Abstract
Background and purpose:
Adipocyte differentiation in vitro is coordinately activated by two transcription factors, peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT enhancer binding protein α (C/EBPα), but it is inhibited by preadipocyte factor-1 (pref-1). Statins, inhibitors of HMG-CoA reductase and de novo cholesterol synthesis, can have pleiotropic effects which influence adipocyte phenotype by ill-defined mechanisms. We investigated the effects of pitavastatin (NK-104) on adipocyte differentiation and the transcriptional pathways involved.
Experimental approach:
The effects of pitavastatin on adipocyte differentiation were evaluated by the formation of oil droplets, content of cellular triglyceride and expression of adipocyte-specific genes. Regulatory mechanisms were assessed by analysis of PPARγ, C/EBPα and pref-1 expression.
Key results:
Pitavastatin significantly inhibited adipocyte differentiation of 3T3-L1 preadipocytes in response to adipogenic inducers. Evidence for inhibition included fewer Oil Red O positive droplets, less cellular triglyceride and decreased expression of adipocyte-specific genes, including fatty acid binding protein (aP2), CD36, adipsin and glucose transporter 4 (GLUT4). The inhibitory effects of pitavastatin on adipocyte differentiation of 3T3-L1 preadipocytes were time and concentration dependent. Pitavastatin significantly blocked induction of PPARγ expression, but not C/EBPα expression or DNA binding activity of PPARγ. Also, pitavastatin induced pref-1 expression in preadipocytes and maintained expression of pref-1 at high levels in differentiated cells.
Conclusions and implications:
Our data suggest that pitavastatin inhibits adipocyte differentiation by blocking PPARγ expression and activating pref-1 expression. These studies may have implications in the regulation of adipogenesis in response to statins.
Keywords: adipocyte differentiation, C/EBPα, fibroblasts, pitavastatin, PPARγ, pref-1, triglycerides
Introduction
Adipocytes are a major site for energy storage (in the form of triglycerides) for use during periods of caloric insufficiency. In addition, adipocytes secrete numerous important regulatory molecules (Cornelius et al., 1994; Gregoire et al., 1998). Adipocyte differentiation has important implications for human disease states. For instance, the overabundance of fat cells causing obesity is considered to be a major risk factor for type II diabetes and hypertension. It is also linked to some types of cancers, immune dysfunction and cardiovascular diseases (Moller and Flier, 1991; Spiegelman et al., 1993).
Because of the difficulty in studying adipocyte differentiation in vivo, much of our knowledge of adipocyte differentiation has been obtained from the well-established in vitro models of adipogenesis (Gregoire et al., 1998). Among these models, the 3T3 preadipocyte cell line is the best characterized. For example, 3T3-F442A fibroblasts injected into mice can differentiate and form fat pads, which are similar to normal adipose tissue (Green and Kehinde, 1979). Differentiated 3T3-L1 preadipocytes possess most of the ultrastructural properties of adipocytes from animal tissue. The formation and appearance of fat droplets in these differentiated cells are also similar to adipose tissue in vivo (Green and Kehinde, 1979; Novikoff et al., 1980).
Differentiation of 3T3-L1 fibroblast/preadipocytes is initiated by an adipogenic mixture of isobutylmethylxanthine, dexamethasone and insulin (IDI). In the course of differentiation, addition of IDI to confluent fibroblasts completes the postconfluent mitosis and subsequent growth arrest within the first 2 days (Bernlohr et al., 1985). Following growth arrest, cells are committed to becoming adipocytes. A series of molecular events occur in the progression of adipocyte differentiation (Gregoire et al., 1998; Farmer, 2006; Rosen and MacDouglad, 2006). The very early markers of adipocyte differentiation are the expression of c-fos, c-jun, junB, c-myc and CCAAT enhancer-binding proteins (C/EBP)β and -δ (Cornelius et al., 1994). Among these molecules, only C/EBPβ and -δ are directly and specifically implicated in differentiation and are induced primarily in response to dexamethasone and isobutylmethylxanthine, respectively. Homodimers or heterodimers of C/EBPβ/δ stimulate expression of C/EBPα and peroxisome proliferator-activated receptor-γ (PPARγ) (Yeh et al., 1995; Rosen and MacDouglad, 2006), and expression of C/EBPβ and -δ decline at late stages of differentiation (Clarke et al., 1997; Lane et al., 1999). Once activated, C/EBPα and PPARγ cross-regulate each other to maintain high expression, and, in a cooperative and synergistic manner, they induce the expression of many adipocyte-specific proteins and enzymes involved in creating and maintaining the adipocyte phenotype (Shao and Lazar, 1997; Gregoire et al., 1998; Farmer, 2006; Rosen and MacDouglad, 2006). Ectopic expression of either of these two transcription factors, however, still promotes adipogenesis, but at a slower rate and to a lesser extent (Mandrup and Lane, 1997).
Preadipocyte factor-1 (pref-1) is an epidermal growth factor (EGF) repeat domain-containing transmembrane protein and exists as multiple discrete forms with a molecular weight range of 45–60 kDa in preadipocytes. However, expression of pref-1 is totally abolished after adipocyte differentiation (Smas and Sul, 1993). Constitutive expression of pref-1 in preadipocytes blocks adipogenic agent-induced adipocyte differentiation, suggesting a regulatory role of pref-1 in this biological process (Smas and Sul, 1993). In fact, addition of the larger soluble form of pref-1 inhibits adipocyte differentiation (Mei et al., 2002). The induction of adipocyte differentiation by glucocorticoids is also partially due to the suppression of pref-1 expression (Smas et al., 1999). A recent study suggests that pref-1 inhibits expression of PPARγ and C/EBPα (Kim et al., 2007).
Statins, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, significantly reduce serum low-density lipoprotein (LDL) cholesterol levels and decrease the incidence of coronary heart disease. In addition, statins have pleiotropic effects on many cell types, including endothelial cells, smooth muscle cells and monocyte/macrophages (LaRosa, 2001; Takemoto and Liao, 2001). Previously, it has been reported that lovastatin impaired adipocyte differentiation in 3T3-L1 cells in a dose-dependent manner, while simvastatin inhibited adipocyte differentiation in mouse bone marrow stromal cells (Nishio et al., 1996; Song et al., 2003). However, the mechanisms by which statins alter adipogenesis are incompletely understood.
