Abstract
Aims
To examine the impact on heart rate variability (HRV), of agonism or antagonism at the cardiac β2-adrenoceptor in healthy volunteers, using standard time-domain summary statistics and non-linear methods (scatterplot and quadrant analysis).
Methods
Under double-blind and randomised conditions (Latin square design), 17 normal volunteers received placebo, salbutamol (β2-adrenoceptor partial agonist), ICI 118,551 (specific β2-adrenoceptor antagonist), or salbutamol plus ICI 118,551. Single oral doses of medication (at weekly intervals) were administered at 22.30 h, with HRV assessed from the sleeping heart rates.
Results
Salbutamol reduced the long-term (SDNN: 135 ms [120, 156], SDANN: 107 ms [89, 124]) time-domain indicators of HRV compared with placebo (SDNN: 39 [24, 55], SDANN 42 [29, 56], {mean difference [95% confidence intervals of difference]}). Alone, ICI 118,551 did not effect HRV, but in combination blocked the actions of salbutamol. Scatterplot length (944 ms [869, 1019]) and area (222*103 ms2 [191, 253]) were reduced by salbutamol compared with placebo; (length difference (164 [98, 230]) and area difference 59 [36, 83]). Scatterplot width (dispersion) was lower at both low (width RR-1 25% salbutamol 277 ms [261, 293]: salbutamol minus placebo 14 ms [0, 28]) and high (width 75% salbutamol 417 [391, 443]: salbutamol minus placebo 41 [20, 62]) heart rates. ICI 118,551 alone did not alter scatterplot parameters but in combination blocked the effect of salbutamol. Cardiac acceleration episodes (i.e. consecutive ΔRR and ΔRRn+1 shorten) were increased following salbutamol 7288 [6089, 8486] compared with placebo −1890 [−2600, −1179]; the beat-to beat difference (ΔRRn+1) was reduced after salbutamol compared with the other treatments. ICI 118,551 did not effect acceleration episodes but reduced the effect of salbutamol when used in combination.
Conclusions
Agonism at the cardiac β2-adrenoceptor in healthy volunteers with salbutamol altered autonomic balance towards sympathetic dominance; this re-balancing was blocked by ICI 118,551 given in combination with salbutamol. However antagonism at the β2-adrenoceptor with ICI 118,551 alone did not significantly alter the HRV. The β2-adrenoceptor modulates HRV in healthy volunteers; the implications of agonism and antagonism at the β2-adrenoceptor in cardiovascular disease states warrants further investigation.
Keywords: heart rate variability, scatterplot, non-linear, β-adrenoceptor, partial agonist
Introduction
Heart-rate variability (HRV) is a powerful predictor of outcome in established cardiovascular disease [1–3]; disturbed autonomic balance contributes to the impaired prognosis [4, 5]. Drugs that oppose cardiac sympathetic stimulation, such as the β-adrenoceptor antagonists, have improved patient survival following myocardial infarction [6, 7]. It is unclear whether secondary prevention benefits result from a re-balancing of the autonomic nervous system; however HRV has been augmented following β-adrenoceptor antagonism in cardiovascular disease states [8, 9].
The relationship between the ancillary pharmacological properties of a β-adrenoceptor drug [10] and HRV is a neglected area. We have demonstrated that the partial β-adrenoceptor agonist celiprolol reduced time-domain and non-linear measures of heart rate variability; a component of the reduced variability appeared mediated through the β2-adrenoceptor [11]. We therefore sought to extend these observations on HRV effects of agonism and antagonism at the β2-adrenoceptor in normal volunteers under randomised, double-blind and placebo controlled conditions. The actions of salbutamol (a β2-adrenoceptor partial agonist), ICI 118,551 (a specific β2-adrenoceptor antagonist) and the combination of salbutamol plus ICI 118,551 have been studied.
HRV may be assessed by different methods; these have been critically evaluated [12]. The time-domain summary statistics provide information on both short and long-term heart rate variations; however, the pattern of these fluctuations is not evident. Spectral methods provide a time-averaged estimate of cyclic variability and are most useful in the assessment of sinusoidal or near-sinusoidal variations in HR [13, 14]. It has been suggested that non-linear phenomena are involved in the genesis of HRV (i.e. the heart rate shows non-linear complexity or ‘chaotic’ behaviour [15]); analyses based on non-linear dynamics may prove superior and allow more precise outcome prediction [16].
We have therefore, in addition to the standard time-domain statistics, used the Poincaré plot and quadrant methods to determine HRV with non-linear techniques in this study. The Poincaré plot is a ‘scatterplot’ (return map) of current cycle is plotted against the previous beat (RR vs RRn−1); scatterplot length is correlated with long-term (SDNN and SDANN) time domain statistics [17] while scatterplot width appears a specific measure of parasympathetic nervous system activity [17, 18]. Quadrant analysis is another non-linear method proposed to investigate the nature of cardiac interval sequencing [13]; here the interval difference between two consecutive beats and the next pair is evaluated (ΔRR vsΔRRn+1). Quadrant analysis removes the dominant characteristics apparent in the Poincaré plot, namely the high correlation between one interval and the next.
