Abstract
Background and purpose:
Melanin-concentrating hormone (MCH) is a cyclic orexigenic neuropeptide predominantly expressed in the lateral hypothalamus. We investigated the roles of MCH1 receptor signalling in ovariectomy (OVX)-induced obesity in female C57BL/6J mice, an animal model of postmenopausal obesity.
Experimental approach:
The effects of blocking signalling via the MCH1 receptor on OVX-induced obesity was investigated by using Mch1r deficient (KO) mice and chronic treatment with a selective MCH1 receptor antagonist.
Key results:
OVX induced body weight gain and increases in the weight of visceral fat and of liver; these effects were attenuated following OVX in Mch1r KO mice. OVX-induced triglyceride (TG) accumulation and elevated expression of lipogenic genes were significantly ameliorated in the liver of Mch1r KO mice. In agreement with these results, chronic i.c.v. infusion of a selective MCH1 receptor antagonist significantly reduced body weight gain, visceral fat and liver weights in OVX mice, and hepatic TG contents and lipogenic gene expression levels were normalized.
Conclusion and implications:
Our results indicate that MCH1 receptor signalling is involved in the development of fatty liver, as well as obesity, in OVX mice, and suggest a therapeutic potential for MCH1 receptor antagonists in the treatment of obesity and fatty liver.
Keywords: liver, melanin-concentrating hormone, obesity, ovariectomy
Introduction
Menopause is frequently associated with increased body mass in humans. Likewise, withdrawal of oestrogen after bilateral ovariectomy (OVX) in rodents causes a temporal increase in food intake and decreases in motor activity and thermogenesis, resulting in obesity (Landau and Zucker, 1976; McElroy and Wade, 1987; Yoshioka et al., 1988; Shimomura et al., 1990). Oestrogen directly modulates excitability of neurons in the hypothalamus (Minami et al., 1990) and gene expression of neuropeptides that regulate energy homeostasis, such as neuropeptide Y (NPY) and melanin-concentrating hormone (MCH) (Shimizu et al., 1996; Mystkowski et al., 2000).
MCH, a cyclic neuropeptide, was initially identified as a skin colour regulator in fish (Kawauchi et al., 1983). It is reported that MCH in mammals is expressed in the lateral hypothalamic area (Nahon, 1994), which is one of the brain regions well known to play key roles in energy homeostasis. Upregulation of prepro-MCH mRNA was reported in several obese animals (Qu et al., 1996; Rovere et al., 1996; Mizuno et al., 1998; Hanada et al., 2000; Tritos et al., 2001). Intracerebroventricular (i.c.v.) injection of MCH triggered increased food intake in rats (Rossi et al., 1997, 1999; Ludwig et al., 1998; Tritos et al., 1998; Chaffer and Morris, 2002; Abbott et al., 2003; Kela et al., 2003) and disruption of the prepro-MCH gene reduces food intake and increases metabolic rate, which resulted in a lean phenotype (Shimada et al., 1998). In contrast, overexpression of prepro-MCH gene causes moderate obesity and insulin resistance in mice (Ludwig et al., 2001). In addition, we showed recently that chronic i.c.v. infusion of MCH caused obesity not only by increasing food intake, but also by decreasing energy expenditure in mice (Gomori et al., 2003; Ito et al., 2003).
The pharmacological effects of MCH are thought to be mediated through two G protein-coupled receptors, the MCH1 receptor and the MCH2 receptor. Since MCH2 receptor does not exist in rodents (Sailer et al., 2001), the MCH1 receptor is predominantly responsible for MCH-related obesity in rodents. Indeed, Mch1r knockout (KO) mice are resistant to diet-induced obesity (DIO; Marsh et al., 2002), and chronic administration of an MCH1 receptor antagonist, which did not show any effects in Mch1r KO-DIO mice, significantly suppressed food intake and body weight gain in DIO mice (Mashiko et al., 2005).
Recently, several reports suggested that oestrogen influences the gene expression of MCH. Oestrogen administration decreases hypothalamic MCH mRNA levels in OVX rats (Murray et al., 2000). Hyperoestrogenemia selectively and completely suppresses the expected increase of MCH mRNA expression in response to progressive weight loss (Mystkowski et al., 2000), although the physiological relevance of this finding remains to be explored. Moreover, it was recently reported that a physiological dose of oestradiol significantly decreased MCH-induced food intake in OVX rats (Messina et al., 2006). In addition, oestrogen receptors are localized in the lateral hypothalamus close to MCH-producing cells, which suggests that oestrogen may directly modulate MCH production and release (Blaustein, 1992). These findings suggest that MCH might be one of the specific targets for oestrogen to regulate energy homeostasis. Therefore, we hypothesized that the deregulation of MCH could be involved in menopause-related obesity and metabolic changes and thus investigated the metabolic effects of deficiencies in signalling via the MCH1 receptor in OVX mice. In the present study, we found that blockade of MCH signalling by receptor KO or a chronic treatment with a selective MCH1 receptor antagonist strikingly ameliorated the obesity and development of fatty liver, induced by OVX. These results suggest a possible role of MCH1 receptor antagonists in the treatment of obesity and fatty liver.
Methods
Animals
Female C57BL/6J mice (12 weeks old) were purchased from CLEA Japan Inc. (Tokyo, Japan). Female Mch1r KO mice (14–16 weeks old) were generated as described previously (Marsh et al., 2002) and they were backcrossed for seven generations onto a C57BL/6J background (wild type, WT) using a speed congenics technique (Wong, 2002). Mice were housed individually in plastic cages under controlled temperature, humidity (23±2°C, 55±15%) and 12 h light–dark cycle (lights on from 0700 to 1900 hours) with ad libitum access to regular diet (CE-2, CLEA Japan Inc.) and tap water. All experimental procedures followed the Japanese Pharmacological Society Guidelines for Animal Use.