In this report, we tested the hypothesis that statins can alter transcriptional pathways critical for adipocyte differentiation. Our data demonstrate that pitavastatin inhibits adipocyte differentiation by blocking PPARγ, but not C/EBPα, expression. The inhibition of adipocyte differentiation by pitavastatin is also due to induction of pref-1 expression in preadipocytes and the maintenance of pref-1 in differentiated cells. These findings may have implications in the regulation of adipogenesis and, potentially, obesity-related diseases.
Methods
Cell culture
3T3-L1 cells, a murine fibroblast/preadipocyte cell line, were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in 100 mm Petri dishes with Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum, 50 μg ml−1 of penicillin/streptomycin and 2 mM glutamine. Cells were induced to differentiate at the initial confluence in complete medium as follows. On the first day of confluence, a mixture (IDI) of isobutylmethylxanthine (0.5 mM), dexamethasone (1 μM) and insulin (1 μM) in complete DMEM medium was added to the 3T3-L1 cells for 3 days. Cells then were switched to complete medium containing insulin (1 μM) alone for another 3 days, followed by incubation in complete medium for additional 1–2 days. All control cells received the corresponding solvent(s) treatments.
Extraction of total RNA, isolation of poly(A+) RNA and Northern blotting
The poly (A+) RNA was isolated from 100 μg of total RNA and used to determine the expression of fatty acid-binding protein (aP2), CD36, PPARγ, C/EBPα and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by Northern blot as described (Han et al., 2004). The template DNA for aP2 or PPARγ was generated by reverse transcription-polymerase chain reaction (RT-PCR) based on the published sequences. The sequences of 5′- and 3′- of oligonucleotides used for aP2 were GATGCCTTTGTGGGAACC (307–325 on exon 1) and AACTCTTGTGGAAGTCACG (28–47 on exon 4), respectively, with a PCR product size of 377 bp (Phillips et al., 1986). The sequence of 5′- and 3′- of oligonucleotides used for PPARγ were CAGAGATGCCATTCTGGC (140–157) and TTTCCTGTCAAGATCGCCC (824–842), respectively, with a PCR product size of 703 bp (Zhu et al., 1993). The probe for mouse CD36 is an NsiI–BglII digest (193–805 bp). The probe for C/EBPα was a BamHI digest of C/EBPα plasmid DNA purchased from ATCC.
Oil Red O staining and triglyceride assay
The formation of oil droplets in cells was analysed by Oil Red O staining as follows. After removal of culture medium, cells were washed once with phosphate-buffered saline (PBS), then fixed for at least 1 h with pre-chilled 10% formaldehyde in PBS. Cells were stained with Oil Red O solution (a mixture of three parts of 0.5% (w/v) Oil Red O in isopropanol and two parts of water) for 2 h at room temperature followed by washing with PBS twice, ethanol once and water twice. Cells were kept in water and photographed.
To analyse the content of cellular triglycerides, cells were washed with PBS and then scraped into 1 ml PBS and homogenized by sonication for 1 min. The lysate was assayed for total triglycerides by using assay kits from Sigma (St Louis, MO, USA) (GPO-Trinder) and for cellular protein by the Lowry method.
Determination of aP2, GLUT4, adipsin, PPARγ and C/EBPα protein expression, and PPARγ DNA-binding activity
Total cellular protein was extracted and used to determine the expression of aP2 and glucose transporter 4 (GLUT4) by Western blot as described (Han et al., 2004). Cell culture medium from all steps of adipocyte differentiation was pooled and mixed well. The levels of adipsin in the pooled medium were also analysed by Western blot.
Nuclear proteins were extracted and used to determine the expression of PPARγ and C/EBPα by Western blot. Nuclear proteins were also used to determine the binding activity of PPARγ to PPARγ response element (PPRE) by electrophoretic mobility shift assay (EMSA) and by using a kit from Active Motif (Han et al., 2004).
Construction and determination of PPARγ promoter activity
PPARγ promoter (from −1022 to +46) was generated by PCR with following primers, pPPARγ-f, 5′-TAGCCTCGAGGTCACTGAATTATATTAGGTACCTTATG-3′, pPPARγ-r, 5′-TGCCAAGCTTTCAGCGAAGGCACCATGCTCTGGGTCAAC-3′ and the genomic DNA isolated from 3T3-L1 preadipocytes. The primers contained restriction sites for Xho (5′primer) and HindIII (3′primer), respectively. After sequence confirmation, the PCR product was digested by enzymes and then sub-cloned into pGL4.10 luciferase reporter to generate plasmid of PPARγ promoter-luciferase reporter (pPPAR-Luc) and amplified.
The initial confluent 3T3-L1 preadipocytes in 24-well plates were transfected with pPPAR-Luc and Renilla luciferase reporter (RLuc, for internal control) by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After transfection, cells were incubated in adipogenic-inducing medium in the presence or absence of pitavastatin for 48 h. Cells were lysed and activity of pPPAR-Luc and RLuc were determined according to the manufacturer's instruction (Promega, Madison, WI, USA).
Data analysis
All the experiments were repeated at least three times and representative results are presented. In the triglyceride assay, the data were analysed by paired Student's t-test; with P<0.05 showing significant differences.