Methods
Seventeen healthy male volunteers (age 30±3 years; weight 71±10 kg) were studied. Overnight tape records were retrieved from archive from two placebo-controlled, double-blind, balanced (Latin square design) studies [19, 20]. The original objective of these studies was to evaluate the properties of some β-adrenoceptors, using the sleeping hourly heart rate as one of the assessment parameters. The effects of agonists of the β-adrenoceptor can most readily be assessed at night, due to the low level of prevailing sympathetic drive and cathecholamine levels. The nocturnal parasympathetic dominance results in a progressive fall in the heart rate that reaches a nadir approximately 6 h after onset of sleep; sleeping heart rate is further reduced by β-adrenoceptor antagonists and this contrasts with the effects of partial agonists of the β-adrenoceptor that increase or prevent the normal fall in the sleeping heart rate [21]. When the studies were originally undertaken, it was considered that the limitations imposed by the available technology, precluded detailed HRV analyses.
Among the treatments each volunteer had received (with an interval of at least 1 week), was single oral doses of placebo, salbutamol 8 mg, ICI 118,551 25 mg, or salbutamol 8 mg plus ICI 118,551 25 mg. Medication was administered between 22.15 and 22.45 h. Salbutamol behaves either as a full or partial agonist at the β2-adrenoceptor [22]; it is 107 times more selective for the β2 compared with the β1-adrenoceptor (comparison of EC50 values for the relative effects of an agonist on β1 and β2 mediated responses) [23]. ICI 118,551 possessed a high degree of selectivity and specificity for the β2-adrenoceptor; in comparison with propranolol (β2/β1 selectivity ratio 2.2), ICI 118,551 had a ratio of 123; it was devoid of partial agonist activity [24]. Thus it was a useful drug for examining the role of the β2-adrenoceptor in physiological control mechanisms and in disease states. ICI 118,551 at doses above 50 mg (particularly with chronic dosing) had demonstrable effects on β1-adrenoceptors; however at 20 mg there were no, or minimal effects on dobutamine induced positive inotropic responses [25]. Doses of ICI 118,551 of 20 mg orally had minimal effects on peak exercise heart rate or on the BP response to infused isoprenaline in normal volunteers [20], further evidence of its specificity for the β2-adrenoceptor. ICI 118,551 25 mg had no effect on the sleeping heart rate [19].
Heart rate methods
The HR was recorded (modified lead II position) using an Oxford Medilog 2–24 minature analogue tape recorder, with a phase-locked loop motor speed control to ensure speed stability. The recording speed was 2 mm s−1. The Holter tape records were analysed using a neural network classification (Biomedical Systems Colortrace™—St Louis, MO, 63146, USA). HRV was assessed from RR interval files generated from 22.00 to 08.00 h, analysed in hourly epochs. Aberrant data values were excluded; further filtering used a validated operator independent method for establishing HRV from continuous extended ECG recordings [26]. This method accepted only consecutive beats of normal morphological characteristics (N-N) with cycle lengths within 17.5% of the preceding cycle length.
Time-domain summary statistics
The summary time-domain statistical measures of HRV calculated included the standard deviation of all NN intervals (SDNN), the standard deviation of the averages of the NN intervals in all 5 min segments of the recording (SDANN), the mean of the standard deviations of all NN intervals for all 5 min segments of the recording (SDNN index), the square root of the mean of the sum of the squares of differences between adjacent NN intervals (rmsSD) and the number of pairs of adjacent NN counts differing by more than 50 ms divided by the total number of all NN intervals (pNN50). These measures have been summarised [12]; SDNN reflects all cyclic components responsible for variability in the recording, SDANN and SDNN index represent variability over cycles longer and shorter than 5 min respectively while rmsSD and pNN50, derived from interval differences, are short-term HRV measures estimating high frequency variations in heart rate.
Scatterplot methods (Figure 1)
Figure 1.
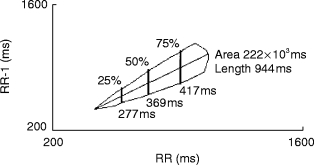
The length of the Poincaré plot (scatterplot) was operator defined. The area was calculated by performing width measurements (10 msec interval) with subsequent rectangular integration. Width measurements were standardised at 10%, 25%, 50%, 75% and 90% of the scatterplot length.
Scatterplots were constructed by plotting each RR interval against the preceding RR interval, as previously described [17]. Unlike previous descriptions of the method, where dedicated commercial scanner software produced hard copy of the scatterplots requiring manual measurements, we developed an automated computer method. Scatterplots were initially produced using the charting feature of SPSS (Release 6.1) [27], with its ability to handle large data-sets, and then stored in bitmap format (Figure 1). A digitising programme (TechDig V2.0a; Ron Jones (ronjones@xnet.com) Mundelein, IL) was customised, to permit automated technician analysis of the Poincaré plot. Initially the bounds of the X and Y axis were defined (reference points and the numeric values for the start and end of each axis entered), using a mouse and fine cross-hairs. The limits of the scatterplot length were defined by clicking on the extremes of the scatterplot diagonal. The computer calculated the scatterplot length (between the two calibration points), width (at 10 ms intervals) and area; these results were available through the Windows™ clipboard to be pasted into other spreadsheet or data management programmes for statistical processing. The key to the width measurements was a test to determine if a given pixel fell inside or outside the boundaries of the scatterplot. The test counted the coloured pixels in the plot in a 9×21 pixel window centred at the pixel of interest. If more than 20 pixels in this window were coloured, the pixel was considered to be within the scatterplot. The width measurement was made at a given RR interval by performing this test at each pixel along the line perpendicular to the RR axis at the interval of interest. The width was defined by the difference between the first pixel in the scatterplot and the next pixel not in the scatterplot. The area was calculated by performing width measurements at 10 ms intervals and subsequent rectangular integration. Width measurements were also automatically performed at 10%, 25%, 50%, 75% and 90% of the scatterplot length as previously described [28]; the average width of the scatterplot was the measure of variance [17] rather than calculating the variance around the perpendicular to the line of identity (i.e. scatterplot length) [18]. The reproducibility of the method was determined in 12 volunteers, with repeat analysis of a scatterplot on 5 consecutive occasions; the reproducibility for the length, area and width was calculated. The average coefficient of variation (range) was for length 1.0% (0.3–1.8), area 1.3% (0.4–3.1), P10% 3.6% (0–8.9), P25% 3.3% (0–9.0), P50% 1.4% (0–3.3), P 75% 1.3% (0.2–3.8) and P90% 2.0% (0.5–4.9).