Surgical procedure and experimental designs
Experiment 1: Effect of OVX in WT and Mch1r KO mice
Mice were randomly divided into two groups. One group received bilateral OVX (WT: N=12, KO: N=14) and the other was sham-operated (WT: N=9, KO: N=15). The operation was performed under ether anaesthesia. Body weight was measured once a week for 8 months. Cumulative food intake for 1 month was measured starting at 4 months after the OVX operation. Eight months after OVX, mice were fasted for 2 h and blood samples were collected from the infraorbital vein for measurement of plasma glucose, insulin and leptin levels. Afterwards, the mice were killed by collecting whole blood from the heart under ether anaesthesia. The blood samples were used for measurement of blood parameters. The liver and mesenteric adipose tissue were excised and weighed. Liver samples were snap-frozen in liquid nitrogen and stored at −80°C before measuring hepatic lipids and mRNAs.
Experiment 2: I.c.v. infusion of MCH1 receptor antagonist in OVX-induced obese mice
Mice were randomly divided into two groups. One group received bilateral OVX (N=20) and the other was sham-operated (N=7). Operations were performed under ether anaesthesia. Five months after OVX, mice were anaesthetized with sodium pentobarbital (80 mg kg−1, intraperitoneally; Dainabot, Osaka, Japan). A sterile brain infusion cannula (28 G; Durect Corporation, Cupertino, CA, USA) was connected to an osmotic minipump (model no. 2004, Durect Corporation) filled with 30% propylene glycol (PG) in distilled water with polyvinylchloride tubing. The osmotic minipump was implanted under the skin of the back. Then, the infusion cannula was stereotaxically implanted into the right lateral ventricle and fixed to the skull with medical adhesive and dental cement. The stereotaxic coordinates used were 0.4 mm posterior to the bregma, 0.8 mm lateral to the midline and 2.0 mm from the surface of the skull using a flat skull position. Finally, the incision was closed with sutures and antibiotic (Cefamezin-α, 50 mg kg−1, Astellas Pharma Inc., Tokyo, Japan) was subcutaneously injected once at the end of the surgery. The placement of the cannula was confirmed at the end of the experiment by the injection of 0.5% Evans blue dye. Following the 4-week recovery period, OVX mice were divided into two groups to match average body weight and food intake (n=10). The infusion pump was replaced with a new pump (model no. 2004, Durect Corporation) filled with the MCH1 receptor antagonist (7.5 μg day−1 for 4 weeks) or its vehicle (30% PG) under ether anaesthesia. This dose was selected because the MCH1 receptor antagonist at 7.5 μg day−1 produced anti-obesity effects in DIO, but not in DIO-Mch1r KO mice (Mashiko et al., 2005). Food intake and body weight were measured daily. After a 4-week i.c.v. infusion, mice were fasted for 2 h, and blood samples were collected from the infraorbital vein for measurement of plasma glucose, insulin and leptin levels. The mice were then killed by collecting whole blood from the heart under ether anaesthesia. The blood samples were used for measuring blood parameters including triglyceride (TG), total cholesterol and free fatty acid (FFA). Perimetric, retroperitoneal and mesenteric adipose tissues and liver were excised and weighed. Liver samples were snap-frozen in liquid nitrogen and stored at −80°C before measuring hepatic lipids and mRNAs.
Motor activity
Six months after OVX, we examined the effect of OVX on locomotor activity in sham or OVX-operated WT and KO mice. Motor activity was measured for 3 consecutive days using activity-monitoring system (NS-AS01, Neuroscience Inc., Tokyo, Japan) in home cages. In brief, the activity monitor consisted of an infrared radiation sensor placed over a home cage (21 × 32 × 12.5 cm), a signal amplification circuit and a control unit. The sensor detected the movement of animals based on the infrared radiation associated with their body temperature (Mori et al., 2000; Narita et al., 2002). Motor activity data were collected in 10 min intervals and analysed with a computer-associated system (AB System-24A, Neuroscience Inc.).
Measurement of plasma hormone levels
Plasma leptin and insulin levels were measured using ELISA (enzyme-linked immunosorbent assay) kits (Morinaga, Kanagawa, Japan). Plasma glucose, TG, total cholesterol and FFA levels were measured using commercial kits (Determiner GL-E, L TGII, L TCII, Kyowa Medex Co. Ltd, Tokyo, Japan; NEFA-HA Testwako (II), Wako Pure Chemical Industries Ltd, Osaka, Japan).
Measurement of liver lipid contents
Total lipids were extracted from 50 mg of liver by the method of Folch et al. (1957) and dried under N2 gas. The TG contents of liver were measured using Determiner L TGII kit (Kyowa Medex).
