Abstract
Background and Purpose:
The large-conductance Ca2+-activated K+ channel (BKCa, KCa1.1) links membrane excitability with intracellular Ca2+ signaling and plays important roles in smooth muscle contraction, neuronal firing, and neuroendocrine secretion. This study reports the characterization of a novel BKCa channel blocker, 2,4-dimethoxy-N-naphthalen-2-yl-benzamide (A-272651).
Experimental Approach:
86Rb+ efflux in HEK-293 cells expressing BKCa was measured. Effects of A-272651 on BKCa α- and BKCa αβ1-mediated currents were evaluated by patch-clamp. Effects on contractility were assessed using low-frequency electrical field stimulated pig detrusor and spontaneously contracting guinea pig detrusor. Effects of A-272651 on neuronal activity were determined in rat small diameter dorsal root ganglia (DRG).
Key Results:
A-272651 (10 μM) inhibited 86Rb+ efflux evoked by NS-1608 in HEK-293 cells expressing BKCa currents. A-272651 concentration-dependently inhibited BKCa currents with IC50 values of 4.59 μM (Hill coefficient 1.04, measured at +40 mV), and 2.82 μM (Hill coefficient 0.89), respectively, for BKCa α and BKCa αβ1-mediated currents. Like iberiotoxin, A-272651 enhanced field stimulated twitch responses in pig detrusor and spontaneous contractions in guinea pig detrusor with EC50 values of 4.05±0.05 and 37.95±0.12 μM, respectively. In capsaicin-sensitive DRG neurons, application of A-272651 increased action potential firing and prolonged action potential duration.
Conclusions and Implications:
These data demonstrate that A-272651 modulates smooth muscle contractility and neuronal firing properties. Unlike previously reported peptide BKCa blockers, A-272651 represents one of the first small molecule BKCa channel blockers that could serve as a useful tool for further characterization of BKCa channels in physiological and pathological states.
Keywords: A-272651, potassium channel, large-conductance Ca2+-activated K+ channel, bladder, BKCa, dorsal root ganglia, smooth muscle, sensory neurons, detrusor
Introduction
Ca2+-activated K+ channels (KCa), which are Ca2+-sensitive and voltage-gated, play important roles in regulating the activity of smooth muscle tissues including that of the urinary bladder. On the basis of Ca2+-sensitivity, voltage dependency and conductance, three subtypes of KCa channels – large-conductance Ca2+-activated K+ channel (KCa1.1; BKCa), intermediate-conductance (KCa3.1; IKCa) and small-conductance (KCa2.1–KCa2.3; SKCa) channels have been described (Toro et al., 1998; Stocker, 2004; Turner and Shieh, 2006). In smooth muscles such as those from the urinary bladder, BKCa channels regulate resting membrane potential and initiate action potential repolarization to limit contraction frequency and amplitude, thus playing significant roles in cholinergic and purinergically mediated contractility (Heppner et al., 1997; Werner et al., 2007). Smooth muscle BKCa channels are composed of the pore-forming KCMA1 (α-subunit) and KCMB1 encoding an auxiliary β1-subunit that modulates kinetics properties and Ca2+/voltage-sensitivity (Hanner et al., 1997; Toro et al., 1998). In β1-subunit knockout mice where BKCa activities in bladder smooth muscle cells were greatly reduced, this smooth muscle displayed elevated phasic contraction amplitude and decreased frequency compared to bladder smooth muscle strips from the wild-type mice (Petkov et al., 2001). Likewise, ablation of the α-subunit leads to enhanced myogenic and nerve-mediated contractility and increased urination frequency (Meredith et al., 2004; Thorneloe et al., 2005). In contrast, injection with KCMA1 gene into obstructed rats ameliorated the hyperactivity of the urinary bladder (Christ et al., 2001). These results suggest that BKCa channels play significant roles in the regulation of phasic activity of the urinary bladder.
In addition to smooth muscle contractility, BKCa channels also play important roles in action potential duration and shaping spiking pattern of neurons in hippocampus (Shao et al., 1999) and dorsal root ganglia (DRG) (Scholz et al., 1998; Zhang et al., 2003). In mice lacking BKCa channels, cerebellar ataxia in the form of abnormal conditioned eye-blink reflex, abnormal locomotion and pronounced deficiency in motor coordination were observed (Sausbier et al., 2004). Lack of precise timing frequency in inner hair cells and reduction in maximum spike rates in auditory nerve fibres were also observed in the mice with ablation of the BKCa α-subunit that leads to progressive hearing loss (Ruttiger et al., 2004; Oliver et al., 2006).