Cardiac sequence (quadrant) analysis
The non-linear cardiac sequencing or quadrant method is as previously described [13]. Consecutive ΔRR and ΔRRn+1 differences are compared (i.e. three consecutive RR values; two differences RR1–RR2 (ΔRR) and RR2–RR3: (ΔRRn+1)). This method removes the dominant characteristic apparent in comparing each beat with the next—namely the high correlation between one interval and the next. Four patterns can be identified; +/+ (a lengthening sequence—cardiac deceleration), +/− or −/+ (balanced sequences), and finally −/− (a shortening sequence—cardiac acceleration). Events of each type were counted by quadrant; in addition the average duration (i.e. of the second difference ΔRRn+1) of the difference within each quadrant was calculated. When the ANS is balanced, one would expect the quadrant b/c patterns to predominate (+/− or −/+) in contrast to parasympathetic (quadrant a; +/+) or sympathetic dominance (quadrant d; −/−).
Statistical methods
Tabular summary statistics are presented as mean [95% confidence intervals]. There is some uncertainty about the linear nature of the scaling of HRV parameters, for this reason the overall significance for each variable was calculated using non-parametric two way analysis of variance (Friedman’s procedure); where significance was indicated, the data-sets were then examined using the Wilcoxon sign test to determine the between group P values [29]. Differences between groups were considered significant at the P < 0.01 level.
Results
Effects on heart rate (Table 1: Figure 2)
Table 1.
Mean values [95% confidence intervals] for HRV variables.
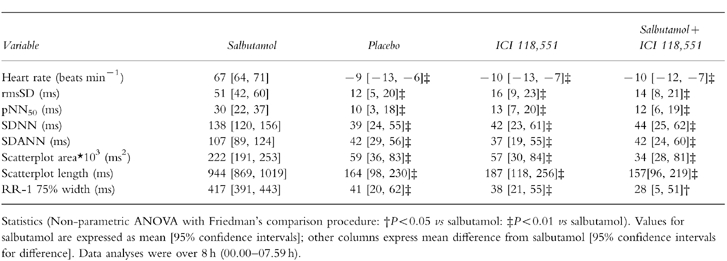
Figure 2.
Effects on heart rate (over 8 h) following placebo (▴), salbutamol 8 mg (□) ICI 118,551 25 mg (◊) and salbutamol 8 mg plus ICI 118,551 25 mg (•). Statistics related to between group comparisons: non-parametric ANOVA followed by Wilcoxon’s rank sign test. ‡P < 0.01 vs salbutamol. Data are mean±95% confidence interval.
Over the course of the night the sleeping heart rate decreased significantly. Commencing 2 h post-dosing differences between the drugs were apparent; between 2 and 8 h the heart rate reduction following salbutamol 8 mg was significantly less than the comparable fall after placebo. ICI 118,551 alone and salbutamol plus ICI 118,551 were not different from placebo.
Time-domain methods (Table 1: Figure 3)
Figure 3.
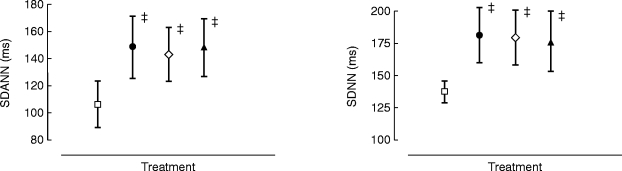
Effects on SDNN and SDANN (over 8 h) following placebo (▴), salbutamol 8 mg (□), ICI 118,551 25 mg (◊) and salbutamol 8 mg plus ICI 118,551 25 mg (•). Statistics related to between group comparisons: non-parametric ANOVA followed by Wilcoxon’s rank sign test. ‡P < 0.01 vs salbutamol. Data are mean±95% confidence interval.
There were significant between treatment differences for actions on the long (SDNN, SDANN) and short-term (rmsSD) HRV summary statistics. The long-term measurements SDNN and SDANN were significantly reduced (vs placebo) by salbutamol 8 mg, unaffected by ICI 118,551, and the reduction in HRV attributable to salbutamol was prevented when given in combination with ICI 118,551.
The short-term HRV parameters of rmsSD and pNN50 were significantly reduced (vs placebo) by salbutamol 8 mg; ICI 118,551 alone had no effect but when given in combination with salbutamol, it prevented the fall in these short-term HRV parameters.
Effects on scatterplot parameters (Table 1: Figures 4, 5)
Figure 4.