Materials
Pitavastatin was obtained from the Pharmaceutical Division, Kowa Company Ltd (Tokyo, Japan). All other chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA). Anti-pref-1 antibody was purchased from Chemicon International Inc. (Temecula, CA, USA). Rabbit anti-aP2 serum was generated by immunization with murine aP2 peptide of DGKSTTIKRKRDGDKLV (Sun et al., 2003). All other antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Results
Pitavastatin inhibits adipocyte differentiation, accumulation of neutral lipids and triglycerides
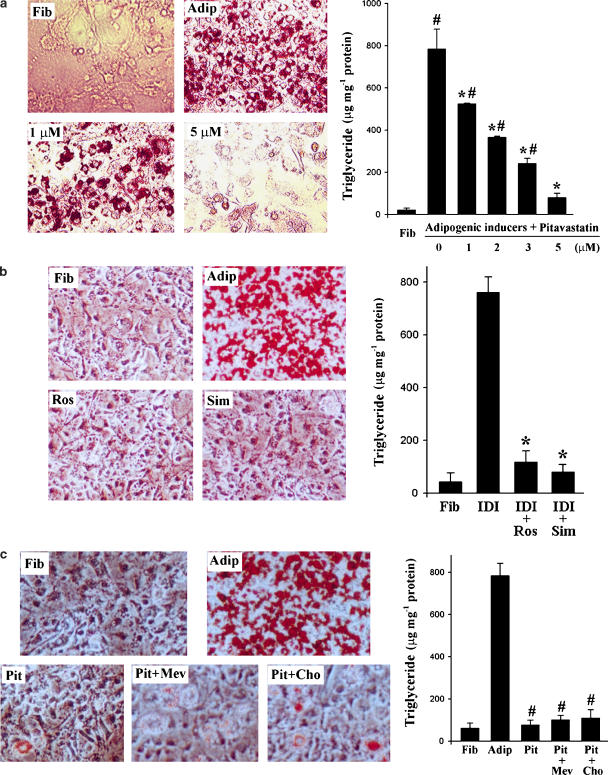
To determine the effect of pitavastatin on adipocyte differentiation, pitavastatin (1–10 μM) was added to 3T3-L1 fibroblasts at the initiation of differentiation induced by the IDI mixture (see Methods). Treatment was continued until differentiation was complete. Cellular toxicity, as determined by microscopic observation and trypan blue exclusion, was not observed with concentrations of pitavastatin up to 10 μM (data not shown). The effects of pitavastatin on adipocyte differentiation were evaluated by Oil Red O staining and by analysis of cellular triglyceride content. Oil droplets were not seen in fibroblasts but were abundant in differentiated adipocytes. Pitavastatin (1 μM) significantly reduced the number of oil droplets and completely prevented the formation of oil droplets at 5 μM (Figure 1a). Consistent with the appearance of the formed oil droplets, cellular triglyceride levels were very low in fibroblasts and this level was elevated a few hundred-fold after differentiation. Pitavastatin significantly reduced cellular triglyceride levels in a concentration-dependent manner (Figure 1a). The level of cellular triglyceride in the presence of 5 μM pitavastatin was about 2- to 3-fold of that in fibroblasts, but was not significantly different.
Figure 1.
Statins inhibit adipocyte differentiation. (a) At the first day of confluence, 3T3-L1 fibroblast/preadipocytes were induced toward adipocyte differentiation in the presence of pitavastatin at the indicated concentrations for 8 days. Neutral lipid in fibroblasts or the differentiated cells was assessed by Oil Red O staining. Cells were also used to determine the cellular triglyceride content as described in the Methods. #,*Significantly different from fibroblasts and adipocytes, respectively (P<0.05 by Student's t-test, n=4). (b) Fibroblasts were induced toward adipocyte differentiation (IDI) in the presence of rosuvastatin (Ros) or simvastatin (Sim; 5 μM of each) for 8 days. Neutral lipid and cellular triglyceride contents were determined as described above. (c) Fibroblasts were induced toward adipocyte differentiation in the presence of pitavastatin (Pit; 5 μM) or pitavastatin plus mevalonate (Mev; 10 μM) or cholesterol (Cho; 10 μM) for 8 days. Neutral lipid and cellular triglyceride contents were analysed as described in the Methods.
The effects of other statins, such as rosuvastatin and simvastatin, on adipocyte differentiation were also evaluated by determination the formation of oil droplets and analysis of cellular triglycerides. Both rosuvastatin and simvastatin (5 μM) also significantly blocked the formation of oil droplets and reduced the accumulation of cellular triglyceride during differentiation (Figure 1b).
To determine if the inhibitory effects of pitavastatin on adipocyte differentiation was dependent on HMG-CoA reductase activity, we added mevalonate (10 μM) or cholesterol (10 μM) to the differentiating cells in the presence of pitavstatin. Compared with pitavastatin alone, co-treatment with mevalonate or cholesterol did not alter the effect of pitvastatin on Oil Red O staining or cellular triglyceride content (Figure 1c) suggesting pitavastatin inhibition of adipocyte differentiation was independent of its effect on HMG-CoA reductatse.
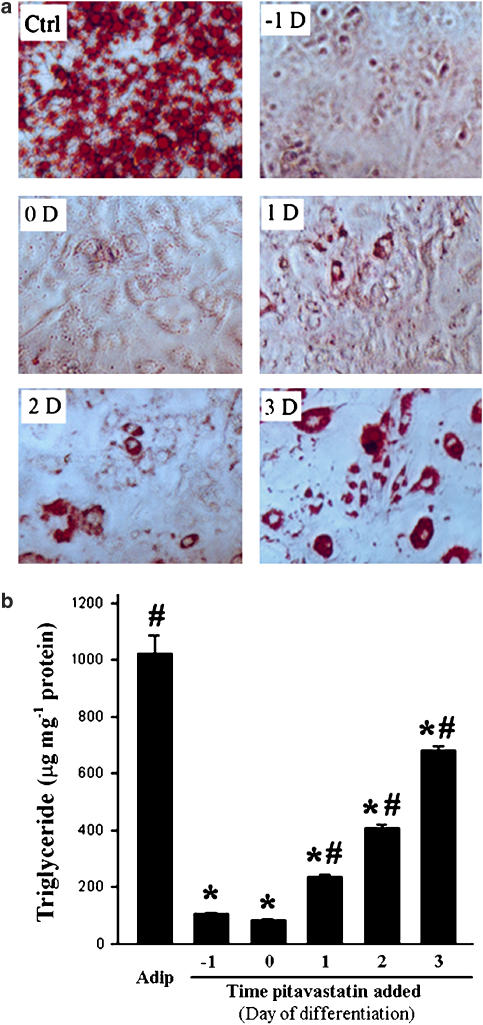
In vitro adipocyte differentiation is completed in about 1 week following the initiation of IDI treatment. Associated with this differentiation, a series of molecular events occur at different times, which determine the phenotype of adipocytes. Thus, the time of addition of pitavastatin in this procedure could have an important influence on adipocyte differentiation. To test this, pitavastatin (5 μM) was added to differentiated cells on days −1, 0, 1, 2 and 3 before and during IDI-induced differentiation. Both Oil Red O staining and the triglyceride assay demonstrated that lipid accumulation was dependent on the time of treatment with pitavastatin (Figure 2). Compared with untreated adipocytes, few oil droplets formed in cells when pitavastatin was added 1 day ahead or on the day of induction of differentiation. This suggests that pitavastatin completely blocked adipogenesis. Although pitavastatin reduced formation of oil droplets when added to cells after the initiation of differentiation, the effect was diminished as the time of addition was delayed after the initiation of differentiation (Figure 2a). Similarly, no triglyceride accumulated within cells when pitavastatin was added at the early stages of differentiation. However, significant amounts of triglycerides were present in cells when pitavastatin was added on day 1 or more after induction of differentiation (Figure 2b).