Real-time reverse transcription-PCR
TaqMan analysis was used to determine mRNA levels of sterol regulatory element-binding protein-1a, -1c (SREBP1a, -1c), fatty acid synthase (FAS), acetyl-CoA carboxylase 1 and 2 (ACC1, 2), acyl-CoA oxidase (ACO), peroxisome proliferator-activated receptor α (PPARα) and liver type carnitine palmitoyltransferase I (CPT1L) in the liver as described previously (Ito et al., 2003). Briefly, total RNA was purified using Isogen (Nippon Gene, Tokyo, Japan). Quantity and purity were determined by absorbance at 260 and 280 nm. cDNA was synthesized from 1 μg of total RNA using TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA, USA). The following primers and probes were used in the real-time PCR. For FAS, the primers were forward GGCTCAGCATGGTCGCTT, reverse CTCCCGCCAGCTGTCATT and probe AACCACCCTCTGGGCATGGCTATCTTCT. For ACC1 (GenBank accession no. NM133360), the primers were forward TTCTGAATGTGGCTATCAAGACTGA, reverse TGCTGGGTGAACTCTCTGAACA and probe CGATATTGAGGATGACAGGCTTGCAGCT. For ACC2 (GenBank accession no. NM133904), the primers were forward ACAGAGATTTCACCGTTGCGT, reverse CGCAGCGATGCCATTGT and probe ACTCGCTTTGGAGGCAACAGGGTCAT. For SREBP1a (GenBank accession no. BC056922), the primers were forward CCGAGATGTGCGAACTGGA, reverse AAGTCACTGTCTTGGTTGTTGATGA and probe TTTTGAACGACATCGAAGACATGCTCCA. For SREBP1c (GenBank accession no. AB046200), the primers were forward GTAGCGTCTGCACGCCCTA, reverse CTTGGTTGTTGATGAGCTGGAG and probe ACGGAGCCATGGATTGCACATTTGAAG. For PPARα (GenBank accession no. NM011144), the primers were forward CGCGTGTGATAAAGCCATTG, reverse CACGATGCTGTCCTCCTTGA and probe CGTACGCGATCAGCATCCCGTCTTT. For ACO, the primers were forward GCCTTTGTTGTCCCTATCCGT, reverse CGATATCCCCAACAGTGATGC and probe AGATTGGGACCCACAAGCCTCTGCC. For CPT1L, the primers were forward CCTGCAACTTTGTGCTGGC, reverse TGAACAGCTTGAGCCTCTGCT and probe ATGATGGACCCCACAACAACGGCA. A primer and VIC-labeled probe set for β-actin were synthesized based on sequences by Niiya et al. (2001).
In a typical reaction, 5 ng of cDNA was mixed with 5 μl of TaqMan Universal PCR Master Mix (Applied Biosystems); 900 nM of forward and reverse primers; 250 nM of probe complementary to the gene of interest, which was labeled with FAM dye; 60 nM of each primer; and 250 nM of probe complementary to β-actin labeled with VIC dye in a 10 μl total volume. The reaction was performed in a 384-well optical reaction plate using PRISM 7900HT Sequence Detection System (Applied Biosystems). The expression data were normalized to β-actin expression level.
Statistical analyses
Data are expressed as means±s.e. Significant differences in body weight changes were analysed by repeated-measures one-way analysis of variance (ANOVA), followed by Bonferroni/Dunn test. For initial body weights, total body weight changes, cumulative food intake, blood parameters, tissue weights, mRNA levels and lipid parameters in the liver unpaired t-tests were performed. In the Mch1r KO mice study, two-way ANOVA was performed for the interaction between factors of OVX- and MCH deficiency phenotype. P-values <0.05 were considered to be significant.
Materials
The MCH1 receptor antagonist was synthesized at Banyu Pharmaceutical Co. Ltd (Ibaraki, Japan). This compound has previously been characterized by Bednarek et al. (2002), ‘Compound 30'; and Shearman et al. (2003), ‘Compound B'. The antagonist has high affinity for MCH1 receptor with a Ki value of 9.9 nM and about 1000-fold selectivity over MCH2 receptor (Mashiko et al., 2005). All other chemicals were of analytical grade.
Results
Changes in body weight and food intake after OVX in WT and Mch1r KO mice
We used female Mch1r KO mice whose background was C57BL/6J and female C57BL/6J mice as WT mice. We evaluated the response of Mch1r KO and WT mice to OVX. Initial body weights of the WT and Mch1r KO mice differed by about 2 g (P<0.001, Figure 1a). Eight months after OVX operation, the body weight change did not differ between sham-operated WT and KO mice (Figure 1b). OVX significantly increased body weight change in both WT and Mch1r KO mice, but the body weight change was significantly attenuated in Mch1r KO mice (Figure 1b). There was a significant interaction between OVX surgery and MCH deficiency in the total body weight change (F(1, 46)=8.80, P=0.0048). Mch1r KO significantly increased food intake in sham-operated mice, but not in OVX mice at 4 months after the operation (Table 1).
Figure 1.
Body weight (a) and total body weight change at 8 months after operation (b) in OVX-WT and Mch1r KO mice. Data shown are means±s.e. Numbers in parentheses indicate the numbers of animals. ***P<0.001 vs sham-operated group. ###P<0.001 vs WT mice receiving the same operation (sham or OVX). KO, knockout; OVX, ovariectomy; WT, wild type.
Table 1.
Cumulative food intake, tissue weights and blood parameters after OVX operation in wild-type and Mch1r KO mice (Experiment 1)
|
Wild type |
Mch1r KO |
|||
|---|---|---|---|---|
| Sham | OVX | Sham | OVX | |
| Cumulative food intake (g) | 113.2±3.3 | 113.7±2.7 | 125.7±3.3# | 113.1±4.2* |
| Tissue weights | ||||
| Mesenteric fat weight (g) | 0.22±0.03 | 0.45±0.08* | 0.09±0.02### | 0.17±0.03*,## |
| Liver weight (g) | 1.30±0.04 | 1.43±0.03* | 1.02±0.04### | 1.09±0.04### |
| Liver TG (mg g liver−1) | 11.3±0.5 | 14.5±1.3* | 7.8±0.7## | 8.6±0.8### |
| Blood parameters | ||||
| Glucose (mM) | 8.2±0.2 | 10.2±0.2*** | 7.9±0.2 | 9.2±0.2***,## |
| Insulin (ng ml−1) | 0.6±0.1 | 1.4±0.2*** | 0.3±0.0## | 0.7±0.1**,## |
| Leptin (ng ml−1) | 4.7±0.7 | 12.2±1.4*** | 1.0±0.1### | 4.7±0.8***,### |
| Total CHL (mM) | 1.5±0.0 | 1.8±0.0*** | 1.2±0.0### | 1.3±0.1### |
| TG (mg l−1) | 401±48 | 375±38 | 259±21## | 250±20## |
| FFA (μeq l−1) | 621±36 | 603±32 | 612±22 | 696±33* |
Abbreviations: CHL, cholesterol; FFA, free fatty acid; KO, knockout; TG, triglyceride.