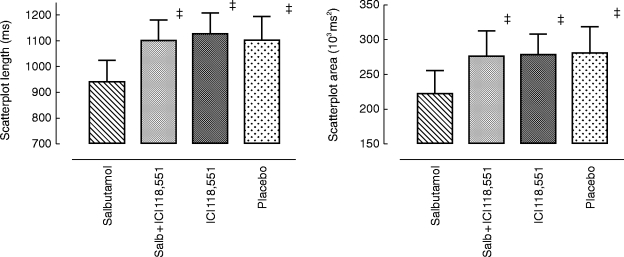
Effects on scatterplot length and area (over 8 h) following placebo, salbutamol 8 mg, ICI 118,551 25 mg and salbutamol 8 mg plus ICI 118,551 25 mg. Statistics related to between group comparisons: non-parametric ANOVA followed by Wilcoxon’s rank sign test. ‡P < 0.01 vs salbutamol. Data are mean±95% confidence interval.
Figure 5.
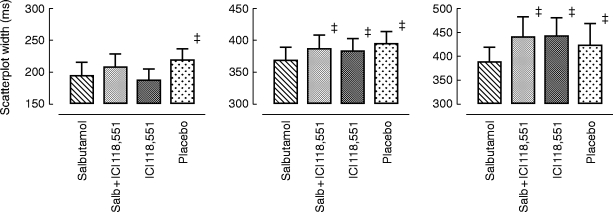
Effects on scatterplot width (over 8 h) at the 10th, 50th and 90th percentile following placebo, salbutamol 8 mg, ICI 118,551 25 mg and salbutamol 8 mg plus ICI 118,551 25 mg. Statistics related to between group comparisons: non-parametric ANOVA followed by Wilcoxon’s rank sign test. ‡P < 0.01 vs salbutamol. Data are mean±95% confidence interval.
A significant reduction in scatterplot length and area (Figure 4) followed salbutamol 8 mg compared with placebo, ICI 118,551 alone did not affect scatterplot length or area but in combination with salbutamol prevented the reduction in these scatterplot indices.
The geometric analysis of the scatterplots allowed assessment of HRV effects at fixed RR intervals. Differences between the drugs (Figure 5) were present over the entire scatterplot length. Salbutamol 8 mg significantly reduced the width of the scatterplot across the whole range (P10%, P25%, P50%, P75%, and P90%) compared with placebo. ICI 118,551 alone had no effect on scatterplot widths but prevented the actions of salbutamol when given in combination.
Quadrant analysis: effect of treatment on total counts (Figure 6)
Figure 6.
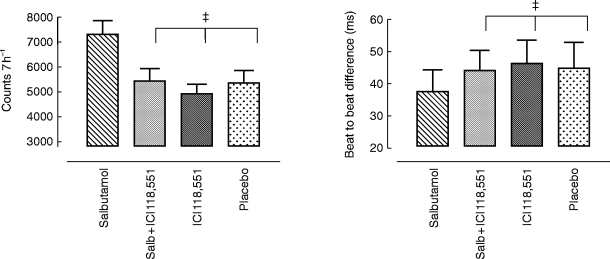
Effects on quadrant −/− (d: acceleration/acceleration) (over 7 h) counts and mean interval difference following placebo, salbutamol 8 mg, ICI 118,551 25 mg and salbutamol 8 mg plus ICI 118,551 25 mg. Statistics related to between group comparisons: non-parametric ANOVA followed by Wilcoxon’s rank sign test. ‡P < 0.01, all three vs salbutamol. Data are mean±95% confidence interval.
Analysis of each quadrant for total counts over 7 hr (00.00–06.59 h), considered in turn intervals of type +/+ (a), +/− (b), −/+ (c), or −/− (d); salbutamol shifted the autonomic balance towards sympathetic dominance. Cardiac acceleration episodes (i.e. no. of times ΔRR and ΔRRn+1 were both altered in the same direction) were increased in quadrant a and d following salbutamol. In quadrant d for example (Figure 6), the count frequency increased after salbutamol 8 mg compared with placebo, ICI 118,551 25 mg alone had no effect, but in combination blocked the shift attributable to salbutamol. The absolute magnitude of the sequence difference was also determined. Salbutamol 8 mg reduced the average duration of the beat-to-beat difference; for quadrant d for example, the bias towards cardiac acceleration was reflected in the shortened beat-to-beat difference (ΔRRn+1) compared with placebo, ICI 118,551 or ICI 118,551 plus salbutamol 8 mg (38 [31, 44] vs 44 [38, 52], 47 [40, 54] and 45 [37, 53] ms respectively; P < 0.01 for each comparison).
Discussion
Although many studies have considered the impact of the β-adrenoceptor group on autonomic balance and the regulation of HRV, the relative role and importance of the β-adrenoceptor subtypes in the modulation of HRV is not established. The interaction between receptor subtype and stimulation or blockade of the β-adrenoceptor has received limited consideration, although it may be relevant to secondary prevention goals after myocardial infarction [6, 7]. We specifically considered the relevance of agonism and antagonism at the β2-adrenoceptor. We felt that comparison of the β2 agonist effects of salbutamol and the antagonist effects of the highly β2-selective adrenoceptor antagonist ICI 118,551 on HRV would be instructive in this regard. Salbutamol has, depending on the model used, either partial or full agonist activity at the β2-adrenoceptor [22, 30]; its chronotropic actions are consistent with effects at the cardiac β2-adrenoceptor [31, 32]. On the other hand, ICI 118,551 possessed a high degree of selectivity and specificity for the β2-adrenoceptor; it caused a dose-dependent parallel shift to the right of the isoprenaline vasodilator curve without alteration of the corresponding isoprenaline chronotropic curve. Additionally ICI 118,551 possessed at least a 250 fold greater affinity for the vascular β2-adrenoceptor than the cardiac β1-adrenoceptor [24]. Thus it represented a useful drug for examining the role of the β2-adrenoceptor in physiological control mechanisms and in disease states. The dose of ICI 118,551 used in this study had no or minimal effects on the β1-adrenoceptor [20, 25] or on the sleeping heart rate [19].