Although gene knockout experiments have indicated a clear role of BKCa channels in smooth muscle and neuronal function, pharmacological tools for the study of BKCa channels have thus far been limited. Compounds initially claimed as BKCa channel openers are relatively weak or are known to possess ancillary pharmacology, which limits their utility as probes to study therapeutic relevance of BKCa channels (Edwards et al., 1994; Schroder et al., 2001). Earlier known openers include the glycosylated triterpene activator, dehydrosoyasaponin-I extracted from the medicinal herb, Desmodium adscendens (McManus et al., 1993) and several benzimidazolone analogues such as NS-004 and NS-1619 (Olesen et al., 1994), which stimulate BKCa activity leading to membrane hyperpolarization. More recently, the BKCa channel-opening activity of several fluorooxindole, bisphenol, quinolinone, triazolone analogues have been reported (reviewed in Wu, 2003; Turner and Shieh, 2006), and analogues continue to be optimized in terms of both potency and selectivity for potential utility in neurodegenerative and smooth muscle diseases with erectile dysfunction and urinary incontinence.
In contrast to openers, few non-peptide blockers of BKCa channels are known at present. Iberiotoxin (IbTx) is a selective, high-affinity blocker that served as a valuable tool to characterize BKCa channels (Kaczorowski et al., 1996). Alkaloids such as paxilline, penitrem A and verruculogen are blockers of smooth muscle maxi-K+ channels and have been shown to increase spontaneous contractility in smooth muscles including those from the urinary bladder (DeFarias et al., 1996; Molinari et al., 2000). Although a variety of nonpeptide blockers with range of IKCa/SKCa selectivities have been identified at the intermediate/small conductance Ca2+-activated K+ channels (Campos Rosa et al., 2000; Malik-Hall et al., 2000), organic small molecule blockers of BKCa channels yet remain to be identified. This study reports on the identification and characterization of A-272651 (2,4-dimethoxy-N-naphthalen-2-yl-benzamide) as a novel blocker of BKCa channels.
Materials and methods
Animal studies
All studies were carried out in accordance with guidelines outlined by the Animal Welfare Act, the Association for Assessment and Accreditation of Laboratory Animals (AAALAC) and the Institutional Animal Care and Use Committee of Abbott Laboratories.
Radioligand binding
Binding of radiolabelled IbTx – [125I]IbTx-D19Y/Y36F (NEN Life Science Products, Boston, MA, USA) was carried out as described previously (Molinari et al., 2000) by incubation in a final volume of 500 μl using about 10–20 μg protein per tube at room temperature. In competition experiments, membranes were preincubated with varying concentrations of compounds for 2 h followed by an additional incubation for 2.5 h in the presence of [125I]IbTx-D19Y/Y36F (8 pM). Incubations were terminated by rapid vacuum filtration over GF/B glass fibre filters presoaked in 0.5% polyethyleneimine and filters washed three times with 1.5 ml of ice-cold 50 mM Tris buffer (pH 7.2). Bound radioactivity was quantitated by γ counting spectroscopy at an efficiency of 80%.
Cation flux assays
Rubidium (86Rb+) flux assays were conducted as described previously (Parihar et al., 2003) using HEK-293 cells expressing human BKCa α-subunits. Briefly, cells were loaded with 1.0–2.0 μCi per well of the radiotracer 86Rb+ (NEN Life Science Products, Boston, USA), incubated for 18–24 h in culture media and then washed three times with assay buffer containing (mM) 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES), 20; NaCl, 120; KCl, 5; CaCl2, 2; MgCl2, 1; MgSO4, 0.4; D-glucose, 20; ouabain, 0.01; and adjusted with NaOH to pH 7.4. Assays were initiated by the addition of appropriate concentrations of test compounds. In cases where inhibitors were assessed, assays were initiated after a 10-min preincubation with the inhibitor followed by 30 min incubation with the test compounds. For the measurements of 86Rb+ efflux, supernatants were harvested and saved in 96-well Opti-Plates (Packard Bioscience, Meriden, CT, USA). Cells were subsequently lysed with 1.0 N NaOH and the supernatants again saved in another 96-well Opti-Plate. EcoLume liquid scintillation fluid (ICN, Costa Mesa, CA, USA) was added in both sets of supernatants (efflux and lysate), and the plates were counted on a Packard TopCount (Perkin-Elmer Life Sciences, Downers Grove, IL, USA). Each test concentration of compounds was tested in duplicate wells.
Electrophysiological recordings
BKCa currents in HEK-293 cells
The patch-clamp technique was used to measure effects on membrane currents in whole-cell configurations as described previously (Parihar et al., 2003). Fire-polished patch electrodes had a resistance of 2–5 MΩ when filled with pipette solution, which contained (mM): KCl, 140; MgCl2, 1; CaCl2, 0.1; ethylene glycol bis(2-aminoethylether)-N,N,N′,N′,-tetraacetic acid (EGTA), 1; HEPES, 10; (pH 7.2 with 5 N KOH, 285 mOsm). The bath solution contained (mM): NaCl, 135; KCl, 5; CaCl2, 2.5; MgCl2, 1.2; HEPES, 5 (pH 7.4 with 5 N NaOH, 310 mOsm). HEK-293 cells transfected with BKCa α and BKCa αβ1-subunits were voltage-clamped at a holding potential of −80 mV and the ionic current was measured at test potentials from −40 to +100 mV for 400 ms (20 mV each step). The whole-cell currents were amplified using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA) and low-pass filtered at 5 kHz (−3 db, four-pole Bessel filter) before digitization by Digidata 1200B at a sampling rate of 10 kHz.