Figure 2.
Pitavastatin inhibits adipocyte differentiation in a time-dependent manner. Pitavastatin (5 μM) was added to cells on the indicated day of induction (before, at same time or after) of adipocyte differentiation. Both Oil Red O staining and triglyceride assay were conducted on day 8 of differentiation. #,*Significantly different from fibroblasts (day 0 sample) and adipocytes, respectively (P<0.05 by Student's t-test, n=4).
Pitavastatin inhibits expression of adipocyte-specific genes during adipocyte differentiation
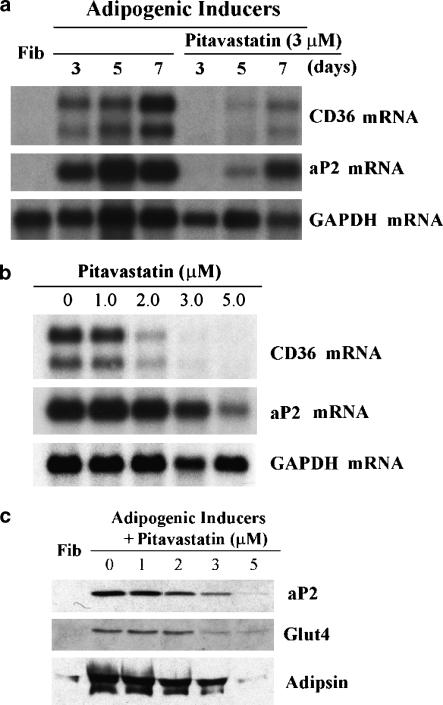
Adipocyte differentiation from fibroblast/preadipocytes is associated with expression of several adipocyte-specific genes including those for aP2, CD36, adipsin and GLUT4. The inhibitory actions of pitavastatin on adipocyte differentiation were further characterized by determining its effect on expression of these genes. Initially, expression of two PPARγ responsive genes, CD36 and aP2, was evaluated by Northern blot analysis in differentiated cells in the presence or absence of pitavastatin. Both aP2 and CD36 mRNA were undetectable in fibroblasts. After 3 days of induction, aP2 and CD36 mRNA were abundant. Their expression levels were maximal at 1 week of differentiation (Figure 3a). Expression of aP2 or CD36 mRNA in the presence of pitavastatin at day 3 of differentiation was markedly reduced. Although both CD36 and aP2 mRNA were detected on days 5 and 7 in differentiated cells in the presence of pitavastatin, the levels were much less than that in untreated differentiated cells. The effects of pitavastatin concentration on CD36 and aP2 mRNA expression in adipocyte differentiation were also determined. Pitavastatin (1–5 μM) was added at the onset of differentiation until completion of differentiation (1 week). An inverse relationship between the level of CD36/aP2 mRNA and the concentration of pitavastatin was observed (Figure 3b).
Figure 3.
Pitavastatin inhibits aP2 and CD36 mRNA expression, ap2 and GLUT4 protein expression, and secretion of adipsin during adipocyte differentiation. Adipocyte differentiation of confluent fibroblasts was induced with IDI for the indicated days in the presence or absence of 3 μM pitavastatin (a) or for 8 days in the presence or absence of pitavastatin at the indicated concentrations (b, c). Total RNA was extracted and analysed for CD36 and aP2 mRNA expression by Northern blot as described in the Methods. The same blot was re-probed for GAPDH mRNA. Cellular lysates and pooled culture medium were collected as described in the Methods. Cellular proteins (40 μg) or culture medium (30 μl) were evaluated for aP2, GLUT4 and adipsin by Western blot.
The effects of pitavastatin on protein expression of aP2 and GLUT4 in differentiated cells and the level of secreted adipsin in culture medium after adipocyte differentiation were determined by Western blot analysis. In fibroblasts, expression of aP2, GLUT4 and secreted adipsin were undetectable. Expression of each was very high in adipocytes, but significantly inhibited in differentiated cells in the presence of pitavastatin (Figure 3c).
