, we explored the notion that apoptotic mimicry may modify the duration of inflammation. Indeed, we demonstrated that the administration of PS-containing liposomes reduced the delayed phase of carrageenan-triggered mouse paw oedema. Moreover, we showed that the effects of PS liposomes were partly dependent on PPAR activation. Our study reinforces the concept that exploiting mechanisms of inflammation resolution may lead to the development of novel anti-inflammatory strategies.
Acknowledgments
The skillful technical assistance of Mrs Adriane Madeira is gratefully acknowledged. We also thank Dr João Batista Calixto (UFSC) for allowing the use of his facilities and Dr Fernando Cunha (USP) for the gift of GW9662. Work in JA laboratory is supported by grants and fellowships from CNPq, CAPES, PRONEX and FAPESC, Brazil.
Abbreviations
- BADGE
bisphenol-A diglycidyl ether
- GW9662
2-chloro-5-nitro-N-phenylbenzamide
- HTAB
hexadecyltrimethylammonium bromide
- IL-1β
interleukin-1β
- MPO
myeloperoxidase
- NAG
N-acetyl-b-D-glucosaminidase
- PAF
platelet-activating factor
- PC
phosphatidylcholine
- PGE2
prostaglandin E2
- PPAR
peroxisome proliferator-activated receptor
- PS
phosphatidylserine
- TGF-β
transforming growth factor-β
- TMB
3,3-5,5-tetramethylbenzidine
- TNF-α
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Aramaki Y. Liposomes as immunomodulator-inhibitory effect of liposomes on NO production from macrophages. Biol Pharm Bull. 2000;23:1267–1274. doi: 10.1248/bpb.23.1267. [DOI] [PubMed] [Google Scholar]
- Barcelos LS, Talvani A, Teixeira AS, Cassali GD, Andrade SP, Teixeira MM. Production and in vivo effects of chemokines CXCL1-3/KC and CCL2/JE in a model of inflammatory angiogenesis in mice. Inflamm Res. 2004;53:576–584. doi: 10.1007/s00011-004-1299-4. [DOI] [PubMed] [Google Scholar]
- Bertrand C, Geppetti P, Baker J, Yamawaki I, Wandel J. Role of neurogenic inflammation in antigen-induced vascular extravasation in guinea pig trachea. J Immunol. 1993;150:1479–1485. [PubMed] [Google Scholar]
- Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Maffia P, Pattel NSA, et al. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-γ, reduces acute inflammation. Eur J Pharmacol. 2004;483:79–93. doi: 10.1016/j.ejphar.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Modalities for reducing interleukin 1 activity in disease. Immunol Today. 1993;14:260–264. doi: 10.1016/0167-5699(93)90042-J. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGFβ, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, deCathelineau A, Deleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- Fernandes D, Assreuy J. Involvement of guanylate cyclase and potassium channels on the delayed phase of mouse carrageenan-induced paw oedema. Eur J Pharmacol. 2004;501:209–214. doi: 10.1016/j.ejphar.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Ferreira SH. A new method for variations of rat paw volume. J Pharm Pharmacol. 1979;31:648. doi: 10.1111/j.2042-7158.1979.tb13616.x. [DOI] [PubMed] [Google Scholar]
- Freire-de-Lima CG, Nascimento DO, Soares MBP, Bozza PT, Castro-Faria-Neto HC, Mello FG, et al. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- Frode TS, Souza GE, Calixto JB. The modulatory role played by TNF-alpha and IL-1 beta in the inflammatory responses induced by carrageenan in the mouse model of pleurisy. Cytokine. 2001;13:162–168. doi: 10.1006/cyto.2000.0816. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR. New insights into the role of COX 2 in inflammation. J Mol Med. 2000;78:121–129. doi: 10.1007/s001090000094. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clarck MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nature Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Hallegua DS, Weisman MH. Potential therapeutic uses of interleukin 1 receptor antagonists in human diseases. Ann Rheum Dis. 2002;61:960–967. doi: 10.1136/ard.61.11.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques MG, Silva PM, Martins MA, Flores CA, Cunha FQ, Assreuy-Filho J, et al. Mouse paw edema. A model for inflammation. Braz J Med Biol Res. 1987;20:243–249. [PubMed] [Google Scholar]
- Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27:244–250. doi: 10.1016/j.it.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann PR, Kench JA, Vondracek A, Kruk A, Daleke DL, Jordan M, et al. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol. 2005;174:1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher LC, Savill JS, Baker A, Fuller RW, Haslett C. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. J Leukoc Biol. 1992;52:269–273. [PubMed] [Google Scholar]
- Meier P, Finch A, Evan G. Apoptosis in development. Nature. 2000;407:796–801. doi: 10.1038/35037734. [DOI] [PubMed] [Google Scholar]
- Nencioni A, Wesselborg S, Brossart P. Role of peroxisome proliferator-activated receptor gamma and its ligands in the control of immune responses. Crit Rev Immunol. 2003;23:1–13. doi: 10.1615/critrevimmunol.v23.i12.10. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Tsuchiya S, Aramaki Y. Involvement of ERK, a MAP kinase, in the production of TGF-beta by macrophages treated with liposomes composed of phosphatidylserine. Biochem Biophys Res Commun. 2004;324:1400–1405. doi: 10.1016/j.bbrc.2004.09.198. [DOI] [PubMed] [Google Scholar]
- Pinho V, Souza DG, Barsante MM, Hamer FP, De Freitas MS, Rossi AG, et al. Phosphoinositide-3 kinases critically regulate the recruitment and survival of eosinophils in vivo: importance for the resolution of allergic inflammation. J Leuk Biol. 2005;77:800–810. doi: 10.1189/jlb.0704386. [DOI] [PubMed] [Google Scholar]
- Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nature Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- Savill J, Fadok VA. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- Sawatzky DA, Willoughby DA, Colville-Nash PR, Rossi AG. The involvement of apoptosis-modulating proteins ERK, Bcl-XL, and Bax in the resolution of acute inflammation in vivo. Am J Pathol. 2006;168:33–41. doi: 10.2353/ajpath.2006.050058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol. 2004;122:305–321. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LAJ, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nature Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Strandiford TJ, Keshamouni VG, Reddy RC. Peroxisome proliferator-activated receptor-{gamma} as a regulator of lung inflammation and repair. Proc Am Thorac Soc. 2005;2:223–231. doi: 10.1513/pats.200501-010AC. [DOI] [PubMed] [Google Scholar]
- Twomey C, McCarthy JV. Pathways of apoptosis and importance in development. J Cell Mol Med. 2005;9:345–359. doi: 10.1111/j.1582-4934.2005.tb00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- Werner U, Szelenyi I.Measurement of MPO activity as model for detection of granulocyte infiltration in different tissues Agents Actions 1992C101–C103.Spec No: [PubMed]
- Zhang Y, Wang JZ, Wu YJ, Li WG. Anti-inflammatory effect of recombinant human superoxide dismutase in rats and mice and its mechanism. Acta Pharmacol Sin. 2002;23:439–444. [PubMed] [Google Scholar]
- Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation. Shock. 2005;23:393–399. doi: 10.1097/01.shk.0000160521.91363.88. [DOI] [PubMed] [Google Scholar]