Abstract
Background and purpose:
Specific and selective inhibitors for mGlu1 receptors are presently inadequate. A new generation of non-competitive mGlu1 antagonists with low nanomolar potencies is emerging. We evaluated two new compounds, YM-298198 and JNJ16259685, for effectiveness, potency and specificity for the first time in a brain slice preparation.
Experimental approach:
Patch-clamp recording of Purkinje neurones in cerebellar slices were obtained. The slow mGlu1-mediated EPSP was used to establish a concentration-response curve. Fast excitatory synaptic inputs were tested for non-specific effects.
Key results:
YM-298198 and JNJ16259685 inhibited the synaptic activation of mGlu1 in a concentration-dependent manner (IC50 values of 24 nM and 19 nM, respectively). The antagonists were slow to inhibit and to reverse on washout, probably due to their lipophilic nature. There were no non-specific effects on fast AMPA receptor-mediated synaptic transmission in the cerebellum.
Conclusions and implications:
These compounds are more than a thousand-fold more potent than previously available compounds. Their selectivity and specificity will be very useful for studying the role of mGlu1 receptors both in vitro and in vivo.
Keywords: metabotropic glutamate receptors, cerebellum, Purkinje cell
Introduction
The excitatory amino acid neurotransmitter glutamate is known to act via three types of ionotropic (AMPA, NMDA and kainate) and eight types of metabotropic (mGlu1-8) receptor (for a review see Kew and Kemp, 2005). The heterogeneous distribution (Martin et al., 1992; Shigemoto et al., 1992; Baude et al., 1993) throughout the nervous system of mGlu1, the first mGlu receptor to be cloned (Houamed et al., 1991; Masu et al., 1991), already suggested its role in multiple brain functions. Functional evidence for the involvement of mGlu1 receptors has been obtained using two techniques: targeted disruption of the gene (Aiba et al., 1994; Conquet et al., 1994; Ichise et al., 2000) and pharmacological manipulation of the receptor (Conn and Pin, 1997). Both techniques have advantages and disadvantages but for the pharmacological approach, availability of subtype selective ligands, in particular antagonists, with minimal side effects is paramount. The first antagonists with action at mGlu1 receptors were all competitive, conformationally constrained glutamate analogues based around 4-carboxyphenylglycine. However, it has proved difficult to develop such compounds with satisfactory selectivity and potency. In addition, these amino acids are unlikely to have good central bioavailability when administered systemically, thereby limiting their therapeutic usefulness. Recently, non-competitive mGlu1 antagonists have been developed and they are promising. The first discovered was 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt; Annoura et al., 1996; Hermans et al., 1998), but a lack of potency and recent doubts about its specificity (Fukunaga et al., 2007) make this compound less attractive. Fortunately, a new generation of more potent mGlu1-selective non-competitive antagonists is emerging. From those that are commercially available, we chose to evaluate 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-α]benzimidazole-2-carboxamide hydrochloride (YM-298198; Kohara et al., 2005) and (3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone (JNJ16259685; Lavreysen et al., 2004; see Figure 1a for structures). There were two linked aims to this study. The first was to test, for the first time, the effectiveness of the antagonists in a brain slice preparation using a well-characterized mGlu1-mediated synaptic response. The second aim was to test supra-maximal concentrations for non-specific effects on fast glutamatergic transmission.
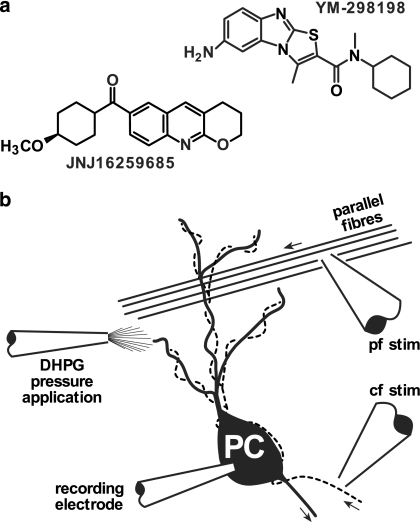
Figure 1.