Inhibition of adipocyte differentiation by pitavastatin is dependent on the suppression of PPARγ and the activation of pref-1, but not C/EBPα expression
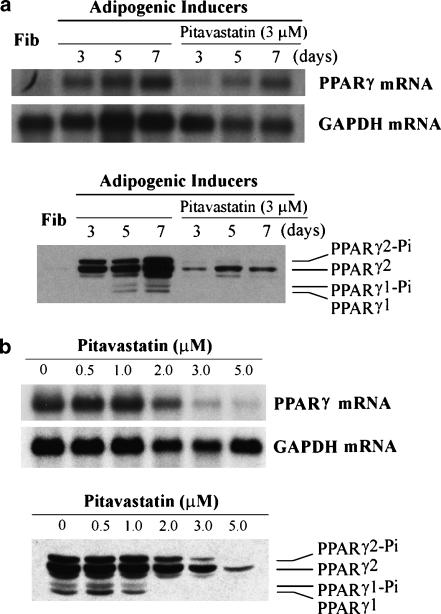
Adipocyte differentiation is activated by the coordinate actions of PPARγ and C/EBPα, but inhibited by expression of pref-1. To study the mechanism(s) by which pitavastatin inhibits adipocyte differentiation, the effects of pitavastatin on expression of PPARγ and C/EBPα was assessed. Both PPARγ mRNA and protein were undetectable in fibroblasts. The adipogenic mixture (IDI) significantly induced expression of PPARγ mRNA and protein (PPARγ1/2 and phosphorylated PPARγ1/2) after 5 days of induction (Figure 4a). Expression of PPARγ mRNA and protein was maximal after completion of differentiation (1 week). In the presence of pitavastatin, on day 3 of induction, PPARγ mRNA was still undetectable and protein was much lower than that in corresponding untreated differentiated cells. In the presence of pitavastatin, although both PPARγ mRNA and PPARγ2 protein were increased at the late stages of differentiation, expression was markedly reduced relative to untreated cells and expression of PPARγ1 protein was still undetectable (Figure 4). Further studies indicated that pitavastatin inhibited expression of PPARγ mRNA and protein in a dose-dependent manner during adipocyte differentiation (Figure 4b).
Figure 4.
Pitavastatin inhibits PPARγ expression during adipocyte differentiation. Fibroblasts were induced toward adipocyte differentiation for the indicated days in the presence of 3 μM pitavastatin (a) or for 8 days in the presence of indicated concentrations of pitavastatin (b). RNA was extracted to determine PPARγ mRNA expression by Northern blot. Nuclear proteins were isolated to determine PPARγ1/2 and phospho-PPARγ1, 2 (PPAR-γ1/2-Pi) protein expression by Western blot.
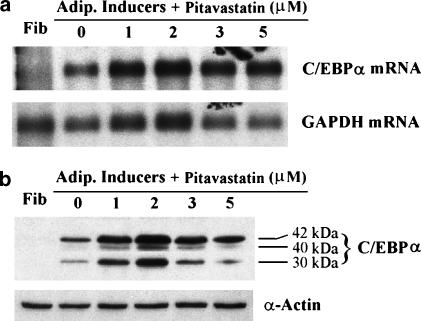
In contrast to the effects on PPARγ expression in adipocyte differentiation, pitavastatin increased C/EBPα expression (Figure 5). C/EBPα mRNA and protein were undetectable in fibroblasts by Northern and Western blots but were abundant in adipocytes. Pitavastatin-induced C/EBPα expression at different concentrations and maximal induction was seen at a concentration of 2 μM.
Figure 5.
Pitavastatin induces C/EBPα expression in adipocyte differentiation. Confluent fibroblasts were induced toward adipocyte differentiation for 8 days in the presence of pitavastatin at the indicated concentrations. RNA and nuclear protein were extracted to determine C/EBPα mRNA expression by Northern blot (a) and C/EBPα protein expression by Western blot (b), respectively.
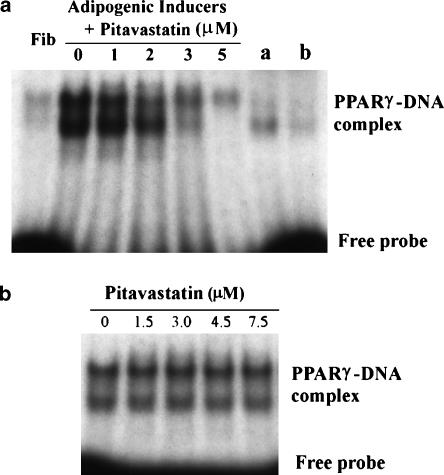
In addition to the regulation of PPARγ expression, the effects of pitavastatin on the binding activity of PPARγ protein to PPRE were evaluated (Figure 6). 3T3-L1 fibroblasts were induced to differentiate in the presence of varying concentrations of pitavastatin. At the completion of differentiation, nuclear proteins were collected and the DNA-binding activity of PPARγ was determined by EMSA. EMSA revealed a prominent PPARγ protein–DNA complex in adipocytes but a DNA–protein complex was barely detectable in fibroblasts. Changes in the PPARγ–DNA complex in response to pitavastatin reflected the PPARγ protein expression pattern (Figures 4b and 6a). Therefore, pitavastatin did not affect the affinity for the binding of PPARγ protein to its response element. To further confirm this, different concentrations of pitavastatin were added to the nuclear protein–DNA reaction system and the formation of PPARγ–DNA complexes was evaluated. Pitavastatin did not interfere with the reaction of PPRE (Figure 6b).
Figure 6.
Effect of pitavastatin on PPARγ DNA-binding activity. Confluent fibroblasts were induced toward adipocyte differentiation in the presence of pitavastatin at the indicated concentrations for 8 days. Nuclear proteins were collected and used to analyse DNA-binding activity of PPARγ protein by EMSA as described in the Methods. (a) Nuclear proteins (4 μg) from fibroblasts (Fib), differentiated cells or samples a/b (nuclear extract of THP-1/PMA macrophages supplied by the manufacturer of the assay kits) were used to conduct EMSA. Anti-PPARγ (1:5 diluted) was added to sample b reaction system. (b) Pitavastatin at the indicated concentrations was added to the reaction system of adipocyte nuclear proteins with PPARγ probe. The complex of PPARγ–DNA was determined by EMSA.
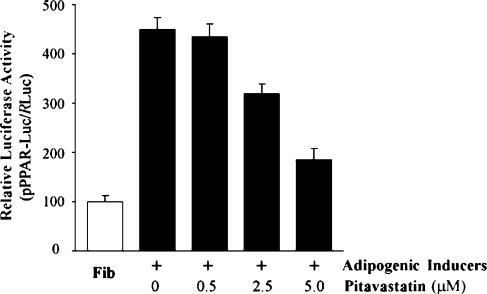
To directly investigate the effect of pitavastatin on PPARγ transcription, a pPPAR-Luc was constructed and used to transfect into preadipocytes. The effect of pitavastatin on activity of the PPARγ promoter was evaluated in the presence of IDI (Figure 7). With the progression of adipocyte differentiation, PPARγ promoter activity was significantly elevated. However, pitavastatin suppressed this activation suggesting a direct effect on transcription of PPARγ.