Food intake was monitored for 1 month starting at 4 months after OVX. Tissue weights and blood parameters were measured at 8 months after OVX. Data shown are means±s.e.
P<0.05, 0.01, 0.001 vs sham-operated group.
P<0.05, 0.01, 0.001 vs sham-operated group.
P<0.05, 0.01, 0.001 vs sham-operated group.
P<0.05, 0.01, 0.001 vs WT mice receiving the same operation (sham or OVX).
P<0.05, 0.01, 0.001 vs WT mice receiving the same operation (sham or OVX).
P<0.05, 0.01, 0.001 vs WT mice receiving the same operation (sham or OVX).
Changes in tissue weights after OVX in Mch1r KO mice
Mesenteric fat weight was significantly increased by OVX in both WT and Mch1r KO mice, but the fat weight was significantly less in OVX-Mch1r KO than OVX-WT mice (Table 1). Comparing the sham-operated mice, the liver of Mch1r KO mice weighed less than that of WT mice. OVX significantly increased liver weight in WT mice. In Mch1r KO mice, liver weight was not significantly changed by OVX (Table 1). OVX significantly increased liver TG contents by 1.3-fold in WT mice, while OVX did not cause significant changes of hepatic TG accumulation in Mch1r KO mice (Table 1).
Molecular profiling in liver after OVX in Mch1r KO mice
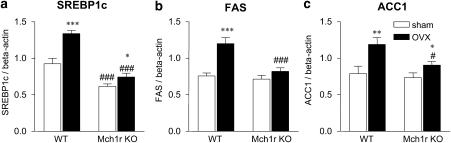
We measured expression of genes related to lipogenesis in the liver. As shown in Figure 2, expression of SREBP1c, which is a key transcriptional factor for fatty acid synthesis, was significantly elevated in OVX mice. After the sham operation, the expression of SREBP1c was lower in Mch1r KO mice compared to WT mice. Moreover, OVX-induced hyperexpression of SREBP1c was diminished in Mch1r KO mice (Figure 2a). Expression of FAS and ACC1 mRNAs, which are regulated by SREBP1c, were comparable between sham-operated WT and Mch1r KO mice. OVX increased the expression of these mRNAs in WT, but they were significantly inhibited in OVX-Mch1r KO mice (Figure 2). There were significant interactions between OVX treatment and MCH deficiency in the expression levels of SREBP1c mRNA (F(1, 46)=8.05, P=0.0068) and FAS mRNA (F(1, 46)=8.07, P=0.0067). In contrast, no significant interaction was observed in the expression levels of ACC1. Expression levels of genes related to fatty acid oxidation including PPARα and ACO were comparable in both phenotypes and were not affected by OVX (data not shown).
Figure 2.
Expression of hepatic SREBP1c (a), FAS (b) and ACC1 (c) mRNAs in WT and Mch1r KO mice measured by the TaqMan system. The expression of mRNA was measured at 8 months after OVX. Data shown are means±s.e. *,**,***P<0.05, 0.01, 0.001 vs sham-operated group. #,###P<0.05, 0.001 vs WT mice receiving the same operation (sham or OVX). ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; KO, knockout; OVX, ovariectomy; SREBP1c, sterol regulatory element-binding protein; WT, wild type.
Plasma biochemical parameters after OVX in Mch1r KO mice
In sham-operated mice, plasma insulin and leptin levels of Mch1r KO mice were significantly less than that of WT mice. In both WT and Mch1r KO mice, OVX significantly increased the plasma insulin and leptin levels relative to sham-operated mice. The insulin and leptin levels of OVX-Mch1r KO mice were comparable to the levels of sham-treated WT mice. There was a significant interaction between OVX treatment and MCH deficiency for leptin levels (F(1, 46)=4.91, P=0.0317) and a tendency to interact for plasma insulin levels (F(1, 46)=3.13, P=0.0837). Plasma glucose levels were significantly elevated by OVX in both WT and Mch1r KO mice, but the increment of plasma glucose was significantly higher in WT mice than in Mch1r KO mice. OVX significantly increased plasma total cholesterol levels in WT mice, but OVX did not affect cholesterol levels in Mch1r KO mice. The total cholesterol levels of Mch1r KO mice were less than that of WT mice, regardless of treatment. Plasma TG levels were significantly reduced in Mch1r KO mice, but the levels were not affected by OVX. Plasma FFA levels was significantly increased in Mch1r KO mice, but not in WT mice (Table 1).
Spontaneous locomotor activity in Mch1r KO mice
Locomotor activity was measured 6 months after OVX in WT and Mch1r KO mice (Figure 3). Compared with sham-operated WT mice, the sham-operated Mch1r KO mice tended to show an increased locomotor activity (P=0.06), as reported previously (Marsh et al., 2002). OVX significantly decreased locomotor activity in Mch1r KO mice, but not in WT mice. Cumulative activity for 24 h in OVX-Mch1r KO mice was similar to WT mice.
Figure 3.
Spontaneous locomotor activity in OVX-WT and Mch1r KO mice. Locomotor activity was measured at 6 months after OVX. Data are represented as means±s.e. *P<0.05 vs sham-operated group. KO, knockout; OVX, ovariectomy; WT, wild type.