(a) Structures of YM-298198 and JNJ16259685. (b) Recording arrangement. Whole-cell recordings were made from cerebellar Purkinje cells (PC). Stimulating electrodes were placed in the molecular layer to stimulate parallel fibres (pf stim) or in the granule cell layer in the vicinity of recorded PC to stimulate the climbing fibres (broken line; cf stim). Pressure application of DHPG was made through a glass pipette positioned in proximity to the PC dendrites. Arrows indicate normal direction of action potential travel.
Methods
Slice preparation
Parasagittal slices (250 μm thick) were cut from the mid-vermal cerebellum of 21–30-day-old Sprague–Dawley rats, which had been killed in accordance with the Animals (Scientific Procedures) Act, 1986. The slicing was performed using a Vibroslice (TPI 1000+, Intracel, Royston, UK) in cold (4–8°C) artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl 120; KCl 2; CaCl2 2; NaHCO3 26; MgSO4 1.19; KH2PO4 1.18; D-glucose 11 (pH 7.4-when equilibrated with 5% CO2 in O2). The slices were placed in a recovery chamber for about 1 h at 35°C and then allowed to cool to room temperature (∼21°C).
Electrophysiological recording
A slice was held down in a recording chamber (volume ∼300 μl) with a slice weight and perfused at 1.5 ml min−1 with warmed aCSF (29–31°C). Purkinje cells were identified visually and by their characteristic electrophysiological properties. Current-clamp (Axoclamp 2A, Molecular Devices, Sunnyvale CA, USA) and voltage-clamp (Axopatch 200B) recordings were performed using whole-cell patch electrodes at a membrane potential of –70 mV. Electrodes had resistances of 2–4 MΩ when filled with the following solution (in mM): KCH3SO3 135; NaCl 4; KCl 10; ethylene glycol bis(β-aminoethylether)-N,N,N',N',-tetraacetic acid 1; 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid 10; NaGTP 0.4 and MgATP 4 (290 mosM kg−1; pH 7.3 adjusted with KOH).
Parallel and climbing fibres were stimulated via glass microelectrodes (∼1 MΩ when filled with aCSF) with rectangular voltage pulses (100 μs wide and 5–70 V amplitude; DS2, Digitimer, Welwyn Garden City, UK). For parallel fibre stimulation, the electrode was positioned in the molecular layer at approximately 100 μm from the surface of the slice (Figure 1b). For climbing fibre stimulation, the electrode was positioned in the granule cell layer close to the recorded Purkinje cell (Figure 1b). The position was optimised such that at threshold stimulation amplitude, the failures produced no inadvertent effects in the recorded Purkinje cell. Pharmacologically, isolated mGlu-EPSPs were evoked with brief trains of stimuli (8–10 stimuli at 50 Hz) applied to the parallel fibres and repeated every 60 s. These experiments were performed in aCSF containing (in μM) bicuculline (30), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulphonamide (NBQX) (10), D-(−)-2-amino-5-phosphonopentanoic acid (AP5) (30) and (2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid (CGP55845) (10) to block GABAA, AMPA, NMDA and GABAB receptors, respectively. (S)-3,5-Dihydroxyphenylglycine (DHPG)-induced inward currents were recorded in the presence of TTX (1 μM; to block voltage-gated Na channels), CsCl (2 mM; to block Ih), bicuculline (30 μM) and NBQX (10 μM). DHPG (200 μM) was applied from a glass pipette onto the dendritic region by a short (50–500 ms; 20 kPa) pressure pulse. Post-tetanic depression (PTD) was induced by a brief tetanic stimulation of the parallel fibres (10 pulses at 100 Hz) in the presence of bicuculline (30 μM), AP5 (30 μM) and CGP55845 (10 μM). The response to a single parallel fibre stimulation, 4 s after the tetanus, was compared with the response to a control stimulus, 2 s before the tetanus. The PTD protocol was repeated every 60 s.
Data analysis
Data were analysed using Clampfit 9.2 (Molecular Devices) and Origin 6 (OriginLab, Northampton MA, USA). The peak of the slow, pharmacologically isolated mGlu-EPSP, which occurred around 300–500 ms, was measured relative to the extrapolated pre-stimulus baseline. The potential, owing to raised extracellular K+, which rises during the burst stimulation of the parallel fibres and decays over 200–300 ms, sometimes overlapped with the peak of the mGlu-EPSP. In a few cells, this led to an error in the peak measurement of less than 20% and was ignored (see Figure 2a for example). DHPG-induced inward currents were measured by zeroing the baseline and integrating the current over 6 s. Climbing fibre responses were quantified by integrating the initial 100 ms of the response (Carta et al., 2006). For PTD, the ratio of the initial slope (10–60%) of the post- and the pre-tetanus EPSPs was calculated. Data were normalized against the mean value for the control period (10 min).