Figure 7.
Pitavastatin inhibits PPARγ transcription. Confluent 3T3-L1 preadipocytes in 24-well plates were transfected with pPPAR-Luc or RLuc by Lipofectamine 2000. After transfection, cells were induced toward adipocyte differentiation with IDI in the presence or absence of the indicated concentration of pitavastatin for 48 h. Activity of pPPAR-Luc was determined and normalized to the activity of RLuc (n=4).
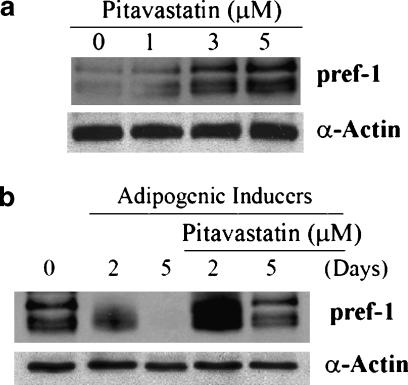
The effects of pitavastatin on expression of pref-1 were evaluated by two experiments. First, the effect of pitavastatin on pref-1 expression in preadipocytes was studied. As reported by others, multiple bands of pref-1 were observed by Western blot analysis (Smas and Sul, 1993). Pitavastatin increased pref-1 expression in preadipocytes in a concentration-dependent manner (Figure 8a). Second, the effect of pitavastatin on pref-1 expression in differentiated cells was investigated. During adipocyte differentiation, expression of pref-1 declined rapidly. After 5 days of differentiation, pref-1 was undetectable. In contrast, pitavastatin still induced pref-1 expression at the earlier stages of differentiation. Even at the later stages, pitavastatin still maintained pref-1 expression at levels comparable with those observed in preadipocytes (Figure 8b).
Figure 8.
Activation of pref-1 expression by pitavastatin. (a) Pitavastatin induces pref-1 expression in preadipocytes. 3T3-L1 preadipocytes were treated with pitavastatin at the indicated concentrations for 24 h. Total cellular proteins were extracted and expression of pref-1 protein was determined by Western blot. (b) Pitavastatin maintains pref-1 expression in differentiated cells. 3T3-L1 preadipocytes were induced toward adipogenesis with IDI in the presence or absence of pitavastatin (5 μM) for 2 and 5 days, respectively. Expression of pref-1 protein in preadipocytes and differentiated cells was determined by Western blot.
Discussion
Adipocyte differentiation is predominantly regulated by two transcription factors, PPARγ and C/EBPα. Selective regulation of PPARγ or C/EBPα expression can alter this differentiation process. Here, we demonstrate that pitavastatin and other statins inhibited adipocyte differentiation by inhibiting PPARγ, but not C/EBPα expression, and by increasing expression of an important inhibitor of adipocyte differentiation, pref-1.
Activation or inhibition of PPARγ affects adipocyte differentiation (Spiegelman, 1998; Tamori et al., 2002). Both natural (prostaglandin J2 series and some oxidized lipids) and synthetic (thiazolidinediones (TZDs)) PPARγ ligands activate PPARγ and promote adipogenesis in primary cells and cell lines (Kliewer et al., 1995; Gimble et al., 1996). In contrast, binding of bisphenol A diglycidyl ether does not activate PPARγ but blocks the binding of PPARγ ligands and significantly inhibits adipocyte differentiation (Wright et al., 2000). Decreased PPARγ expression also negatively influences adipocyte differentiation. Phosphatidylinositol 3-kinase inhibitors, LY294002 and wortmannin, inhibit PPARγ expression and block adipocyte differentiation (Xia and Serrero, 1999). Tumor necrosis factorα inhibits adipocyte differentiation in preadipocytes and reverses differentiation in differentiated adipocytes by suppressing PPARγ expression without affecting pref-1 expression (Xing et al., 1997; Kurebayashi et al., 2001). In these experiments, the expression of C/EBPα is either not changed or changed in a trend similar to PPARγ in response to treatment. In contrast, our studies demonstrate that pitavastatin-induced inhibition of adipocyte differentiation is associated only with decreased PPARγ, not C/EBPα expression. These findings suggest that inhibition of PPARγ is essential for blocking adipocyte differentiation in response to the statins. Furthermore, our data are consistent with work of Li et al. (2003) who evaluated adipogenesis in D1 cells, a multipotential cell line that can differentiate into cells of osteoblast or adipocyte lineage. They demonstrated that lovastatin inhibited adipogenesis in D1 cells by inhibiting expression of PPARγ2. Similarly, atorvastatin was recently reported to inhibit 3T3-L1 adipocyte differentiation by significantly reducing expression of PPARγ and moderately reducing expression of C/EBPα (Nakata et al., 2006).
C/EBPα is crucial for adipocyte differentiation. Expression of C/EBPα is triggered by C/EBPβ/δ and occurs 3 days after the initiation of adipogenic differentiation (Cornelius et al., 1994; Clarke et al., 1997; Lane et al., 1999). In vivo, C/EBPα is essential for differentiation of white, but not of brown adipose tissue. Lack of C/EBPα in knockout mice results in death of the mice shortly after birth (Linhart et al., 2001). Transfection of a fibroblastic cell line with a C/EBPα containing retrovirus promotes adipogenesis, while antisense C/EBPα RNA suppresses adipocyte differentiation of 3T3-L1 cells (Lin and Lane, 1992; Freytag et al., 1994). Several molecules, such as chlorinated cyclodiene pesticides and retinoic acid, block adipogenesis through C/EBPα-mediated transcription but also suppress expression of PPARγ (Schwarz et al., 1997; Moreno-Aliaga and Matsumura, 1999). We showed that inhibition of adipocyte differentiation by pitavastatin did not require the suppression of C/EBPα. In support of these data, others have shown that in the absence of C/EBPα, TZDs can induce adipogenesis (Gustafson et al., 2003).
