Abstract
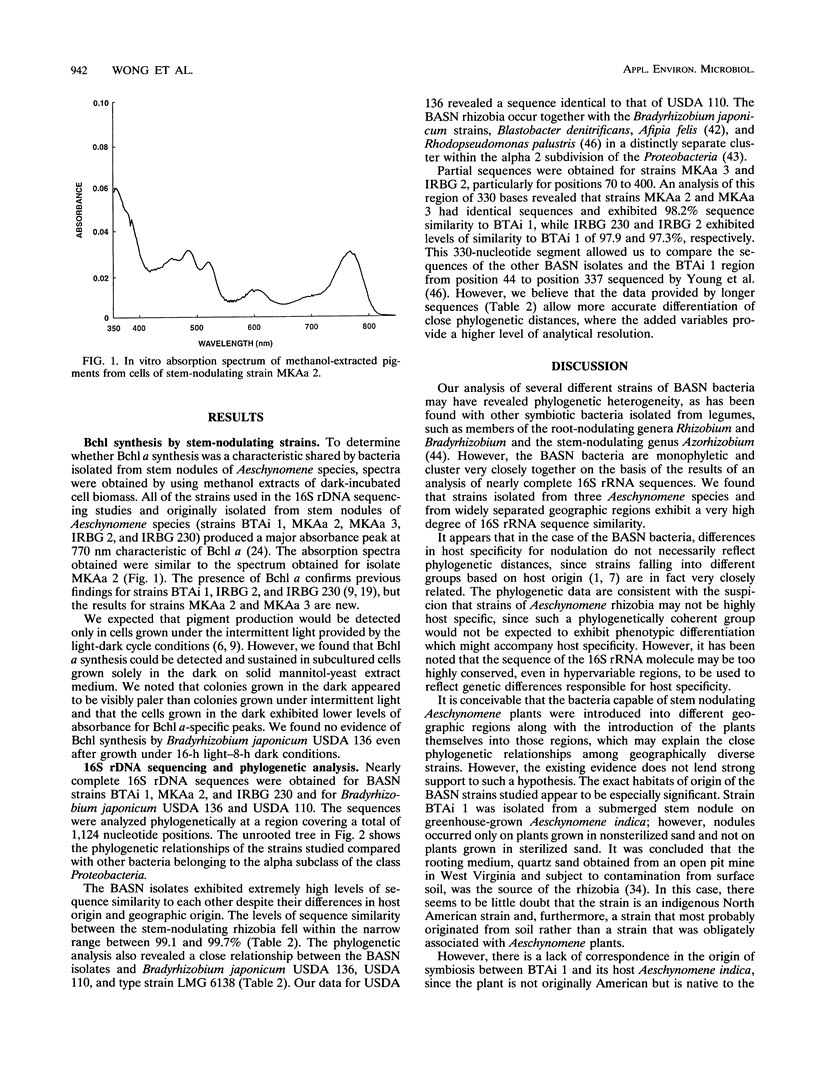
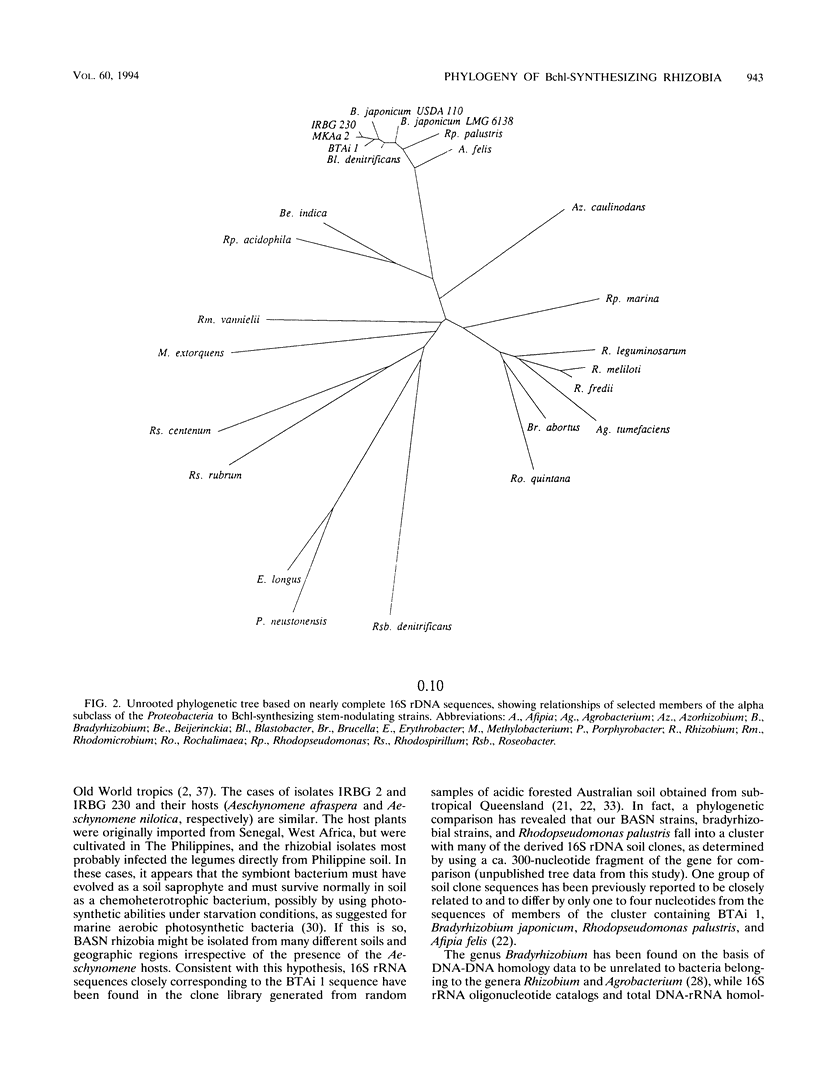
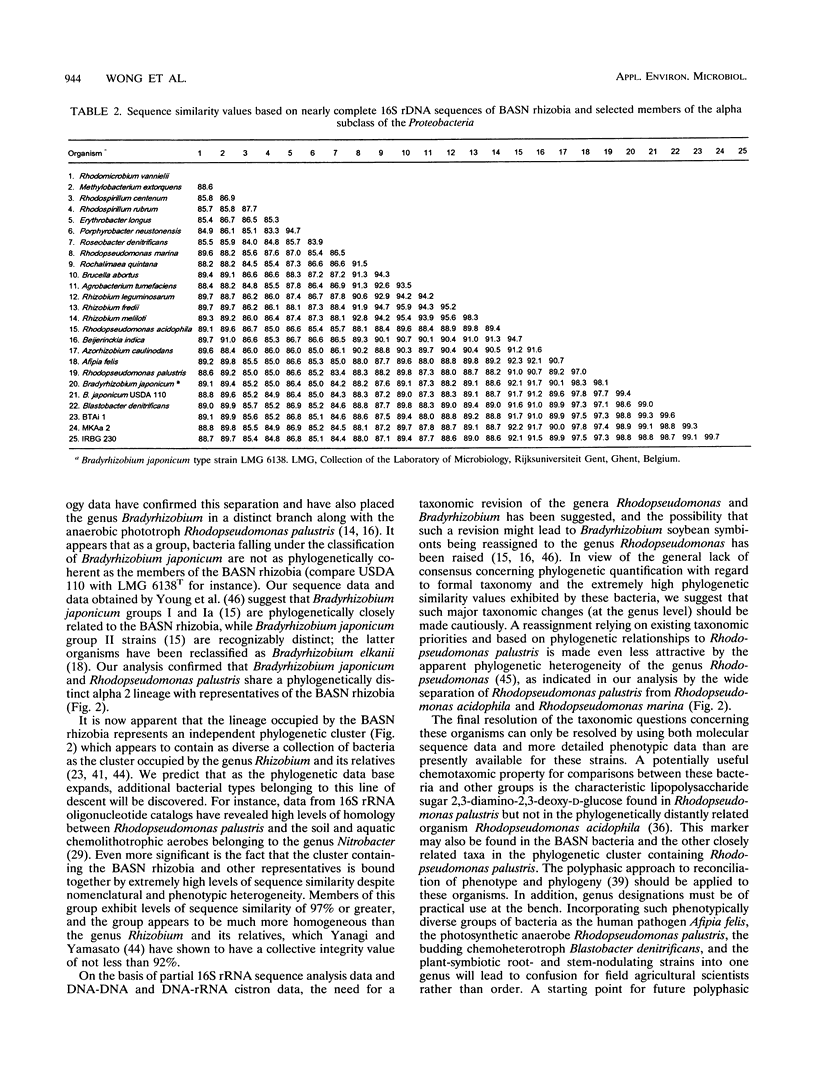
Nearly complete and short partial 16S rRNA sequences were derived from PCR-amplified ribosomal DNAs of Bradyrhizobium japonicum USDA 136 and USDA 110 and five strains of bacteriochlorophyll-synthesizing bacteria isolated from stem nodules of Aeschynomene indica and other Aeschynomene species growing in different geographic regions, including India, The Philippines and North America. We confirmed that the five stem-nodulating strains examined synthesize bacteriochlorophyll a, and the absorption spectra of methanol-extracted cells contained a major absorbance peak at 770 nm. Strains isolated on different continents and from different Aeschynomene species were found to be phylogenetically homogeneous and exhibited levels of sequence similarity of more than 99%. The bacteriochlorophyll-synthesizing rhizobia, Bradyrhizobium japonicum, Blastobacter denitrificans, Afipia felis, and Rhodopseudomonas palustris exhibited levels of sequence similarity of 97% or greater and belong to a distinct line of descent within the alpha-2 subdivision of the Proteobacteria. Variable regions between positions 995 and 1045 provide potential target sites for design of a probe that is able to distinguish the photosynthetic rhizobia from closely related taxa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alazard D. Stem and Root Nodulation in Aeschynomene spp. Appl Environ Microbiol. 1985 Sep;50(3):732–734. doi: 10.1128/aem.50.3.732-734.