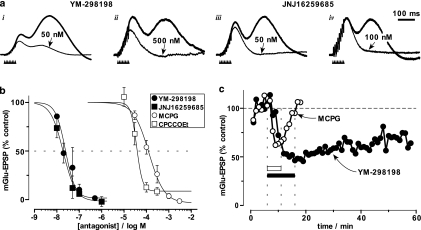
Figure 2.
YM-298198 and JNJ16259685 inhibit synaptic activation of mGlu1 in cerebellar slices. (a) Brief tetanic stimulation of the parallel fibres (indicated by arrowheads) evoked a delayed, slow EPSP in Purkinje cells which peaks around 330 ms. Control mGlu EPSP amplitudes (thick traces) were (i) 10.5 mV, (ii) 2.8 mV, (iii) 3.5 mV and (iv) 4.1 mV. Application of YM-298198 (i & ii) or JNJ16259685 (iii & iv), at the concentrations indicated, for 8–10 min, inhibited the mGlu EPSP (thin traces). (b) Concentration-response relationship for YM-298198 (n=3) and JNJ16259685 (n=3). For comparison, MCPG and CPCCOEt are also shown (data from Batchelor et al., 1997). Data points are means±s.e. fitted with sigmoid curves fixed with a higher asymptote of 100%. (c) Comparison of the time course of inhibition by concentrations of YM-298198 and MCPG around their IC50 values. Application of MCPG (100 μM; 6 min; hollow bar) caused rapid inhibition (to 62% of control) which recovered completely within ∼6 min of washout. In contrast, the inhibition due to application of YM-298198 (20 nM; 10 min; filled bar; maximum inhibition=48% of control) was much slower, taking 10 min to reach equilibrium and recovering to only 68% of the control amplitude with 42 min washing.
Where appropriate, mean and s.e.m. of the data were obtained and P-values calculated by two-tailed t-tests.
Materials
All compounds were added to the superfusing aCSF except DHPG. AP5, CGP55845, DHPG, JNJ16259685, (S)-α-methyl-4-carboxyphenylglycine (MCPG) and NBQX were purchased from Tocris Bioscience (Bristol, UK). Tetrodotoxin and YM-298198 hydrochloride were from Latoxan (Valence, France) and Ascent Scientific (Weston-Super-Mare, UK), respectively.
Results
Potency of YM-298198 and JNJ16259685 in a brain slice preparation
mGlu1 Receptor-mediated EPSPs recorded from Purkinje cells in cerebellar slices were used to assess the actions of YM-298198 and JNJ16259685. Brief trains of parallel fibre stimulation, in the presence of appropriate antagonists, elicits isolated slow mGlu-EPSPs peaking at 300–400 ms (Batchelor et al., 1994). An earlier depolarising component that develops during the stimulation and peaks at ∼150 ms is most likely due to raised extracellular K+ (Batchelor and Garthwaite, 1993). After 10 min of baseline recording, a range of concentrations of the two compounds was applied for 10 min causing a concentration-dependent reduction in the size of mGlu-EPSP (Figure 2a and b). Each concentration was applied to three separate slices generating IC50 values of 24 and 19 nM and Hill coefficients of 2.0 and 1.6 for YM-298198 and JNJ16259685, respectively (Figure 2b). To aid comparison, the data are plotted alongside previously published data (Batchelor et al., 1997) for the commonly used mGlu1 non-competitive antagonist CPCCOEt (IC50=37 μM; Hill coefficient=3.3), and the competitive and non-selective mGlu antagonist MCPG (IC50=130 μM; Hill coefficient=1.3).