Our analyses suggest a role for the β2-adrenoceptor in the modulation of HRV; the variability was reduced by the β2-adrenoceptor agonist salbutamol in contrast to β2-adrenoceptor antagonism with ICI 118,551, that was devoid of any such action. However when ICI 118,551 was administered together with salbutamol, the reductions in HRV parameters, induced by salbutamol alone, were abolished. Therefore stimulation of the β2-adrenoceptor increased HR and reduced HRV; however blockade of the β2-adrenoceptor did not by itself alter HR or HRV.
At apparent variance with these findings is the literature data, that have largely suggested improved HRV parameters following β-adrenoceptor blockade. However most studies have utilised β1-adrenoceptor antagonists; the implications for HRV of agonism at a receptor have received little emphasis. Cook et al. [33] demonstrated that atenolol in normal volunteers improved time and frequency HRV measures; measures of tonic vagal activity rmsSD, pNN50 and high frequency power increased by 61%, 69% and 84% respectively. Schweizer et al. [34], also in normal volunteers, found that metoprolol 100 mg significantly lowered power spectral density in the low frequency band (0.03 Hz to 0.15 Hz) and concluded that this agent inhibited cardiac sympathetic activation. Niemela et al. [35] reported that β-adrenoceptor blockade, with 200 mg controlled release metoprolol or atenolol 100 mg for 2 weeks in 18 patients with stable coronary disease, similarly increased heart rate variability; the average 24-h high frequency power (atenolol +64%; metoprolol +62%), the rmsSD (atenolol +70%; metoprolol +62%) and SDRR (atenolol +20%; metoprolol +16%) were increased. Sandrone et al. [9], with spectral techniques, demonstrated that atenolol or metoprolol reduced sympathetic activation and increased vagal tone in 20 patients, 4 weeks after the first uncomplicated myocardial infarction. Atenolol and metoprolol exerted more pronounced effects in the daytime compared with the night; there was also a marked attenuation in the circadian variation of the low-frequency component after each drug.
Hohnloser et al. [36] investigated HRV, assessed by time-and frequency-domain measures, after sotalol in 28 patients with chronic ventricular arrhythmia. Therapy with sotalol produced a significant improvement in indices of parasympathetic tone (SDNN, rmsSD, pNN50 and high-frequency power spectrum). Other studies in patients with congestive heart failure have shown that 2 months treatment with the β1-selective adrenoceptor antagonist, bisoprolol (5 mg day−1), have increased rMSSD, pNN50, SDNN and high frequency spectral power during the daytime [28]; metoprolol has also improved short-term time domain HRV variables in coronary disease [37, 38]. Taken together this substantial body of evidence suggests that antagonism at the β1-adrenoceptor increased time and frequency measures of HRV. These findings are consistent with a shift in autonomic balance, representing an enhanced parasympathetic and attenuated sympathetic state.
In contrast, Stein et al. [39] determined the effects of pindolol (5 mg twice daily) and demonstrated a reduced HRV in normal subjects. Pindolol (agonist activity predominately at the β2-adrenoceptor [40]) reduced the HR extremes and decreased SDNN, SDANN, ultra-low frequency and total spectral power. It was only with the reduced sympathetic drive at night that pindolol’s effects in reducing the time domain indices of HRV (SDNN, SDANN, rMSSD and pNN50) were apparent. Consistent with this finding, that agonist activity at the β2-adrenoceptor with pindolol reduced HRV, was that salbutamol, a β2-selective adrenoceptor agonist, shifted cardiovascular autonomic balance towards sympathetic dominance and decreased cardio-vagal nervous responsiveness [41]. Another selective β2-adrenoceptor agonist, terbutaline, also decreased the RR interval variability indices related to vagal tone; the parasympathetic nervous reactivity decreased [42]. Celiprolol (dose-dependent β2-adrenoceptor partial agonist [43]) reduced short and long-term time domain statistics together with scatterplot length, area and width [11]. In addition sequence analysis demonstrated an increased frequency of acceleration episodes, in accord with the hypothesis of a shift from balanced autonomic control to sympathetic dominance. These effects of celiprolol (800 mg), in reducing HRV indices, were attenuated by β1- and β2-adrenoceptor blockade with propranolol 160 mg but not by β1-adrenoceptor blockade with 50 mg atenolol [11]. Therefore one can conclude that agonism at the β2-adrenoceptor reduces HRV in contrast to antagonism at the β1-adrenoceptor, when HRV indices are increased. In the present study antagonism at the β2-adrenoceptor with ICI 118,551 did not increase HRV; however the lack of any HRV effect could merely reflect the absence of tonic tone at the β2-adrenoceptor at night. Whether agonist activity at the β1-adrenoceptor would similarly reduce HRV and the relative importance of the β1- and β2-adrenoceptors in the modulation of HRV cannot be inferred from the available evidence.