Action potential recordings in dorsal root ganglion neurons
DRG from L6 and S1 were harvested from male Sprague–Dawley rats (200–250 g, Charles River, Wilmington, MA, USA) (Zhang et al., 2003). Briefly, DRG neurons were dissociated enzymatically with Dulbecco's modified Eagle's medium (DMEM, Gibco-BRL, Rockville, MD, USA) containing 0.1% collagenase for 20 min. This was followed by incubation at 37°C with 0.1% collagenase/dispase for 10 min and then with 2.5% trypsin for 10 min. Individual DRG neurons were suspended in enzyme-free DMEM, triturated with fire-polished pipettes and plated in polyethyleneimine treated 24 well-plates supplemented with 10% fetal bovine serum, 50 nM nerve growth factor, 2 mM glutamine and 100 U ml−1 penicillin–streptomycin. Neurons were cultured at 37°C in an atmosphere of 5% CO2/95% O2 and 90% humidity for electrophysiological characterization within 48 h. Whole-cell current clamp was used to record changes in action potential firing (Zhang et al., 2003). Current clamp recording was obtained by switching to current clamp mode after a stable whole-cell configuration was established in voltage-clamp mode. Pipette solution contained (mM): KCl 140, MgCl2 2, EGTA 10, HEPES 10, pH 7.2 adjusted with KOH (osmolarity, 285 mOsm). The external solution contained (mM): NaCl 140, KCl 5, MgCl2 2, CaCl2 2, HEPES 10, pH 7.4 adjusted with NaOH (osmolarity, 310 mOsm). Action potentials were evoked by 300 ms depolarizing pulses from 0.1 up to 0.6 nA at 0.1 nA steps and were filtered at 2 kHz and sampled at 10 kHz. Only cells with a stable resting membrane potential (more negative than −50 mV) were used in this study.
Isolated bladder smooth muscle studies
Studies on detrusor preparations were carried out as described previously (Buckner et al., 2000; Shieh et al., 2001). Briefly, urinary bladders from Landrace pigs (9–25 kg, Wilson's PrairieView Farm, Burlington, WI, USA) killed with pentobarbital, 150–200 mg kg−1, Somlethol (JA Webster Inc., Sterling MA, USA) or guinea pigs (250–300 g, Hartley, Charles River Laboratories) were removed and immediately placed in Krebs–Ringer–bicarbonate solution. Muscle strips, 3–5 mm in width and 10 mm in length, dissected free of mucosa were prepared from the bladder tissue by cutting in a circular manner. One end of the strip was fixed to a stationary glass rod and the other was attached to a Grass FT03 transducer at a basal preload of 1.0 g in a 10 ml organ bath chamber. This preload proved to be the best condition for a steady-state baseline. Tissues were allowed to equilibrate for at least 60 min before the assays. In studies with pig detrusor smooth muscle strips, electrical field stimulation (0.05 Hz, 0.5 ms, 20 V) was applied after equilibration via two parallel platinum electrodes included in the stationary rod. In studies with guinea-pig tissues, following equilibration, an additional 15 mM K+ was added (from stock solution of 3 or 4 M KCl) to maintain a final extracellular K+ of 20 mM, which sustained regular phasic activity of the smooth muscle (Malysz et al., 2004). Cumulative concentration–response curves were generated for each tissue and each tissue was exposed to only one test compound (Buckner et al., 2000).
Pharmacological selectivity assays
The activity of A-272651 (10 μM) was evaluated in assays to assess pharmacological selectivity relative to other cell-surface receptors, ion channels, transport sites and enzymes (a total of 75 targets) by use of standardized assay protocols (CEREP, Poitiers, France) as described previously (El Kouhen et al., 2005).
Data analysis
The percent Rb release was defined by measuring the amount of 86Rb+ released into the well after stimulation divided by the total amount of 86Rb+ loaded per well (%86Rb+ release=(86Rb+ in buffer of stimulated cells/86Rb+ in buffer from lysed cells) × 100). The concentration dependence of changes in 86Rb+ efflux or tension responses was fitted by nonlinear regression analysis (GraphPad Prism, San Diego, CA, USA) to obtain IC50 values. Guinea-pig spontaneous myogenic activity was analysed for changes in the area under the contractile response curve during a 15 min interval. In electrical field-stimulated tissues of pig, the concentration-dependent change in the peak amplitude of the response (measured in grams) was used for calculating potencies. Results are expressed as means±s.e.m. Significant differences between groups of means were assessed by the unpaired Student's t-test.
Materials
2,4-Dimethoxy-N-naphthalen-2-yl-benzamide (A-272651), related analogues (see Table 1) and NS-1608 were synthesized at Abbott Laboratories (Abbott Park, IL, USA). All other chemicals and reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA) unless specified otherwise. Compounds were prepared in DMSO (Sigma) as 10 mM stock solutions unless indicated otherwise, kept protected from light and serial dilutions were prepared in appropriate assay buffer just before use.
Table 1.