YM-298198 and JNJ16259685 are slow to act
Relative to other compounds that have been tested in our system, YM-298198 and JNJ16259685 acted slowly to inhibit the mGlu-EPSP. We compared the kinetics of YM-298198 with MCPG at concentrations close to their IC50 values. The non-selective mGlu antagonist, MCPG, was chosen because of its known rapid onset and washout in slices (Batchelor et al., 1994). The inhibition of the mGlu-EPSP by MCPG (100 μM; n=2) reached equilibrium within ∼3 min, whereas, in contrast, YM-298198 (20 nM; n=3) took ∼10 min (Figure 2c).
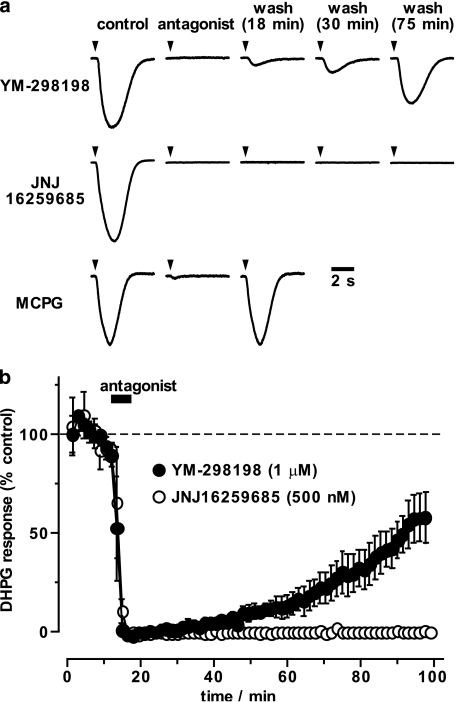
YM-298198 and JNJ16259685 are poorly reversible on washout
On return to control solution, the inhibition of the mGlu-EPSP by YM-298198 and JNJ16259685 either did not recover, or only recovered very slowly, during the remainder of the recording (Figure 2c). The mGlu-EPSP is not amenable to stable, long-term recordings, which may, in part, be due to plastic changes induced by the trains of synaptic stimuli. Instead, to study the reversibility of the antagonists, we recorded the inward currents induced by brief pressure application of the mGlu1 agonist DHPG (200 μM; Figure 3). These currents proved to be more stable. Addition of MCPG (1 mM; n=2), YM-298198 (1 μM; n=3) or JNJ16259685 (500 nM; n=3), abolished the response to DHPG (Figure 3a and b). On washing, the DHPG response recovered with a half-time of about 9 min and 74 min for MCPG and YM-298198, respectively, whereas JNJ16259685 showed no recovery (−0.1±0.2% at 80 min wash; Figure 3a and b).
Figure 3.
Washout of YM-298198 and JNJ16259685 studied with agonist application. (a) Brief pressure application of DHPG (200 μM; at arrowheads) induced robust inward currents (peak control currents were: top, 1.41 nA; middle, 0.61 nA and bottom, 0.40 nA). Application of mGlu antagonists, for 6 min, each abolished the response to DHPG application. The effect of YM-298198 (1 μM) reversed slowly on washout (11% of control peak current at 18 min; 21% at 30 min; 65% at 75 min). The inhibition induced by JNJ16259685 (500 nM) did not show any sign of recovery whereas the inhibition by MCPG (1 mM) had fully recovered by 18 min of washing. (b) Summary of the timecourse of recovery of the DHPG-induced current (normalised area under the curve). Mean±s.e.m. data for YM-298198 and JNJ16259685, (n=3 each) are shown.
Specificity of YM-298198 and JNJ16259685
Once the potency for blocking a mGlu1-mediated response was established, we investigated the specificity of the antagonists in our preparation. The glutamatergic parallel fibre and climbing fibre inputs onto Purkinje cells were tested for actions of the antagonists. It has been reported recently that CPCCOEt affects the response to climbing fibre activation (Fukunaga et al., 2007). Since YM-298198 and JNJ16259685 appear to share a binding site on mGlu1 receptors with CPCCOEt, we paid particular attention to their effects on the climbing fibre response.