The optimum method to investigate heart rate variability during drug intervention is uncertain. The standard time-domain methods include the SDNN, reflecting all cyclic components responsible for variability in the recording, whereas the SDANN and SDNN index represent variability over cycles longer and shorter than 5 min respectively. Non-linear methods have been suggested as an alternative approach, the disadvantages of the time-domain based methods namely the strong correlation between adjacent beats, can be to some extent circumvented. The geometric scatterplot method investigates beat-to-beat variations in heart rate using a visual or pattern recognition approach; it is based on non-linear considerations and assesses non-periodic fluctuations in the heart rate. The scatterplot (Poincaré plot) had independent prognostic value for all-cause and sudden death in heart failure [44]. Human research investigations have correlated scatterplot length with long-term variability indices [17] whereas scatterplot width was correlated with short-term HRV and parasympathetic nervous activity [17, 18, 38]. The impact of β-adrenoceptor antagonism on scatterplot measures of HRV is not established; in patients with chronic heart failure bisoprolol did not alter scatterplot length but increased width at the highest percentiles (lowest heart rates) [28]. However metoprolol, in survivors of myocardial infarction, increased time-domain HRV statistics (mean RR interval, rMSSD, and proportion of adjacent RR intervals differing by >50 ms [pNN50 (%)]) at unaltered scatterplot indices [38]. It should be appreciated that the conventional scatterplot does not take into account the density of the paired RR intervals; the same two-dimensional shape may therefore reflect different patterns of HRV. A numeric quantitation of 3D scatterplots has been suggested where the third co-ordinate corresponds to the number of observations at discrete points along the scatterplot length; this approach improves the predictive accuracy of the method [16].
The other non-linear approach, used in this paper, was the ΔRR vsΔRRn+1 analysis; this method of sequence analysis also removes the dominant characteristic of the strong correlation between consecutive intervals present in both the time-domain and scatterplot analyses. Therefore the time-dependent dynamics of HRV may be assessed. Perturbations of autonomic balance, would be expected to alter the likelihood of the occurrence of particular sequences of acceleration (−/−) or deceleration (+/+), reflecting the shift in balance towards sympathetic or parasympathetic predominance. The normal pattern of the ΔRR vsΔRRn+1 analysis in the ‘awake’ state is alternation of increases and decreases in interval length, contrasting with ‘quiet’ sleep where sequences of progressively increasing and decreasing intervals occur [13]. Other suggested non-linear measurements include the approximate entropy and fractal dimension [42]; an advantage of these methods is that the prerequisites of data stationary and sinusoidal nature required for spectral analysis are not so stringent nor are the parameter estimates so strongly correlated with the mean.
The data from these disparate analyses was consistent, whether the HRV estimates were derived from the time-domain, the scatterplot or quadrant analyses. The main disadvantage of the traditional time-domain summary statistics is the strong dependence on the mean heart rate; for agonists and antagonists of the cardiac β-adrenoceptor, it would be preferable for there to be a rate independent measure of variability. The scatterplot analysis provides parameters that can be interrogated at fixed heart rates [28], while the sequence methods is more highly de-correlated from the mean [14]. In purely statistical terms, in the present assessment none of the methods was of proven superiority; although measuring the differing aspects of HRV, all gave a similar estimate of the pharmacodynamic effects.
In conclusion, the non-linear methods of scatterplot and quadrant analysis permitted autonomic balance to be assessed following therapeutic intervention with agents that modulated the β-adrenoceptor in man. The newer methods were at least as robust as the available time-domain methods. Partial agonism with salbutamol 8 mg altered the autonomic balance towards sympathetic dominance; ICI 118,551 25 mg had no effect on HRV abut prevented the HRV reduction seen when salbutamol was given alone. β-adrenoceptor antagonists have improved survival following myocardial infarction [6, 7]; whether the observed effect of a partial agonist, with decreased parasympathetic and enhanced sympathetic responsiveness, would offset the cardio-protective benefits of β-adrenoceptor blockade in secondary prevention is unknown. However these studies highlight such questions and suggest a direction for further research.
Acknowledgments
The authors are grateful for the ‘C’ programming expertise of Ms Therese Rafferty and Ron Jones in developing the programmes to permit these analyses and to Mr William Leahey for technical assistance with the scatterplot analyses.