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. L., Pannetier C., Jaulin C., Kourilsky P. Optimal conditions for directly sequencing double-stranded PCR products with sequenase. Nucleic Acids Res. 1990 Jul 11;18(13):4028–4028. doi: 10.1093/nar/18.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. R., Fleischman D. E., Calvert H. E., Pyati P. V., Alter G. M., Rao N. S. Bacteriochlorophyll and Photosynthetic Reaction Centers in Rhizobium Strain BTAi 1. Appl Environ Microbiol. 1990 Nov;56(11):3445–3449. doi: 10.1128/aem.56.11.3445-3449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- Liesack W., Stackebrandt E. Occurrence of novel groups of the domain Bacteria as revealed by analysis of genetic material isolated from an Australian terrestrial environment. J Bacteriol. 1992 Aug;174(15):5072–5078. doi: 10.1128/jb.174.15.5072-5078.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Stackebrandt E., Dorsch M., Wolters J., Busch M., Mayer H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J Bacteriol. 1990 Jul;172(7):3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Shiba T., Shioi Y., Takamiya K., Sutton D. C., Wilkinson C. R. Distribution and physiology of aerobic bacteria containing bacteriochlorophyll a on the East and west coasts of australia. Appl Environ Microbiol. 1991 Jan;57(1):295–300. doi: 10.1128/aem.57.1.295-300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackebrandt E., Liesack W., Goebel B. M. Bacterial diversity in a soil sample from a subtropical Australian environment as determined by 16S rDNA analysis. FASEB J. 1993 Jan;7(1):232–236. doi: 10.1096/fasebj.7.1.8422969. [DOI] [PubMed] [Google Scholar]