Changes in body weight and food intake after i.c.v. infusion of MCH1 receptor antagonist in OVX-induced obese mice
The initial body weight of mice before OVX was 22.2±0.1 g. Five months after the OVX, their body weights were significantly heavier than sham-operated controls (P<0.01, Figure 4) and they showed slight, but significant hyperphagia (Table 2). Chronic i.c.v. infusion of the MCH1 receptor antagonist (7.5 μg day−1) for 4 weeks significantly decreased body weights and normalized food intake in the OVX mice (Figure 4 and Table 2).
Figure 4.
Effect of 4-week i.c.v. infusion of MCH1 receptor antagonist on body weight in OVX-induced obese mice. Six months after OVX, infusion of the MCH1 receptor antagonist was started. Numbers in parentheses indicate numbers of animals. Data shown are means±s.e. *P<0.05 vs vehicle-treated group. OVX, ovariectomy.
Table 2.
Effect of i.c.v. infusion of the MCH1 receptor antagonist on food intake, tissue weights and plasma blood parameters (Experiment 2)
| Sham |
OVX |
||
|---|---|---|---|
| Vehicle | Antagonist | ||
| Cumulative food intake (g) | 103.1±3.7 | 118.5±4.3# | 103.3±2.2** |
| Tissue weights | |||
| Mesenteric fat weight (g) | 0.34±0.04 | 0.57±0.05## | 0.24±0.03** |
| Liver weight (g) | 1.44±0.02 | 1.77±0.10# | 1.50±0.03* |
| Liver TG (mg g liver−1) | 12.0±1.0 | 15.1±1.1 | 10.3±0.8** |
| Blood parameters | |||
| Glucose (mM) | 9.0±0.2 | 10.1±0.4 | 9.9±0.5 |
| Insulin (ng ml−1) | 0.9±0.2 | 1.4±0.1# | 1.0±0.1** |
| Leptin (ng ml−1) | 7.4±1.0 | 16.7±2.0## | 5.6±0.7** |
| Total CHL (mM) | 1.4±0.1 | 1.7±0.0## | 1.6±0.0** |
| TG (mg l−1) | 487±44 | 486±41 | 411±42 |
| FFA (μeq l−1) | 394±20 | 444±26 | 380±23 |
Abbreviations: CHL, cholesterol; FFA, free fatty acid; i.c.v., intracerebroventricular; TG, triglyceride.
Starting 6 months after OVX, the MCH1 receptor antagonist was administered for the next 4 weeks. Data shown are means±s.e.
P<0.05, 0.01 vs sham-operated group.
P<0.05, 0.01 vs sham-operated group.
P<0.05, 0.01 vs vehicle-treated OVX group.
P<0.05, 0.01 vs vehicle-treated OVX group.
Tissue weights after i.c.v. infusion of MCH1 receptor antagonist
Removal of the ovaries increased the weight of mesenteric fat by 1.5- to 2-fold (Table 2). The 4-week infusion of the MCH1 receptor antagonist significantly decreased the mesenteric fat pad mass in the OVX mice. Similar changes in fat weight were observed in other fat depots including retroperitoneal fat and perimetric fat (data not shown). The OVX also resulted in an increase in liver weight and hepatic TG content, both of which were significantly reduced by the MCH1 receptor antagonist (Table 2).
Plasma biochemical parameters after i.c.v. infusion of MCH1 receptor antagonist
OVX significantly increased the plasma insulin and leptin levels by 1.5- to 2-fold compared with the levels in the sham-operated mice (Table 2). Administration of the MCH1 receptor antagonist significantly improved the hyperinsulinaemia and hyperleptinaemia observed in the OVX mice. OVX and MCH1 receptor antagonist did not affect the plasma glucose levels. OVX increased total cholesterol levels, and the MCH1 receptor antagonist ameliorated the hypercholesterolaemia. Plasma TG levels were not affected by either OVX or the antagonist. Plasma FFA levels tended to be increased by OVX and normalized by the MCH1 receptor antagonist.
Molecular profiling in liver after i.c.v. infusion of MCH1 receptor antagonist
The expression of SREBP1c, FAS and ACC1 mRNAs increased in the OVX group (Figure 5). These increases were clearly inhibited by the MCH1 receptor antagonist (Figure 5). Expression of SREBP1a and ACC2 were not changed by either OVX or the MCH1 receptor antagonist. Expression of other genes related to fatty acid oxidation, including PPARα, CPT1L and ACO, was not affected by OVX and the MCH1 receptor antagonist (data not shown).
Figure 5.
Expression of SREBP1c (a), FAS (b) and ACC1 (c) mRNAs in liver measured by the TaqMan system. Six months after OVX, the MCH1 receptor antagonist was infused (i.c.v) for 4 weeks. Data shown are means±s.e. *P<0.05 vs sham-operated group. #,##P<0.05, 0.01 vs vehicle-treated group. ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; i.c.v., intracerebroventricular; OVX, ovariectomy; SREBP1c, sterol regulatory element-binding protein.
Discussion
In the current study, we demonstrated that deficiencies in signalling via the MCH1 receptor ameliorated the OVX-induced obesity, that Mch1r KO mice gained significantly less weight than WT mice after OVX and that a selective MCH1 receptor antagonist effectively ameliorated OVX-induced obesity. In addition, we found that the MCH1 receptor deficiency had a beneficial effect on the liver. Withdrawal of oestrogen is reported to cause hepatic steatosis (Nemoto et al., 2000, 2002). In the current study, the OVX mice showed increased liver weight and lipid accumulation, while OVX-induced TG accumulation did not occur in OVX-Mch1r KO mice and the MCH1 receptor antagonist reversed the OVX-induced hepatic steatosis. However, female Mch1r KO mice were hyperactive before the OVX surgery (data not shown) as well as at the end point (Figure 3), which might have affected the difference in OVX-induced weight gain and hepatic TG accumulation between Mch1r KO and WT mice. On the other hand, the MCH1 receptor antagonist we used does not affect spontaneous locomotor activity (Mashiko et al., 2005). From these findings, it is suggested that signalling through MCH is an important factor in the development of fatty liver as well as development of obesity associated with oestrogen deficiency. In agreement with the reduction of hepatic TG accumulation, MCH1 receptor deficiency inhibited the OVX-induced increase in mRNA levels of lipogenesis-related enzymes. The OVX mice showed elevated mRNA expressions of FAS, ACC1 and SREBP1c, the last being a key regulator of fatty acid synthesis (Shimomura et al., 1999; Moon et al., 2001), and this elevation was ameliorated in the liver of Mch1r KO and MCH1 receptor antagonist-treated mice. These results suggest that MCH1 receptor deficiency inhibited the upregulation of hepatic lipid synthesis by OVX. This observation is in accordance with the fact that MCH stimulates fatty acid synthesis in the liver (Ito et al., 2003).