References
- 1.Kleiger RE, Miller JP, Bigger JT, Moss AJ. The Multicenter Post-Infarction Research Group. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 2.Malik M, Farrell T, Cripps T, Camm AJ. Heart rate variability in relation to prognosis after myocardial infarction: selection of optimal processing techniques. Eur Heart J. 1989;10:1060–1074. doi: 10.1093/oxfordjournals.eurheartj.a059428. [DOI] [PubMed] [Google Scholar]
- 3.Bigger JT, Fleiss JL, Steinman R, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 4.Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med. 1976;294:1165–1170. doi: 10.1056/NEJM197605202942107. [DOI] [PubMed] [Google Scholar]
- 5.Spiers JP, Silke B, McDermott U, Shanks RG, Harron DWG. Time and frequency domain assessment of heart rate variability: a theoretical and clinical appreciation. Clin Autonom Res. 1993;3:145–158. doi: 10.1007/BF01819000. [DOI] [PubMed] [Google Scholar]
- 6.Norwegian Multicentre Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304:801–807. doi: 10.1056/NEJM198104023041401. [DOI] [PubMed] [Google Scholar]
- 7.Beta-blocker Heart attack Research Group. A randomised trial of propranolol in patients with acute myocardial infarction. J Am Med Ass. 1982;247:1707–1714. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 8.Molgaard H, Mickley H, Pless P, Bjerregaard P, Moller M. Effects of metoprolol on heart rate variability in survivors of acute myocardiol infarction. Am J Cardiol. 1993;71:1357–1359. doi: 10.1016/0002-9149(93)90555-q. [DOI] [PubMed] [Google Scholar]
- 9.Sandrone G, Mortara A, Torzillo D, La Rovere MT, Malliani A, Lombardi F. Effects of beta blockers (atenolol or metoprolol) on heart rate variability after acute myocardial infaction. Am J Cardiol. 1994;74:340–345. doi: 10.1016/0002-9149(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 10.Lipworth BJ, Grove A. Partial beta-adrenoceptor agonists revisited. Br J Clin Pharmacol. 1997;43:9–14. doi: 10.1111/j.1365-2125.1997.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 11.Silke B, Riddell JG. Effects of beta-adrenoceptor agonists and antagonists on heart-rate variability assessed using summary statistics and nonlinear procedures. J Cardiovasc Pharmacol. 1997;30:817–823. doi: 10.1097/00005344-199712000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Camm J, Malik M. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Report of Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 13.Raetz SL, Richard CA, Garfinkel A, Harper RM. Dynamic characteristics of cardiac R-R intervals during sleep and waking states. Sleep. 1991;14:526–533. doi: 10.1093/sleep/14.6.526. [DOI] [PubMed] [Google Scholar]
- 14.Schechtman VL, Raetz SL, Harper RK, Garfinkel A, Wilson AJ, Southall DP. Dynamic analysis of cardiac R-R intervals in normal infants and in infants who subsequently succumbed to the sudden infant death syndrome. Paed Res. 1992;31:606–612. doi: 10.1203/00006450-199206000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Hagerman I, Berglund M, Lorin M, Nowak J, Sylven C. Chaos-related deterministic regulation of heart rate variability in time- and frequency domains: effects of autonomic blockade and exercise. Cardiovasc Res. 1996;31:410–418. [PubMed] [Google Scholar]
- 16.Hnatkova K, Copie X, Staunton A, Malik M. Numeric processing of Lorenz plots of R-R intervals from long-term ECG’s—comparison with time-domain measures of heart rate variability for risk stratification after myocardial infarction. J Electrocardiography. 1995;28:74–80. doi: 10.1016/s0022-0736(95)80020-4. [DOI] [PubMed] [Google Scholar]
- 17.Copie X, Leheuzey JY, Iliow MC, et al. Correlation between time-domain measures of heart rate variability and scatterplots in post-infarction patients. Pace-Pacing and Clinical Electrophysiology. 1996;19:342–347. doi: 10.1111/j.1540-8159.1996.tb03336.x. [DOI] [PubMed] [Google Scholar]
- 18.Kamen PW, Krum H, Tonkin AM. Poincare plot of heart rate variability allows quantitative display of parasympathetic nervous activity in humans. Clin Sci. 1996;91:201–208. doi: 10.1042/cs0910201. [DOI] [PubMed] [Google Scholar]
- 19.McCaffrey PM, Riddell JG, Shanks RG. The selectivity of xamoterol, prenalterol, and salbutamol as assessed by their effects in the presence and absence of ICI 118, 511. J Cardiovasc Pharmacol. 1988;11:543–551. doi: 10.1097/00005344-198805000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Arnold JMO, O’Conner PC, Riddell JG, Harron DWG, Shanks RG, McDevitt DG. Effects of the beta-2 antagonist ICI 118,551 on exercise tachycardia and isoprenaline-induced beta-adrenoceptor responses in man. Br J Clin Pharmacol. 1985;19:619–630. doi: 10.1111/j.1365-2125.1985.tb02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCaffrey PM, Riddell JG, Shanks RG. An assessment of the partial agonist activity of Ro 31 1118, flusoxolol and pindolol in man. Br J Clin Pharmacol. 1987;24:571–580. doi: 10.1111/j.1365-2125.1987.tb03215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner J, Reinhardt D, Schumann HJ. Comparison of the bronchodilator and cardiovascular actions of isoprenaline, Th 1165a, terbutaline and salbutamol in cats and isolated organ preparations. Res Exp Med. 1973;162:49–62. doi: 10.1007/BF01851883. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell SR. An examination of some beta-adrenoceptor stimulants for selectivity using the isolated trachea and atria of the guinea pig. Eur J Pharmacol. 1972;19:371–379. doi: 10.1016/0014-2999(72)90104-5. [DOI] [PubMed] [Google Scholar]