- Tautz D., Renz M. An optimized freeze-squeeze method for the recovery of DNA fragments from agarose gels. Anal Biochem. 1983 Jul 1;132(1):14–19. doi: 10.1016/0003-2697(83)90419-0. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Hatch T. P., Woese C. R. Eubacterial origin of chlamydiae. J Bacteriol. 1986 Aug;167(2):570–574. doi: 10.1128/jb.167.2.570-574.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Woese C. R., Dobson M. E., Weiss E. A common origin of rickettsiae and certain plant pathogens. Science. 1985 Nov 1;230(4725):556–558. doi: 10.1126/science.3931222. [DOI] [PubMed] [Google Scholar]
- Willems A., Collins M. D. Evidence for a close genealogical relationship between Afipia (the causal organism of cat scratch disease), Bradyrhizobium japonicum and Blastobacter denitrificans. FEMS Microbiol Lett. 1992 Sep 15;75(2-3):241–246. doi: 10.1016/0378-1097(92)90411-g. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi M., Yamasato K. Phylogenetic analysis of the family Rhizobiaceae and related bacteria by sequencing of 16S rRNA gene using PCR and DNA sequencer. FEMS Microbiol Lett. 1993 Feb 15;107(1):115–120. doi: 10.1111/j.1574-6968.1993.tb06014.x. [DOI] [PubMed] [Google Scholar]
- Young J. P., Downer H. L., Eardly B. D. Phylogeny of the phototrophic rhizobium strain BTAi1 by polymerase chain reaction-based sequencing of a 16S rRNA gene segment. J Bacteriol. 1991 Apr;173(7):2271–2277. doi: 10.1128/jb.173.7.2271-2277.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