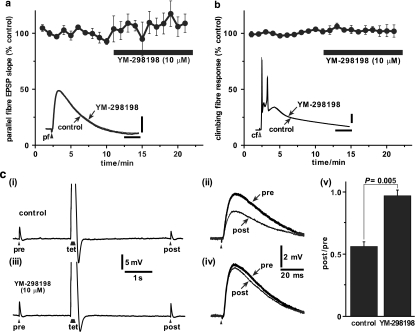
High concentrations (∼500 times IC50) of YM-298198 (10 μM) or JNJ16259685 (10 μM) were applied for 10 min while alternating between parallel fibre and climbing fibre stimulation. The fast, AMPA receptor-mediated, parallel fibre EPSP was unaffected by YM-298198 (Figure 4a; 111±10% of control; P=0.32; n=4) or JNJ16259685 (Figure 5a; 107±4% of control; P=0.25; n=3). Likewise, the complex climbing fibre response, with its underlying AMPA receptor EPSP, was uninfluenced by YM-298198 (Figure 4b; 102±5% of control; P=0.83; n=3) or JNJ16259685 (Figure 5b: 99±1% of control; P=0.69; n=3). In a single example, we recorded the climbing fibre response in the presence of JNJ16259685 (10 μM) and applied CPCCOEt (100 μM). CPCCOEt caused an enhancement of the late part of the complex response recruiting an additional spike (Figure 5c), as it has been shown to do when applied in the absence of JNJ16259685 (Fukunaga et al., 2007). The presence of the antagonists was verified by inducing the mGlu1-dependent post-tetanic depression (Neale et al., 2001) of the parallel fibre EPSP. All cells exhibited PTD (as measured by the ratio of the post- over the pre-tetanic EPSP) at the start of the recording which was inhibited by YM-298198 (Figure 4c) and JNJ16259685 (Figure 5d), as measured at the end of antagonist application.
Figure 4.
Specificity of YM-298198 tested against fast parallel fibre and climbing fibre responses. (a) Normalised initial slopes (10–60%) of fast AMPA receptor-mediated parallel fibre EPSPs were unaffected by application of YM-298198 (filled bar; 10 μM; 10 min; n=4). Inset shows superimposed parallel fibre EPSPs from a single example cell before (thick trace) and during (thin trace) the application of YM-2981980 (scale bars: 2 mV and 25 ms). (b) Normalised integral of climbing fibre response was unaffected by application of YM-298198 (filled bar; 10 μM; 10 min; n=3). Inset shows superimposed climbing fibre responses from a single example cell before (thick trace) and during (thin trace) the application of YM-298198 (scale bars: 2 mV and 25 ms). (c) (i) Under control conditions, the parallel fibre EPSP evoked 4 s after (‘post') a brief tetanus (‘tet'; 8 stimuli at 100 Hz) was smaller than a control EPSP elicited 2 s before (‘pre') the tetanus – post-tetanic depression. (ii) The single EPSPs are shown enlarged and aligned. (iii) The post-tetanic depression protocol was repeated after the experiments shown in panels (a) and (b) and in the continued presence of YM-298198 (10 μM for 12 min). The pre- and post-tetanic EPSPs were of similar amplitudes and are shown enlarged and aligned in (iv). Filled arrowheads represent stimuli to the parallel fibres. (v) All cells (n=4) showed post-tetanic depression of about 50% in control conditions (left column). In the presence of YM-298198 (10 μM) the depression was absent (right column).
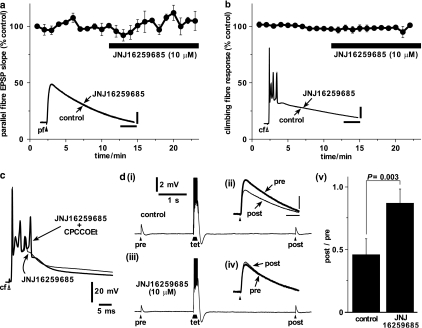
Figure 5.