It is not clear whether the amelioration of fatty liver is a primary outcome of central MCH1 receptor blockade or is secondary to the antiobesity effect. The MCH1 receptor antagonist decreased plasma insulin levels in OVX mice. This may have caused reduction of liver TG contents, since insulin is reported to stimulate fatty acid synthesis via SREBP1c in the liver (Horton et al., 2002). The MCH1 receptor antagonist induced feeding suppression and weight loss, which can also affect the hepatic lipid accumulation. On the other hand, it was recently reported that the development of hepatic steatosis causes insulin resistance (Samuel et al., 2004). Thus, the amelioration of fatty liver is likely to contribute to the antiobesity effect of MCH1 receptor antagonism via amelioration of insulin resistance. In addition, direct connections are reported between hypothalamic centres and the liver via autonomic nerves (Uno et al., 2006), by which MCH1 receptors might affect lipid metabolism. Further examination might be necessary to elucidate the causal relationship between improvement of fatty liver and obesity, in addition to the mechanism as to how MCH and MCH1 receptor antagonists influence the liver.
The MCH1 receptor antagonist significantly suppressed spontaneous food intake in OVX mice and the Mch1r KO mice showed decreased food intake after OVX. Thus, it remains unclear how much the feeding suppression contributes to the changes in tissue weights and hepatic gene expressions. It is important to determine whether the effects of blocking MCH1 receptor signalling on parameters measured are due primarily to decreases in food intake. Pair-feeding with MCH1 receptor antagonists in OVX-induced obesity would be needed to clarify these points. In addition, the OVX-induced changes in body composition are reported to consist of two stages; the rapid weight gaining stage and the weight stabilized stage (McElroy and Wade, 1987). Actually, in this experiment, the most apparent change in body weight was seen within 5 weeks after OVX surgery. It is also reported that the OVX-induced hyperphagia is more apparent during the first several weeks and after that food intake returned to control levels (McElroy and Wade, 1987). Thus, the metabolic profiles in OVX animals may differ between the early stage soon after OVX and the late stage after homeostasis has been re-established. In this experiment, our aim was to examine an effect of long-term blockade of MCH1 receptor signalling, so we measured several metabolic parameters after the OVX-induced changes had been stabilized. It will be interesting and informative, as a next step, to compare the effect of MCH1 receptor antagonism in each stage. Moreover, OVX might result in the changes in levels of many endocrine and neuromodulatory substances such as NPY (Shimizu et al., 1996). Thus, it might be also interesting to compare the effect of MCH1 receptor antagonism with the effect of NPY antagonists in OVX-induced obesity.
Sham-operated female Mch1r KO mice were hyperactive and slightly hyperphagic compared to the WT mice, as reported previously (Marsh et al., 2002). OVX significantly decreased locomotor activity in Mch1r KO mice, but not in WT mice. Similarly, OVX significantly decreased food intake in Mch1r KO mice. The hyperphagic phenotype of Mch1r KO mice compared to the WT mice might be due to compensatory response to hyperactivity (Chen et al., 2002). A possible explanation is that OVX suppressed hyperactivity, and then food intake was normalized in Mch1r KO mice. Alternatively, a decrease in motor activity and food intake in OVX-Mch1r KO mice might be via changes in dopaminergic activity. Dopamine influences feeding and reward as well as motor activity (Nestler and Carlezon, 2006). It is reported that OVX lowers striatal dopamine levels (Ohtani et al., 2001) and that locomotor responses induced by methamphetamine are significantly decreased in OVX rodents (Yu and Liao, 2000; Ohtani et al., 2001; Mickley and Dluzen, 2004). Since Mch1r KO mice have mesolimbic dopamine super-sensitivity (Smith et al., 2005), they might be more sensitive to the influence of dopaminergic changes induced by OVX.
Herein, we have shown that blockade of signalling via the MCH1 receptor ameliorated OVX-induced obesity and hepatic lipid accumulation. This is in agreement with our previous report showing that the MCH1 receptor antagonist blocked DIO (Mashiko et al., 2005). This suggests that the MCH system has a significant role in pathogenesis of obesity. Together with the anticipated beneficial effects on the liver, MCH1 receptor antagonists could be useful agents for treatment of human obesity and the related metabolic diseases.
Acknowledgments
We thank K Watanabe, R Moriya, M Yumoto, R Yoshimoto, T Iguchi, S Ito, Y Takahashi, K Koga and T Umeda (Banyu Pharmaceutical Co. Ltd) for technical assistance.
Abbreviations
- ACC
acetyl-CoA carboxylase
- ACO
acyl-CoA oxidase
- CPT1L
liver type carnitine palmitoyltransferase I
- DIO
diet-induced obesity
- FAS
fatty acid synthase
- FFA
free fatty acid
- KO
knock out
- MCH
melanin-concentrating hormone
- NPY
neuropeptide Y
- OVX
ovariectomy
- PG
propylene glycol
- PPARα
peroxisome proliferator-activated receptor α
- SREBP
sterol regulatory element-binding protein
- TG
triglyceride
- WT
wild type
Conflict of interest
The authors state no conflict of interest.