Electrophysiological assignment of BKCa opening activity in HEK-293 cells stably transfected with BKCa α-subunits
| Compound | Name | R | Effect on outward current in the presence of compound (10 μM) data expressed as % over control |
|---|---|---|---|
| 1 | A-272651 | 2, 4-OCH3 | −69.0±2.4% |
| 2 | 2-OCH3, 4-Cl | −64.6±5.3% | |
| 3 | 2-OCH3, 5-C(CH3)3 | 481±204% | |
| 4 | A-411873 | — | 107.8±24.5%a |
| 5 | NS-8 | — | 21.7±7.0%a |
| 6 | NS-1608 | — | 503.8±18.42% |
Abbreviation: Bkca, large-conductance Ca2+-activated K+ channel.
Previously reported in Turner et al. (2003). Minus sign indicates inhibition of current responses.
Results
A-272651 enhances [125I]IbTx binding
In initial high-throughput screening efforts, A-272651 (Figure 1) was identified as a compound that enhanced the binding of the radioligand, [125I]IbTx-D19Y/Y36F. Unlike unlabelled IbTx that displaced radioligand binding, A-272651 increased specific [125I]IbTx-D19Y/Y36F binding by about 3.5-fold at 1 μM (n=4; EC50=0.29 μM). This increase in binding is akin to that observed previously with indole alkaloids such as paxilline and verruculogen under similar assay conditions (Molinari et al., 2000).
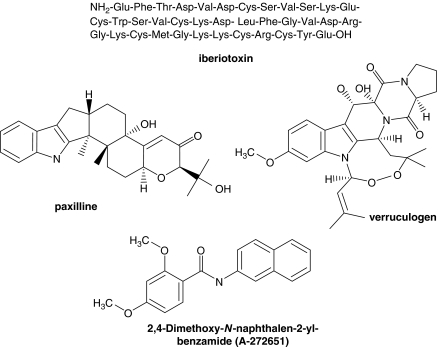
Figure 1.
Structure of A-272651 and other representative large-conductance calcium-activated K+ (BKCa) channel blockers.
Inhibition of BKCa channel-mediated 86Rb+ efflux
As reported previously (Parihar et al., 2003), various structurally diverse BKCa openers belonging to the aryl pyrrole, benzimidazolone, diphenylurea and aryl hydroxy oxindole classes have been shown to stimulate IbTx-sensitive cation flux in HEK-293 cells expressing BKCa α-subunit. Consistent with earlier observations from our laboratory, NS-1608, a diphenylurea BKCa opener, evoked concentration-dependent 86Rb+ efflux from transfected HEK-293 cells with an EC50 value of 2.71±1.36 μM (n=6). As shown in Figure 2, preincubation with 10 μM A-272651, before addition of various concentrations of NS-1608, completely abolished 86Rb+ efflux responses.
Figure 2.
A-272651 suppressed 86Rb+ efflux evoked by NS-1608. Activation of BKCa evoked 86Rb+ efflux by NS-1608 in HEK-293 cells expressing BKCa-α-subunit in the absence and presence of 10 μM A-272651 is shown. Data are from means of 3–4 experiments. Cells were loaded with 86Rb+, as described under Materials and methods and the effects of A-272651 were assessed during 30 min incubation.
Inhibition of BKCa channel-mediated current responses
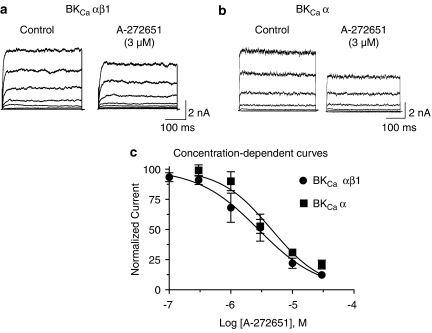
HEK-293 cells expressing BKCa α-subunit were voltage-clamped from a holding potential of −80 mV and ionic currents were measured from test membrane potential of −40 to +100 mV for 400 ms by whole-cell patch clamp. Steep voltage-dependent increases in ionic currents were recorded when test potentials were above −20 mV (Figure 3). Addition of A-272651 resulted in a concentration-dependent inhibition of BKCa currents in HEK-293 cells expressing BKCa α-subunit alone or both α- and β1-subunits (Figure 3). The IC50 values for A-272651 to inhibit BKCa α and BKCa αβ1 currents were 4.59±0.07 μM (Hill coefficient=1.04, measured at +40 mV, n=4) and 2.82±0.09 μM (Hill coefficient=0.89, n=6), respectively (Figure 3c). These results suggest that coexpression of the β1-subunit exerts no substantial effects on the inhibitory effects of A-272651.
Figure 3.
Inhibition of BKCa currents by A-272651. Membrane currents, mediated by BKCa αβ1-subunits (a) and BKCa α-subunit stably transfected in HEK-293 cells (b), were evoked by testing potentials from −40 to +100 mV for 400 ms (20 mV each step) from a holding potential of 80 mV in control and in the presence of 3 μM A-272651. (c) Shows the concentration-dependent inhibition of currents, with IC50 values of 4.59±0.07 μM (Hill coefficient=1.04, measured at +40 mV) for BKCa α and 2.82±0.09 μM (Hill coefficient=0.89) for BKCa αβ1, respectively.