Specificity of JNJ16259685 tested against fast parallel fibre and climbing fibre responses. (a) Normalised initial slopes (10–60%) of fast AMPA receptor-mediated parallel fibre EPSPs were unaffected by application of JNJ16259685 (filled bar; 10 μM; 10 min; n=4). Inset shows superimposed parallel fibre EPSPs from a single example cell before (thick trace) and during (thin trace) the application of JNJ16259685 (scale bars: 2 mV and 25 ms). (b) Normalised integral of climbing fibre response was unaffected by application of JNJ16259685 (filled bar; 10 μM; 10 min; n=3). Inset shows superimposed climbing fibre responses from a single example cell before (thick trace) and during (thin trace) the application of JNJ16259685 (scale bars: 20 mV and 25 ms). (c) Application of CPCCOEt (100 μM; for 4.5 min) in the presence of JNJ16259685 (10 μM for 13 min) enhanced a late component of the spiking phase (thick trace) causing an additional spike to be fired compared to control (thin trace; JNJ16259685 applied for 4 min). (d) (i) Under control conditions, the parallel fibre EPSP evoked 4 s after (‘post') a brief tetanus (‘tet'; 8 stimuli at 100 Hz) was smaller than a control EPSP elicited 2 s before (‘pre') the tetanus – post-tetanic depression. (ii) The single EPSPs are shown enlarged and aligned (scale bars: 2 mV and 20 ms). (iii) The post-tetanic depression protocol was repeated after the experiments shown in panels (a) and (b) and in the continued presence of JNJ16259685 (10 μM for 14 min). The pre- and post-tetanic EPSPs were of similar amplitudes and are shown enlarged and aligned in (iv). Filled arrowheads represent stimuli to the parallel fibres. (v) All cells (n=4) showed post-tetanic depression of about 50% in control conditions (left column). In the presence of JNJ16259685 (10 μM) the depression was much reduced (right column).
Discussion
YM-298198 and JNJ16259685 inhibit the synaptic activation of mGlu1 on Purkinje cells in slices with IC50 values of 24 and 19 nM, respectively. The value for YM-298198 is consistent with those obtained in single cells and membrane preparations (16–20 nM; Kohara et al., 2005). The slight discrepancy between the published data for JNJ16259685 (1.2–3.2 nM; Lavreysen et al., 2004) and our higher value (19 nM) could be explained by difficulty in judging when full equilibrium had been attained in the slices. This was particularly problematic with the low antagonist concentrations. The relatively slow onset and persistent action of these compounds is probably explained by the reluctance of these lipophilic compounds to penetrate into the slice to reach their target. The persistent nature of the antagonist action may, or may not, be advantageous depending on the experimental circumstances. Nonetheless, the potency of these compounds to block the mGlu1 EPSP is more than a thousand times greater than that of the previously characterised mGlu1-selective antagonists 4-carboxyphenylglycine and CPCCOEt (IC50=35 and 40 μM, respectively; Batchelor et al., 1997) or 1-aminoindan-1,5-dicarboxylic acid (AIDA; IC50=200 μM; Tempia et al., 1998).
JNJ16259685 also acts at mGlu5 receptors, but with a much lower affinity (IC50 rat mGlu5=1.3 μM and human mGlu5=28 μM; Lavreysen et al., 2004). At 500 nM, our data suggest that there is a complete inhibition of mGlu1 receptors (Figures 2 and 3). At this concentration, there should be insignificant binding to mGlu5 receptors (Lavreysen et al., 2004). Therefore, under conditions where the concentration can be adequately regulated, JNJ16259685 could be used to test for mGlu1 involvement.
The absence of an effect of YM-298198 and JNJ16259685 on fast parallel fibre EPSPs and climbing fibre responses is consistent with the reported lack of binding to AMPA receptors (Lavreysen et al., 2004; Kohara et al., 2005). It also suggests that these compounds have no significant effects on the many molecules located at pre- and postsynaptic sites that contribute to, or regulate, normal excitatory synaptic transmission, even at the high concentration used (10 μM).
We reported previously that the non-competitive mGlu1 antagonist CPCCOEt enhances the climbing fibre response. This effect was not mediated by mGlu receptors, as judged by the inability of competitive mGlu1 antagonists to mimic or occlude the effect (Fukunaga et al., 2007). Here we show that YM-298198 and JNJ16259685, although sharing a binding site with CPCCOEt on mGlu1 receptors, do not enhance the climbing fibre response. This strengthens further the conclusion that this effect of CPCCOEt is independent of mGlu receptors and is likely to be a non-specific action of this antagonist.
Lower concentrations of YM-298198 and JNJ16259685 are required, compared to previously available mGlu1 antagonists, to inhibit mGlu receptor activation. This, by itself, reduces the likelihood of non-specific effects since, in general, the lower the concentration of compound used, the lower the probability of significant off-target effects. The chance of a non-specific action of the drug generating a false result could be decreased still further by using both compounds in parallel.