References
- Abbott CR, Kennedy AR, Wren AM, Rossi M, Murphy KG, Seal LJ, et al. Identification of hypothalamic nuclei involved in the orexigenic effect of melanin-concentrating hormone. Endocrinology. 2003;144:3943–3949. doi: 10.1210/en.2003-0149. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, Hreniuk DL, Tan C, Palyha OC, MacNeil DJ, Van Der Ploeg LH, et al. Synthesis and biological evaluation in vitro of selective, high affinity peptide antagonists of human melanin-concentrating hormone action at human melanin-concentrating hormone receptor 1. Biochemistry. 2002;41:6383–6390. doi: 10.1021/bi0200514. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Cytoplasmic estrogen receptors in rat brain: immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Morris MJ. The feeding response to melanin-concentrating hormone is attenuated by antagonism of the NPY Y(1)-receptor in the rat. Endocrinology. 2002;143:191–197. doi: 10.1210/endo.143.1.8569. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hu C, Hsu CK, Zhang Q, Bi C, Asnicar M, et al. Targeted disruption of the melanin-concentrating hormone receptor-1 results in hyperphagia and resistance to diet-induced obesity. Endocrinology. 2002;143:2469–2477. doi: 10.1210/endo.143.7.8903. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gomori A, Ishihara A, Ito M, Mashiko S, Matsushita H, Yumoto M, et al. Chronic intracerebroventricular infusion of MCH causes obesity in mice. Melanin-concentrating hormone. Am J Physiol Endocrinol Metab. 2003;284:E583–E588. doi: 10.1152/ajpendo.00350.2002. [DOI] [PubMed] [Google Scholar]
- Hanada R, Nakazato M, Matsukura S, Murakami N, Yoshimatsu H, Sakata T. Differential regulation of melanin-concentrating hormone and orexin genes in the agouti-related protein/melanocortin-4 receptor system. Biochem Biophys Res Commun. 2000;268:88–91. doi: 10.1006/bbrc.1999.2081. [DOI] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Gomori A, Ishihara A, Oda Z, Mashiko S, Matsushita H, et al. Characterization of MCH-mediated obesity in mice. Am J Physiol Endocrinol Metab. 2003;284:E940–E945. doi: 10.1152/ajpendo.00529.2002. [DOI] [PubMed] [Google Scholar]
- Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature. 1983;305:321–323. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- Kela J, Salmi P, Rimondini-Giorgini R, Heilig M, Wahlestedt C. Behavioural analysis of melanin-concentrating hormone in rats: evidence for orexigenic and anxiolytic properties. Regul Pept. 2003;114:109–114. doi: 10.1016/s0167-0115(03)00114-9. [DOI] [PubMed] [Google Scholar]
- Landau IT, Zucker I. Estrogenic regulation of body weight in the female rat. Horm Behav. 1976;7:29–39. doi: 10.1016/0018-506x(76)90002-7. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Mountjoy KG, Tatro JB, Gillette JA, Frenderich RC, Flier JS, et al. Melanin-concentrating hormone: a functional melanocortin antagonist in the hypothalamus. Am J Physiol. 1998;274:E627–E633. doi: 10.1152/ajpendo.1998.274.4.E627. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, et al. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci USA. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiko S, Ishihara A, Gomori A, Moriya R, Ito M, Iwaasa H, et al. Antiobesity effect of a melanin-concentrating hormone 1 receptor antagonist in diet-induced obese mice. Endocrinology. 2005;146:3080–3086. doi: 10.1210/en.2004-1150. [DOI] [PubMed] [Google Scholar]
- Mcelroy JF, Wade GN. Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol Behav. 1987;39:361–365. doi: 10.1016/0031-9384(87)90235-6. [DOI] [PubMed] [Google Scholar]
- Messina MM, Boersma G, Overton JM, Eckel LA. Estradiol decreases the orexigenic effect of melanin-concentrating hormone in ovariectomized rats. Physiol Behav. 2006;88:523–528. doi: 10.1016/j.physbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Mickley KR, Dluzen DE. Dose-response effects of estrogen and tamoxifen upon methamphetamine-induced behavioral responses and neurotoxicity of the nigrostriatal dopaminergic system in female mice. Neuroendocrinology. 2004;79:305–316. doi: 10.1159/000079710. [DOI] [PubMed] [Google Scholar]
- Minami T, Oomura Y, Nabekura J, Fukuda A. 17 Beta-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519:301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47:294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- Moon YA, Shah NA, Mohapatra S, Warrington JA, Horton JD. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J Biol Chem. 2001;276:45358–45366. doi: 10.1074/jbc.M108413200. [DOI] [PubMed] [Google Scholar]
- Mori T, Baba J, Ichimaru Y, Suzuki T. Effects of rolipram, a selective inhibitor of phosphodiesterase 4, on hyperlocomotion induced by several abused drugs in mice. Jpn J Pharmacol. 2000;83:113–118. doi: 10.1254/jjp.83.113. [DOI] [PubMed] [Google Scholar]
- Murray JF, Baker BI, Levy A, Wilson CA. The influence of gonadal steroids on pre-pro melanin-concentrating hormone mRNA in female rats. J Neuroendocrinol. 2000;12:53–59. doi: 10.1046/j.1365-2826.2000.00425.x. [DOI] [PubMed] [Google Scholar]