As shown in Table 1, the 4-chloro analogue of A-272651 (compound 2 in Table 1) also showed comparable inhibition of BKCa current (see Table 1). Interestingly, when a t-butyl substituent was introduced (compound 3, Table 1), the resulting analogue was found to significantly increase BKCa currents (481% at 10 μM). The observed enhancement in current responses is comparable to that observed with NS-1608 (504±18%, n=5), and substantially higher than that observed with the previously reported amino-azaindole A-411873 (108%) (compound 28 in Turner et al., 2003; Turner and Shieh, 2006), and with NS-8 (22%) under similar experimental conditions. Within this structural series, it is thus possible to produce both strong inhibitors and enhancers of BKCa currents by judicious selection of aryl substituents.
A-272651 increases bladder smooth muscle contractility
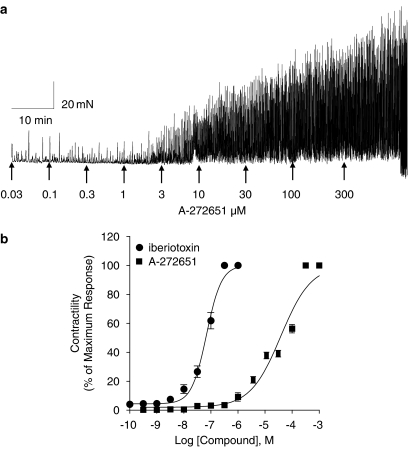
To assess the functional effects of A-272651 on smooth muscle contractility, effects on myogenic phasic activity and on electrical field stimulated bladder smooth muscle were assessed. In the presence of 20 mM extracellular K+, the guinea-pig detrusor smooth muscle segments exhibit spontaneous phasic contractile activity, which can be augmented by IbTx and attenuated by compounds such as NS-8 and NS-1619 suggesting the participation of BKCa channels (Buckner et al., 2002). As illustrated by Figure 4a, A-272651 increased spontaneous bladder contractions in a concentration-dependent manner. The maximal increase in contractility was comparable to that observed with IbTx, whereas the EC50 value was about 500-fold higher than IbTx itself. As reported previously from our laboratory, low-frequency stimulus (0.05 Hz) produced a continuous transient twitch response in the pig detrusor that can be effectively abolished by potassium channel openers such as the potent KATP channel-opener P1075 (Buckner et al., 2000) and BKCa openers such as NS-1619 (Malysz et al., 2004). Under similar conditions, A-272651 increased twitch responses in a concentration-dependent manner with an EC50 value of 4.66±0.06 μM (Figure 5).
Figure 4.
The effect of A-272651 and IbTx on the spontaneous phasic activity of guinea-pig detrusor. (a) A-272651 evoked concentration-dependent increases in spontaneous phasic contractions starting at concentration of 1 μM and attaining maximal levels comparable to IbTx. (b) The EC50 value for A-272651 to enhance spontaneous phasic contraction is 37.95±0.12 μM, which is about 500-fold higher than the EC50 of IbTx (0.068±0.04 μM). The data are mean values from four experiments.
Figure 5.
The effect of A-272651 on electrical field-stimulated contractility of the pig detrusor. A-272651 evoked concentration-dependent increases in contractility evoked by low frequency of field stimulation in pig detrusor with an EC50 value of 4.05±0.05 μM (Hill coefficient=1.11±0.11, n=4).
A-272651 increases action potential firing in DRG neurons
We also examined the effects of A-272651 in DRG neurons. In capsaicin-sensitive small-diameter DRG neurons, action potential responses were evoked by injecting threshold current (200–300 pA). Application of 3 μM A-272651 increased the firing frequency and prolonged action potential duration of DRG neurons (Figure 6). As shown in Figure 6c, the effects were readily reversed upon washout.
Figure 6.
The effect of A-272651 on firing activity of sensory neurons from DRG. Action potentials were evoked in control (a) or in the presence of 3 μM A-272651 (b), in capsaicin-sensitive small-diameter DRG neurons. A-272651 significantly enhanced firing frequency in sensory DRG neurons. Single action potential analysis revealed that A-272651 induced a reversible prolongation of action potential duration (c). The recordings shown are representative of multiple recordings (n=4).
Pharmacological selectivity
To determine the specificity of A-272651, the compound was profiled across a panel of in vitro binding assays (CEREP, Poitiers, France). These assays included G protein-coupled receptors, enzymes, transporters and ion channels. A-272651 (10 μM) was found to be inactive at most of the tested targets including voltage-gated K+ channels, small-conductance Ca2+-activated K+ channels, L-type voltage-gated Ca2+-channels and voltage-gated Na+ channels. Some degree of interaction was observed at the concentration tested (10 μM) with adenosine receptor type 3 (94%; percent inhibition of control specific binding), peripheral (benzodiazepine receptor (86%), human CB2 (cannabinoid receptor, 87%) and human 5HT1A (5-hydroxytryptamine receptor, 81%).