- 24.Bilski AJ, Halliday SE, Fitzgerald JD, Wale JL. The pharmacology of a beta-2 adrenoceptor antagonist (ICI 118,551) J Cardiovasc Pharmacol. 1983;5:430–437. doi: 10.1097/00005344-198305000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Harry JD, Norris SC, Percival GC, Young J. The dose in humans at which ICI 118,551 (a selective beta-2 adrenoceptor blocking agent) demonstrates blockade of beta-1 adrenoceptors. Clin Pharmacol Ther. 1988;43:492–498. doi: 10.1038/clpt.1988.64. [DOI] [PubMed] [Google Scholar]
- 26.Malik M, Cripps T, Farrell T, Camm AJ. Prognostic value of heart rate variability after myocardial infarction. A comparison of different data processing methods. Med Biol Eng Comput. 1989;27:603–611. [Google Scholar]
- 27.SPSS I. SPSS for Windows Users Guide. Chicago, IL 60611: Norusis/SPSS Inc.; 1993. [Google Scholar]
- 28.Pousset F, Copie X, LeChat P, et al. Effects of bisoprolol on heart-rate variability in heart-failure. Am J Cardiol. 1996;77:612–617. doi: 10.1016/s0002-9149(97)89316-2. [DOI] [PubMed] [Google Scholar]
- 29.Altman DG. Practical Statistics for Medical Research. London: Chapman & Hall; 1992. Two way analysis of variance; pp. 334–336. [Google Scholar]
- 30.Grove A, McFarlane LC, Lipworth BJ. Expression of the beta-2 partial agonist/antagonist activity of salbutamol in states of low and high adrenergic tone. Thorax. 1995;50:134–138. doi: 10.1136/thx.50.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall JA, Petch MC, Brown MJ. Intracoronary injections of salbutamol demonstrate the presence of functional beta-2 adrenoceptors in the human heart. Circ Res. 1989;65:546–553. doi: 10.1161/01.res.65.3.546. [DOI] [PubMed] [Google Scholar]
- 32.Lipworth BJ, Brown MJ, McDevitt DG. Assessment of airways, tremor, and chronotropic responses to inhaled salbutamol in the quantification of beta-2 adrenoceptor blockade. Br J Clin Pharmacol. 1989;28:95–102. doi: 10.1111/j.1365-2125.1989.tb03510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook JR, Bigger JT, Kleiger RE, Fleiss JL, Steinman RC, Rolnitzky LM. Effect of atenolol and diltiazem on heart rate variability in normal persons. J Am Coll Cardiol. 1991;17:480–484. doi: 10.1016/s0735-1097(10)80119-6. [DOI] [PubMed] [Google Scholar]
- 34.Schweizer MW, Brachmann J, Kirchner U, et al. Heart rate variability in time and frequency domains: effects of gallopamil, nifedipine, and metoprolol compared with placebo. Br Heart J. 1993;70:252–258. doi: 10.1136/hrt.70.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niemela MJ, Airaksinen KE, Huikuri HV. Effect of beta-blockade on heart rate variability in patients with coronary artery disease. J Am Coll Cardiol. 1994;23:1370–1377. doi: 10.1016/0735-1097(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 36.Hohnloser SH, Klingenheben T, Zabel M, Just H. Effect of sotalol on heart rate variability assessed by Holter monitoring in patients with ventricular arrhythmias. Am J Cardiol. 1993;72:67A–71A. doi: 10.1016/0002-9149(93)90027-a. [DOI] [PubMed] [Google Scholar]
- 37.Brouwer J, Viersma JW, Vanveldhuisen DJ, et al. Usefulness of heart-rate variability in predicting drug efficacy (metoprolol vs diltiazem) in patients with stable angina. Am J Cardiol. 1995;76:759–763. doi: 10.1016/s0002-9149(99)80222-7. [DOI] [PubMed] [Google Scholar]
- 38.Keeley EC, Lange RA, Hillis LD, Joglar JA, Page RL. Correlation between time-domain measures of heart rate variability and scatterplots in patients with healed myocardial infarcts and the influence of metoprolol. Am J Cardiol. 1997;79:412–414. doi: 10.1016/s0002-9149(96)00777-1. [DOI] [PubMed] [Google Scholar]
- 39.Stein PK, Rottman JN, Bosner MS, Conger BM, Kleiger RE. Effects of pindolol and labetalol on heart rate variability in normal subjects. Ambul Monit. 1995;8:171–178. [Google Scholar]
- 40.Hadfield SE, Slee S-J, Snow HM. The cardiovascular pharmacology of xamoterol, cicloprolol, prenalterol and pindolol in the anaesthetised dog. Br J Clin Pharmacol. 1989;28:78S–81S. doi: 10.1111/j.1365-2125.1989.tb03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jartti T, Kaila T, Tahvanainen K, Kuusela T, Vanto T, Valimaki I. The acute effects of inhaled salbutamol on the beat-to beat variability of heart rate and blood pressure assessed by spectral analysis. Br J Clin Pharmacol. 1997;43:421–428. doi: 10.1046/j.1365-2125.1997.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jartti TT, Kuusela TA, Kaila TJ, Tahvanainen KUO, Valimaki AT. The dose-response effects of terbutaline on the variability, approximate entropy and fractal dimension of heart rate and blood pressure. Br J Clin Pharmacol. 1998;45:277–285. doi: 10.1046/j.1365-2125.1998.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wheeldon NM, McDevitt DG, Lipworth BJ. Selectivity of antagonist and partial agonist activity of celiprolol in normal subjects. Br J Clin Pharmacol. 1992;34:337–343. doi: 10.1111/j.1365-2125.1992.tb05640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brouwer J, van Veldhuisen DJ, Man in′t Veld AJ, et al. Prognostic value of heart rate variability during long-term follow-up in patients with mild to moderate heart failure. The Dutch Ibopamine Multicentre Trial Study Group. J Am Coll Cardiol. 1996;28:1183–1189. doi: 10.1016/s0735-1097(96)00279-3. [DOI] [PubMed] [Google Scholar]