- Mystkowski P, Seeley RJ, Hahn TM, Baskin DG, Havel PJ, Matsumoto AM, et al. Hypothalamic melanin-concentrating hormone and estrogen-induced weight loss. J Neurosci. 2000;20:8637–8642. doi: 10.1523/JNEUROSCI.20-22-08637.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahon JL. The melanin-concentrating hormone: from the peptide to the gene. Crit Rev Neurobiol. 1994;8:221–262. [PubMed] [Google Scholar]
- Narita M, Mizuno K, Shibasaki M, Narita M, Suzuki T. Up-regulation of the G(q/11alpha) protein and protein kinase C during the development of sensitization to morphine-induced hyperlocomotion. Neuroscience. 2002;111:127–132. doi: 10.1016/s0306-4522(01)00515-2. [DOI] [PubMed] [Google Scholar]
- Nemoto Y, Saibara T, Ogawa Y, Zhang T, Xu N, Ono M, et al. Tamoxifen-induced nonalcoholic steatohepatitis in breast cancer patients treated with adjuvant tamoxifen. Intern Med. 2002;41:345–350. doi: 10.2169/internalmedicine.41.345. [DOI] [PubMed] [Google Scholar]
- Nemoto Y, Toda K, Ono M, Fujikawa-Adachi K, Saibara T, Onishi S, et al. Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice. J Clin Invest. 2000;105:1819–1825. doi: 10.1172/JCI9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Niiya T, Osawa H, Onuma H, Suzuki Y, Taira M, Yamada K, et al. Activation of mouse phosphodiesterase 3B gene promoter by adipocyte differentiation in 3T3-L1 cells. FEBS Lett. 2001;505:136–140. doi: 10.1016/s0014-5793(01)02807-1. [DOI] [PubMed] [Google Scholar]
- Ohtani H, Nomoto M, Douchi T. Chronic estrogen treatment replaces striatal dopaminergic function in ovariectomized rats. Brain Res. 2001;900:163–168. doi: 10.1016/s0006-8993(01)02276-4. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Rossi M, Beak SA, Choi SJ, Small CJ, Morgan DG, Ghatei MA, et al. Investigation of the feeding effects of melanin concentrating hormone on food intake – action independent of galanin and the melanocortin receptors. Brain Res. 1999;846:164–170. doi: 10.1016/s0006-8993(99)02005-3. [DOI] [PubMed] [Google Scholar]
- Rossi M, Choi SJ, O'shea D, Miyoshi T, Ghatei MA, Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138:351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- Rovere C, Viale A, Nahon J, Kitabgi P. Impaired processing of brain proneurotensin and promelanin-concentrating hormone in obese fat/fat mice. Endocrinology. 1996;137:2954–2958. doi: 10.1210/endo.137.7.8770919. [DOI] [PubMed] [Google Scholar]
- Sailer AW, Sano H, Zeng Z, Mcdonald TP, Pan J, Pong SS, et al. Identification and characterization of a second melanin-concentrating hormone receptor, MCH-2R. Proc Natl Acad Sci USA. 2001;98:7564–7569. doi: 10.1073/pnas.121170598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Qu X, Elder BD, BilzI S, Befroy D, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Camacho RE, Sloan SD, Zhou D, Bednarek MA, Hreniuk DL, et al. Chronic MCH-1 receptor modulation alters appetite, body weight and adiposity in rats. Eur J Pharmacol. 2003;475:37–47. doi: 10.1016/s0014-2999(03)02146-0. [DOI] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Ohtani K, Kato Y, Tanaka Y, Mori M. Withdrawal of [corrected] estrogen increases hypothalamic neuropeptide Y (NPY) mRNA expression in ovariectomized obese rat. Neurosci Lett. 1996;204:81–84. doi: 10.1016/0304-3940(96)12322-3. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Shimizu H, Takahashi M, Sato N, Uehara Y, Fukatsu A, et al. The significance of decreased ambulatory activity during the generation by long-term observation of obesity in ovariectomized rats. Physiol Behav. 1990;47:155–159. doi: 10.1016/0031-9384(90)90055-9. [DOI] [PubMed] [Google Scholar]
- Smith DG, Tzavara ET, Shaw J, Luecke S, Wade M, Davis R, et al. Mesolimbic dopamine super-sensitivity in melanin-concentrating hormone-1 receptor-deficient mice. J Neurosci. 2005;25:914–922. doi: 10.1523/JNEUROSCI.4079-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritos NA, Mastaitis JW, Kokkotou E, Maratos-Flier E. Characterization of melanin concentrating hormone and preproorexin expression in the murine hypothalamus. Brain Res. 2001;895:160–166. doi: 10.1016/s0006-8993(01)02066-2. [DOI] [PubMed] [Google Scholar]
- Tritos NA, Vicent D, Gillette J, Ludwig DS, Flier ES, Maratos-Flier E. Functional interactions between melanin-concentrating hormone, neuropeptide Y, and anorectic neuropeptides in the rat hypothalamus. Diabetes. 1998;47:1687–1692. doi: 10.2337/diabetes.47.11.1687. [DOI] [PubMed] [Google Scholar]
- Uno K, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Imai J, et al. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science. 2006;312:1656–1659. doi: 10.1126/science.1126010. [DOI] [PubMed] [Google Scholar]
- Wong GT. Speed congenics: applications for transgenic and knock-out mouse strains. Neuropeptides. 2002;36:230–236. doi: 10.1054/npep.2002.0905. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Yoshida T, Wakabayashi Y, Nishioka H, Kondo M. Reduced brown adipose tissue thermogenesis of obese rats after ovariectomy. Endocrinol Jpn. 1988;35:537–543. doi: 10.1507/endocrj1954.35.537. [DOI] [PubMed] [Google Scholar]
- Yu L, Liao PC. Estrogen and progesterone distinctively modulate methamphetamine-induced dopamine and serotonin depletions in C57BL/6J mice. J Neural Transm. 2000;107:1139–1147. doi: 10.1007/s007020070027. [DOI] [PubMed] [Google Scholar]