Pref-1, a transmembrane protein, contains six EGF-like repeats in its extracellular domain (Smas and Sul, 1993). Cleavage at two proteolytic processing sites in this domain leads to the generation of a soluble pref-1 protein of approximately 50 kDa corresponding to the ectodomain and smaller products from 24 to 30 kDa (Smas et al., 1997). Pref-1 is abundant in preadipocytes but absent in differentiated adipocytes suggesting that expression of pref-1 is regulated during the differentiation process. More importantly, the regulatory role of pref-1 in adipocyte differentiation has been defined. Both constitutive expression of complete pref-1 in preadipocytes and addition of the ectodomain of pref-1 to preadipocytes can inhibit adipogenic agent-induced adipocyte differentiation (Smas and Sul, 1993; Mei et al., 2002). Attenuation of pref-1 transcription by dexamethasone suggests that the pref-1 gene is an early target for initiation of adipocyte differentiation (Smas et al., 1999). The requirement of serum for adipocyte differentiation is necessary owing to the inhibition of pref-1 expression (Smas et al., 1999). Compared with 3T3-L1 cells, pref-1 is threefold higher in the closely related but differentiation-defective 3T3-C2 cells (Smas and Sul, 1993). High expression of pref-1 in transgenic mice is associated with a substantial decrease in total fat pad weight, reduced expression of adipocyte markers and adipocyte-secreted factors. In contrast, genetic deletion of pref-1 causes obesity in mice (Moon et al., 2002; Lee et al., 2003). Our studies support an important role for pref-1 in the inhibition of adipocyte differentiation as pitavastatin induces pref-1 expression in preadipocytes. More importantly, pitavastatin prevents the suppression of pref-1 by adipogenic-inducing agents.
Statin therapy has proved to be beneficial in reducing the incidence of cardiovascular events through its effect in lowering LDL cholesterol levels. Since obesity is a well-established risk factor for cardiovascular disease and diabetes, our studies suggest that it may be interesting to determine if the statins have a role in adipogenesis in vivo.
Acknowledgments
This work was supported by a sponsored research agreement with Kowa Co. Ltd, Tokyo, Japan. Drs Gotto and Hajjar are consultants to the Kowa Co. This work was also supported by the Abercrombie and Silbermann Foundations.
Abbreviations
- aP2
fatty acid-binding protein
- C/EBPs
CCAAT enhancer-binding proteins
- GLUT4
glucose transporter 4
- IDI
adipogenic inducers (isobutylmethylxanthine, dexamethasone and insulin)
- PPARγ
peroxisome proliferator-activated receptor-γ
- pref-1
preadipocyte factor-1
Conflict of interest
The authors state no conflict of interest.
References
- Bernlohr DA, Bolanowski MA, Kelly TJ, Jr, Lane MD. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1985;260:5563–5567. [PubMed] [Google Scholar]
- Clarke SL, Robinson CE, Gimble JM. CAAT/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor γ2 promoter. Biochem Biophys Res Commun. 1997;240:99–103. doi: 10.1006/bbrc.1997.7627. [DOI] [PubMed] [Google Scholar]
- Cornelius P, Macdougald OA, Lane MD. Regulation of adipocyte development. Annu Rev Nutr. 1994;14:99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- Farmer SR. Transcriptional control of adipocyte formation. Cell Met. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag SO, Paielli DL, Gilbert JD. Ectopic expression of the CCAAT/enhancer-binding protein α promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 1994;8:1654–1663. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, et al. Peroxisome proliferator-activated receptor-γ activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50:1087–1094. [PubMed] [Google Scholar]
- Green H, Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol. 1979;101:169–171. doi: 10.1002/jcp.1041010119. [DOI] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Gustafson B, Jack MM, Cushman SW, Smith U. Adiponectin gene activation by thiazolidinediones requires PPAR γ2, but not C/EBPα-evidence for differential regulation of the aP2 and adiponectin genes. Biochem Biophys Res Commun. 2003;308:933–939. doi: 10.1016/s0006-291x(03)01518-3. [DOI] [PubMed] [Google Scholar]
- Han J, Parsons M, Zhou X, Nicholson AC, Gotto AM, Jr, Hajjar DP. Functional interplay between the macrophage scavenger receptor class B type I and pitavastatin (NK-104) Circulation. 2004;110:3472–3479. doi: 10.1161/01.CIR.0000148368.79202.F1. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kim JH, Wang Y, Sul HS. Preadipocyte factor-1 (Pref-1) activates MEK/ERK pathway to inhibit adipocyte differentiation. Mol Cell Biol. 2007;27:2294–2308. doi: 10.1128/MCB.02207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Kurebayashi S, Sumitani S, Kasayama S, Jetten AM, Hirose T. TNF-α inhibits 3T3-L1 adipocyte differentiation without downregulating the expression of C/EBP β and δ. Endocr J. 2001;48:249–253. doi: 10.1507/endocrj.48.249. [DOI] [PubMed] [Google Scholar]
- Lane MD, Tang QQ, Jiang MS. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem Biophys Res Commun. 1999;266:677–683. doi: 10.1006/bbrc.1999.1885. [DOI] [PubMed] [Google Scholar]
- LaRosa JC. Pleiotropic effects of statins and their clinical significance. Am J Cardiol. 2001;88:291–293. doi: 10.1016/s0002-9149(01)01643-5. [DOI] [PubMed] [Google Scholar]
- Lee K, Villena JA, Moon YS, Kim KH, Lee S, Kang C, et al. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1) J Clin Invest. 2003;111:453–461. doi: 10.1172/JCI15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Cui Q, Kao C, Wang GJ, Balian G. Lovastatin inhibits adipogenic and stimulates osteogenic differentiation by suppressing PPARγ2 and increasing Cbfa1/Runx2 expression in bone marrow mesenchymal cell cultures. Bone. 2003;33:652–659. doi: 10.1016/s8756-3282(03)00239-4. [DOI] [PubMed] [Google Scholar]