In conclusion, YM-298198 and JNJ16259685 are likely to be very useful as specific and potent inhibitors of mGlu1 in vitro and in vivo.
Acknowledgments
This work was supported by a Royal Society University Research Fellowship (AMB), a Wellcome Trust Studentship (IF) and the BBSRC (CHY). We thank John Garthwaite for loan of equipment and Tom Salt and Marianne Löhr for valuable comments on the manuscript. YM-298198 was a kind gift from Steve Roome (Ascent Scientific).
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AP5
D-(−)-2-amino-5-phosphonopentanoic acid
- CGP55845
(2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid
- CPCCOEt
7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester
- DHPG
(S)-3,5-dihydroxyphenylglycine
- JNJ16259685
(3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone
- MCPG
(S)-α-methyl-4-carboxyphenylglycine
- NBQX
2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulphonamide
- PC
Purkinje cell
- PTD
post-tetanic depression
- YM-298198
6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-α]benzimidazole-2-carboxamide hydrochloride
Conflict of interest
The authors state no conflict of interest.
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Annoura H, Fukunaga A, Uesugi M, Tatsuuoka T, Horikawa Y. A novel class of antagonists for metabotropic glutamate receptors, 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylates. Biorg Med Chem Letts. 1996;6:763–766. [Google Scholar]
- Batchelor AM, Garthwaite J. Novel synaptic potentials in cerebellar Purkinje cells: probable mediation by metabotropic glutamate receptors. Neuropharmacology. 1993;32:11–20. doi: 10.1016/0028-3908(93)90124-l. [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Knöpfel T, Gasparini F, Garthwaite J. Pharmacological characterization of synaptic transmission through mGluRs in rat cerebellar slices. Neuropharmacology. 1997;36:401–403. doi: 10.1016/s0028-3908(97)00014-2. [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Madge DJ, Garthwaite J. Synaptic activation of metabotropic glutamate receptors in the parallel fibre-Purkinje cell pathway in rat cerebellar slices. Neuroscience. 1994;63:911–915. doi: 10.1016/0306-4522(94)90558-4. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, McIlhinney RAJ, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol potently modulates climbing fiber-->Purkinje neuron synapses: role of metabotropic glutamate receptors. J Neurosci. 2006;26:1906–1912. doi: 10.1523/JNEUROSCI.4430-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin J-P. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Fukunaga I, Yeo CH, Batchelor AM. The mGlu1 antagonist CPCCOEt enhances the climbing fibre response in Purkinje neurones independently of glutamate receptors. Neuropharmacology. 2007;52:450–458. doi: 10.1016/j.neuropharm.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Hermans E, Nahorski SR, Challiss RA. Reversible and non-competitive antagonist profile of CPCCOEt at the human type 1alpha metabotropic glutamate receptor. Neuropharmacology. 1998;37:1645–1647. doi: 10.1016/s0028-3908(98)00132-4. [DOI] [PubMed] [Google Scholar]
- Houamed KM, Kuijper JL, Gilbert TL, Haldeman BA, O'Hara PJ, Mulvihill ER, et al. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science. 1991;252:1318–1321. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Kohara A, Toya T, Tamura S, Watabiki T, Nagakura Y, Shitaka Y, et al. Radioligand binding properties and pharmacological characterization of 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimidazole-2-carboxamide (YM-298198), a high-affinity, selective, and noncompetitive antagonist of metabotropic glutamate receptor type 1. J Pharmacol Exp Ther. 2005;315:163–169. doi: 10.1124/jpet.105.087171. [DOI] [PubMed] [Google Scholar]
- Lavreysen H, Wouters R, Bischoff F, Nobrega PS, Langlois X, Blokland S, et al. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004;47:961–972. doi: 10.1016/j.neuropharm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Huganir RL, Price DL. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- Neale SA, Garthwaite J, Batchelor AM. mGlu1 receptors mediate a post-tetanic depression at parallel fibre – Purkinje cell synapses in rat cerebellum. Eur J Neurosci. 2001;14:1313–1319. doi: 10.1046/j.0953-816x.2001.01769.x. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol. 1998;80:520–528. doi: 10.1152/jn.1998.80.2.520. [DOI] [PubMed] [Google Scholar]