Discussion
Apart from peptide toxins such as IbTx, some indole alkaloids such as paxilline and verruculogen (Figure 1), are thus far the best characterized blockers of BKCa channels (Turner and Shieh, 2006). In contrast to previously reported blockers of the BKCa channel, A-272651 is a low-molecular-weight biaryl amide. A-272651 and related analogues are prepared by single-step amide coupling reactions from the corresponding naphthyl amine and substituted benzoic acid starting materials. In this study, interactions of A-272651 with BKCa channels were demonstrated across a number of assays including cation flux, whole-cell patch clamp in BKCa expressing cells and primary DRG neurons and tissue reactivity studies in bladder smooth muscle.
The IC50 values of A-272651 to inhibit BKCa α- and αβ1-mediated currents (4.59 and 2.82 μM respectively) are ∼1000-fold higher than that of IbTx (1.7 nM) and paxilline (1.9 nM) (Kaczorowski et al, 1996; Sanchez and McManus, 1996). In addition to modifying channel-gating properties and calcium sensitivity, the β-subunits can influence BKCa pharmacology. For example, it has been shown that β-subunits can influence interactions of toxins such as charybdotoxin (Hanner et al., 1997). Likewise, changes in pharmacological properties of BKCa channels in the presence of the β1-subunit have also been revealed for the peptide toxin IbTx and the alkaloid blocker tetrandrine (Dworetzky et al., 1996). Although A-272651 is 1.6-fold more potent in blocking BKCa αβ1-mediated currents compared with BKCa α-mediated currents, the difference was not statistically significant, suggesting that the β1-subunit has no substantial effects on interaction of A-272651 with the channel.
It is known that membrane-bound Ca2+ can influence the binding interactions of [125I]IbTx-D19Y/Y36F with BKCa channels (Knaus et al., 1994; Molinari et al., 2000). In our previous studies, paxilline and verruculogen both increased [125I]IbTx-D19Y/Y36F binding to some 400% above control values when assays were performed in the absence of ethylenediaminetetraacetic acid (EDTA). Interestingly, in the presence of EDTA, the modulatory effect of these alkaloids was not observed. The binding interactions of A-272651 reported in this study appears to be similar to that reported for the indole alkaloids with significant potentiation of [125I]IbTx-D19Y/Y36F in the presence of EDTA. The fact that removal of membrane-bound Ca2+ by EDTA abolishes the effect of indole alkaloids as reported previously, suggests that Ca2+ is required for the observed potentiatory effect on IbTx binding. While chelating agents (EDTA, EGTA) at micromolar concentrations increase the binding affinity by removal of bound Ca2+, indole alkaloids (paxilline, verruculogen) at nanomolar concentrations may increase the binding affinity by an allosteric interaction that increases the toxin affinity or indirectly by decreasing membrane-bound Ca2+ interactions with the BKCa channel.
While we have not examined functionally for effects on other Ca2+-activated K+ channels, A-272651 was evaluated in a panel of receptor binding assays for more than 70 diverse neurotransmitter receptor and ion channel sites (CEREP, France). No significant displacement of L-type voltage-gated Ca2+-channels (verapamil site) and small conductance Ca2+-activated ligand (apamin) binding sites was noted at 10 μM. In addition, the effect of A-272651 on BKCa channels appears selective as no significant effect on basal 86Rb+ flux, baseline bladder twitch or action potential responses were noted at the concentration ranges examined. It remains to be elucidated whether A-272651 inhibits Ca2+-influx, which could possibly diminish opening probabilities of BKCa channels in an indirect manner. However, A-272651 evoked spontaneous contraction (Figure 4) and prolonged action potential duration (Figure 6), suggesting that A-272651 appears not to alter Ca2+-regulation, which could indirectly modulate BKCa channels.
Although A-272651 increased contractility of smooth muscle under both neurogenic and myogenic conditions, differences in potencies are apparent. A-272651 was about 10-fold more potent in increasing twitch responses in electrically driven bladder smooth muscles (EC50=4 μM) compared to increasing contractility under myogenic conditions (EC50=38 μM). Apart from species differences, the nature of two separate models is likely to account for these observations. In guinea-pig detrusor, the elevation of extracellular K+ is thought to increase contractility by a direct depolarization of the smooth muscle and activation of L-type Ca2+-channels. In contrast, electrical field stimulation excites the intramural nerve to release excitatory neurotransmitters, the action of which elicits characteristic twitches (Buckner et al., 2002). Thus, the contractions evoked by K+-induced depolarization involve only a myogenic component, whereas electrically evoked contractions involve both a neurogenic component and a myogenic component. A likely basis for the differential effect between the two models is the possibility that A-272651 also inhibits neuronal BKCa channels, in addition to those present at the level of the bladder smooth muscle. This is supported by the observations that A-272651 increased firing frequency in DRG neurons (vide infra).