- Lin FT, Lane MD. Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev. 1992;6:533–544. doi: 10.1101/gad.6.4.533. [DOI] [PubMed] [Google Scholar]
- Linhart HG, Ishimura-Oka K, Demayo F, Kibe T, Repka D, Poindexter B, et al. C/EBPα is required for differentiation of white, but not brown, adipose tissue. Proc Natl Acad Sci USA. 2001;98:12532–12537. doi: 10.1073/pnas.211416898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup S, Lane MD. Regulating adipogenesis. J Biol Chem. 1997;272:5367–5370. doi: 10.1074/jbc.272.9.5367. [DOI] [PubMed] [Google Scholar]
- Mei B, Zhao L, Chen L, Sul HS. Only the large soluble form of preadipocyte factor-1 (Pref-1), but not the small soluble and membrane forms, inhibits adipocyte differentiation: role of alternative splicing. Biochem J. 2002;364:137–144. doi: 10.1042/bj3640137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller DE, Flier JS. Insulin resistance – mechanisms, syndromes, and implications. N Engl J Med. 1991;325:938–948. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- Moon YS, Smas CM, Lee K, Villena JA, Kim KH, Yun EJ, et al. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol Cell Biol. 2002;22:5585–5592. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Aliaga MJ, Matsumura F. Endrin inhibits adipocyte differentiation by selectively altering expression pattern of CCAAT/enhancer binding protein-α in 3T3-L1 cells. Mol Pharmacol. 1999;56:91–101. doi: 10.1124/mol.56.1.91. [DOI] [PubMed] [Google Scholar]
- Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia. 2006;49:1881–1892. doi: 10.1007/s00125-006-0269-5. [DOI] [PubMed] [Google Scholar]
- Nishio E, Tomiyama K, Nakata H, Watanabe Y. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitor impairs cell differentiation in cultured adipogenic cells (3T3-L1) Eur J Pharmacol. 1996;301:203–206. doi: 10.1016/0014-2999(96)00063-5. [DOI] [PubMed] [Google Scholar]
- Novikoff AB, Novikoff PM, Rosen OM, Rubin CS. Organelle relationships in cultured 3T3-L1 preadipocytes. J Cell Biol. 1980;87:180–196. doi: 10.1083/jcb.87.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M, Djian P, Green H. The nucleotide sequence of three genes participating in the adipose differentiation of 3T3 cells. J Biol Chem. 1986;261:10821–10827. [PubMed] [Google Scholar]
- Rosen ED, MacDouglad OA. Adipocyte differentiation from the inside out. Nat Rev. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Retinoic acid blocks adipogenesis by inhibiting C/EBPβ-mediated transcription. Mol Cell Biol. 1997;17:1552–1561. doi: 10.1128/mcb.17.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D, Lazar MA. Peroxisome proliferator activated receptor γ, CCAAT/enhancer-binding protein α, and cell cycle status regulate the commitment to adipocyte differentiation. J Biol Chem. 1997;272:21473–21478. doi: 10.1074/jbc.272.34.21473. [DOI] [PubMed] [Google Scholar]
- Smas CM, Chen L, Sul HS. Cleavage of membrane-associated pref-1 generates a soluble inhibitor of adipocyte differentiation. Mol Cell Biol. 1997;17:977–988. doi: 10.1128/mcb.17.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smas CM, Chen L, Zhao L, Latasa MJ, Sul HS. Transcriptional repression of pref-1 by glucocorticoids promotes 3T3-L1 adipocyte differentiation. J Biol Chem. 1999;274:12632–12641. doi: 10.1074/jbc.274.18.12632. [DOI] [PubMed] [Google Scholar]
- Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- Song C, Guo Z, Ma Q, Chen Z, Liu Z, Jia H, et al. Simvastatin induces osteoblastic differentiation and inhibits adipocytic differentiation in mouse bone marrow stromal cells. Biochem Biophys Res Commun. 2003;308:458–462. doi: 10.1016/s0006-291x(03)01408-6. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM. PPAR-γ: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Choy L, Hotamisligil GS, Graves RA, Tontonz P. Regulation of adipocyte gene expression in differentiation and syndromes of obesity/diabetes. J Biol Chem. 1993;268:6823–6826. [PubMed] [Google Scholar]
- Sun L, Nicholson AC, Hajjar DP, Gotto AM, Jr, Han J. Adipogenic differentiating agents regulate expression of fatty acid binding protein and CD36 in the J744 macrophage cell line. J Lipid Res. 2003;44:1877–1886. doi: 10.1194/jlr.M300084-JLR200. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- Tamori Y, Masugi J, Nishino N, Kasuga M. Role of peroxisome proliferator-activated receptor-γ in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes. 2002;51:2045–2055. doi: 10.2337/diabetes.51.7.2045. [DOI] [PubMed] [Google Scholar]
- Wright HM, Clish CB, Mikami T, Hauser S, Yanagi K, Hiramatsu R, et al. A synthetic antagonist for the peroxisome proliferator-activated receptor γ inhibits adipocyte differentiation. J Biol Chem. 2000;275:1873–1877. doi: 10.1074/jbc.275.3.1873. [DOI] [PubMed] [Google Scholar]
- Xia X, Serrero G. Inhibition of adipose differentiation by phosphatidylinositol 3-kinase inhibitors. J Cell Physiol. 1999;178:9–16. doi: 10.1002/(SICI)1097-4652(199901)178:1<9::AID-JCP2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Xing H, Northrop JP, Grove JR, Kilpatrick KE, Su JL, Ringold GM. TNFα-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPARγ without effects on pref-1 expression. Endocrinology. 1997;138:2776–2783. doi: 10.1210/endo.138.7.5242. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Cao Z, Classon M, Mcknight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Alvares K, Huang Q, Rao MS, Reddy JK. Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J Biol Chem. 1993;268:26817–26820. [PubMed] [Google Scholar]