Neuronal BKCa channels, especially those present in CNS and DRG neurons, are a target for the action of BKCa openers such as aryloxindolone and benzimidazole analogues. For example, the benzimidazolone NS-1619 increased opening activity of a IbTx-sensitive Ca2+-dependent channel and reversibly suppressed action potential firing, attributable to increases in threshold for evoking action potentials, reductions in action potential amplitude and increases in amplitude of after-hyperpolarization (Zhang et al., 2003). These effects potentially underlie the reported reduction in transmitter release and neuroprotective effects with BKCa openers (Gribkoff et al., 2001; Hewawasam et al., 2002). In contrast to the observations with BKCa openers, A-272651 was found to increase firing frequency in DRG neurons, which was readily reversible upon washout, unlike IbTx. Collectively, our studies show that A-272651 can modulate BKCa channels in both bladder smooth muscle and neurons, and this compound represents one of the first small-molecule BKCa channel blockers that could serve as useful tools for investigation of BKCa channels in physiological and pathological states.
Abbreviations
- BKCa
large-conductance Ca2+-activated K+ channel
- BZD
benzodiazepine receptor
- DRG
dorsal root ganglia
- hCB2
human cannabinoid receptor
- IbTx
iberiotoxin
- IKCa
intermediate-conductance Ca2+- activated K+ channel
- 86Rb+
rubidium
- SKCa
small-conductance Ca2+-activated K+ channel
Conflict of interest
The authors state no conflict of interest.
References
- Buckner SA, Milicic I, Daza A, Davis-Taber R, Scott VE, Sullivan JP, et al. Pharmacological and molecular analysis of ATP-sensitive K+ channels in the pig and human detrusor. Eur J Pharmacol. 2000;400:287–295. doi: 10.1016/s0014-2999(00)00388-5. [DOI] [PubMed] [Google Scholar]
- Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M. Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol. 2002;135:639–648. doi: 10.1038/sj.bjp.0704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos Rosa J, Galanakis D, Piergentili A, Bhandari K, Ganellin CR, Dunn PM, et al. Synthesis, molecular modeling, and pharmacological testing of bis-quinolinium cyclophanes: potent, non-peptidic blockers of the apamin-sensitive Ca2+-activated K+ channel. J Med Chem. 2000;43:420–431. doi: 10.1021/jm9902537. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Day NS, Day M, Santizo C, Zhao W, Sclafani T, et al. Bladder injection of ‘naked' hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1699–R1709. doi: 10.1152/ajpregu.2001.281.5.R1699. [DOI] [PubMed] [Google Scholar]
- DeFarias FP, Carvalho MF, Lee SH, Kaczorowski GJ, Suarez-Kurtz G. Effects of the K+ channel blockers paspalitrem-C and paxilline on mammalian smooth muscle. Eur J Pharmacol. 1996;314:123–128. doi: 10.1016/s0014-2999(96)00540-7. [DOI] [PubMed] [Google Scholar]
- Dworetzky SI, Boissard CG, Lum-Ragan JT, McKay MC, Post-Munson DJ, Trojnacki JT, et al. Phenotypic alteration of a human BK (hSlo) channel by hSlobeta subunit coexpression: changes in blocker sensitivity, activation/relaxation and inactivation kinetics, and protein kinase A modulation. J Neurosci. 1996;16:4543–4550. doi: 10.1523/JNEUROSCI.16-15-04543.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Niederste-Hollenberg A, Schneider J, Noack T, Weston AH. Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br J Pharmacol. 1994;113:1538–1547. doi: 10.1111/j.1476-5381.1994.tb17171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kouhen R, Surowy CS, Bianchi BR, Neelands TR, McDonald HA, Niforatos W, et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel and selective transient receptor potential type V1 receptor antagonist, blocks channel activation by vanilloids, heat, and acid. J Pharmacol Exp Ther. 2005;314:400–409. doi: 10.1124/jpet.105.084103. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Starrett JE, Jr, Dworetzky SI, Hewawasam P, Boissard CG, Cook DA, et al. Targeting acute ischemic stroke with a calcium-sensitive opener of maxi-K potassium channels. Nat Med. 2001;7:471–477. doi: 10.1038/86546. [DOI] [PubMed] [Google Scholar]
- Hanner M, Schmalhofer WA, Munujos P, Knaus HG, Kaczorowski GJ, Garcia ML. The beta subunit of the high-conductance calcium-activated potassium channel contributes to the high-affinity receptor for charybdotoxin. Proc Natl Acad Sci USA. 1997;94:2853–2858. doi: 10.1073/pnas.94.7.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol. 1997;273:C110–C117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- Hewawasam P, Erway M, Moon SL, Knipe J, Weiner H, Boissard CG, et al. Synthesis and structure-activity relationships of 3-aryloxindoles: a new class of calcium-dependent, large conductance potassium (maxi-K) channel openers with neuroprotective properties. J Med Chem. 2002;45:1487–1499. doi: 10.1021/jm0101850. [DOI] [PubMed] [Google Scholar]
- Kaczorowski GJ, Knaus HG, Leonard RJ, McManus OB, Garcia ML. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J Bioenerg Biomembr. 1996;28:255–267. doi: 10.1007/BF02110699. [DOI] [PubMed] [Google Scholar]
- Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33:5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- Malik-Hall M, Ganellin CR, Galanakis D, Jenkinson DH. Compounds that block both intermediate-conductance (IKCa) and small-conductance (SKCa) calcium-activated potassium channels. Br J Pharmacol. 2000;129:1431–1438. doi: 10.1038/sj.bjp.0703233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malysz J, Buckner SA, Daza AV, Milicic I, Perez-Medrano A, Gopalakrishnan M. Functional characterization of large conductance calcium-activated K+ channel openers in bladder and vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:481–489. doi: 10.1007/s00210-004-0920-y. [DOI] [PubMed] [Google Scholar]
- McManus OB, Harris GH, Giangiacomo KM, Feigenbaum P, Reuben JP, Addy ME, et al. An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 1993;32:6128–6133. doi: 10.1021/bi00075a002. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- Molinari EJ, Sullivan JP, Wan Y, Brioni JD, Gopalakrishnan M. Characterization and modulation of [125I]iberiotoxin-D19Y/Y36F binding in the guinea-pig urinary bladder. Eur J Pharmacol. 2000;388:155–161. doi: 10.1016/s0014-2999(99)00853-5. [DOI] [PubMed] [Google Scholar]
- Olesen SP, Munch E, Moldt P, Drejer J. Selective activation of Ca2+-dependent K+ channels by novel benzimidazolone. Eur J Pharmacol. 1994;251:53–59. doi: 10.1016/0014-2999(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Oliver D, Taberner AM, Thurm H, Sausbier M, Arntz C, Ruth P, et al. The role of BKCa channels in electrical signal encoding in the mammalian auditory periphery. J Neurosci. 2006;26:6181–6189. doi: 10.1523/JNEUROSCI.1047-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar AS, Groebe DR, Scott VE, Feng J, Zhang XF, Warrior U, et al. Functional analysis of large conductance Ca2+-activated K+ channels: ion flux studies by atomic absorption spectrometry. Assay Drug Dev Technol. 2003;1:647–654. doi: 10.1089/154065803770381002. [DOI] [PubMed] [Google Scholar]
- Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol. 2001;537:443–452. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, et al. Deletion of the Ca2+-activated potassium (BK) alpha-subunit but not the BKbeta1-subunit leads to progressive hearing loss. Proc Natl Acad Sci USA. 2004;101:12922–12927. doi: 10.1073/pnas.0402660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, McManus OB. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology. 1996;35:963–968. doi: 10.1016/0028-3908(96)00137-2. [DOI] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci USA. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A, Gruss M, Vogel W. Properties and functions of calcium-activated K+ channels in small neurones of rat dorsal root ganglion studied in a thin slice preparation. J Physiol. 1998;513:55–69. doi: 10.1111/j.1469-7793.1998.055by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder RL, Jespersen T, Christophersen P, Strobaek D, Jensen BS, Olesen SP. KCNQ4 channel activation by BMS-204352 and retigabine. Neuropharmacology. 2001;40:888–898. doi: 10.1016/s0028-3908(01)00029-6. [DOI] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol. 1999;1:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh CC, Feng J, Buckner SA, Brioni JD, Coghlan MJ, Sullivan JP, et al. Functional implication of spare ATP-sensitive K+ channels in bladder smooth muscle cells. J Pharmacol Exp Ther. 2001;296:669–675. [PubMed] [Google Scholar]
- Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol. 2005;289:F604–F610. doi: 10.1152/ajprenal.00060.2005. [DOI] [PubMed] [Google Scholar]
- Toro L, Wallner M, Meera P, Tanaka Y. Maxi-KCa, a unique member of the voltage-gated K channel superfamily. News Physiol Sci. 1998;13:112–117. doi: 10.1152/physiologyonline.1998.13.3.112. [DOI] [PubMed] [Google Scholar]
- Turner SC, Carroll WA, White TK, Gopalakrishnan M, Coghlan MJ, Shieh CC, et al. The discovery of a new class of large-conductance Ca2+-activated K+ channel opener targeted for overactive bladder: synthesis and structure-activity relationships of 2-amino-4-azaindoles. Bioorg Med Chem Lett. 2003;13:2003–2007. doi: 10.1016/s0960-894x(03)00324-x. [DOI] [PubMed] [Google Scholar]
- Turner SC, Shieh CC.Medicinal chemistry of Ca2+-activated K+ channel modulators Voltage-Gated Ion Channels as Drug Targets 2006Wiley-VCH, Verlag GmBH & Co., KGaA, Weinheim; 310–334.In: Triggle DJ, Gopalakrishnan M, Rampe D, Zheng W (eds) [Google Scholar]
- Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol. 2007;292:R616–R624. doi: 10.1152/ajpregu.00036.2006. [DOI] [PubMed] [Google Scholar]
- Wu SN. Large-conductance Ca2+- activated K+ channels:physiological role and pharmacology. Curr Med Chem. 2003;10:649–661. doi: 10.2174/0929867033457863. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Gopalakrishnan M, Shieh CC. Modulation of action potential firing by iberiotoxin and NS1619 in rat dorsal root ganglion neurons. Neuroscience. 2003;122:1003–1011. doi: 10.1016/j.neuroscience.2003.08.035. [DOI] [PubMed] [Google Scholar]