Abstract
Aims
To establish the incidence, time course and risk factors of hyponatraemia complicating treatment with fluoxetine or paroxetine in an elderly population.
Methods
Retrospective descriptive and case control study in an inpatient/outpatient assessment and rehabilitation service for people aged 65 years and over. Fourteen elderly patients with hyponatraemia complicating treatment with fluoxetine or paroxetine, matched with 56 controls drawn from 845 patients treated with fluoxetine or paroxetine over 3.5 years. No other SSRI antidepressants were used over the study period.
Results
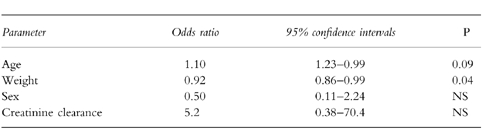
The incidence of hyponatraemia was 4.7/1000 people treated/year (6.3/1000 for fluoxetine and 3.5/1000 for paroxetine). Hyponatraemia was detected at a median 13.5 (mean 18.6, range 4–64) days after commencing the drug. Mean (95% confidence intervals) body weights were lower in cases at 53.0 (95% CI 46.5–59.5) kg compared with controls at 64.5 (95% CI 60.1–68.4) kg (P < 0.01). 71% of cases were women compared with 45% of controls (P = 0.07) but the effect of gender was confounded by body weight. There were trends for cases to be older (odds ratio 1.10: 95% CI 0.99, 1.23) and lighter (odds ratio 0.92, 95% CI 0.86, 0.99).
Conclusions
Approximately 1 in 200 elderly people treated per year with fluoxetine or paroxetine developed complicating hyponatraemia. Low body weight was a particular risk factor. Most cases occurred within 3 weeks of treatment.
Introduction
Selective serotonin reuptake inhibitor (SSRI) antidepressants have become increasingly popular in the treatment of depression. This is partly because they have different side-effects from other classes of antidepressants. Anticholinergic side-effects, urinary hesitancy, cardiotoxicity and/or postural hypotension associated with tricyclic antidepressant use may contribute to the greater use of SSRI antidepressants in the elderly.
Hyponatraemia is a recognised, but rare, complication of tricyclic antidepressant therapy which is thought to be idiosyncratic in type [1]. Hyponatraemia complicating SSRI antidepressant use has now become widely reported [2–6]. A systematic search identified 736 cases by encompassing all published and spontaneously reported cases [4]. Although most cases have occurred in elderly women [4, 8], it is not clear whether this is because they are at particular risk or whether they are the main population group for whom SSRI antidepressants are prescribed. The mechanism of hyponatraemia is thought to be the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) [7].
The department of Health Care of the Elderly at the Princess Margaret Hospital, Christchurch, New Zealand, provides an outpatient and 135 bed inpatient assessment and rehabilitation service for a population of approximately 50,000 people aged 65 years and over. A central database containing patient diagnoses and medications is maintained within the department. The recognition that there were an increasing number of cases of hyponatraemia developing in people taking SSRI antidepressants prompted a systematic search of this database to determine all cases. At the time of the study fluoxetine and paroxetine were the only SSRI antidepressants used.
The primary aims of this study were:
to identify all cases of hyponatraemia occurring in elderly people referred to the service who were taking fluoxetine or paroxetine
to establish the likelihood of hyponatraemia being attributable to fluoxetine or paroxetine, and thereby
to search for risk factors which might predispose to this complication, using a case control method.
Secondary aims were to:
to establish the incidence of hyponatraemia due to fluoxetine or paroxetine in this population, and
to describe the time course of the development of hyponatraemia.
Methods
The denominator population was determined by identifying all people (who were taking fluoxetine or paroxetine) referred to the service over a 3.5 year period. As the service primarily provides a rehabilitation service for elderly people, this population typically comprises people with varying degrees of disability and/or active medical problems.
The numerator population comprised patients from the denominator population who also had hyponatraemia. Patients were included only if the hyponatraemia was considered to be ‘definitely’ or ‘probably’ related to use of the SSRI antidepressant, using the likelihood criteria of Kramer et al. [9, 10]. The relationship was defined as ‘definite’ if hyponatraemia followed a reasonable time course after administration of the drug, resolved after stopping the drug and could not be explained by other means. Patients with uncontrolled heart failure were excluded. The relationship was defined as ‘probable’ if there was an alternative explanation for hyponatraemia but the time course in relation to the drug administration made the SSRI antidepressant the more likely cause. Cases defined as ‘possible’ or ‘unlikely’ were excluded from analysis as either a case or a control.
Potentially important covariates in each case were sought by obtaining the following information: drug (fluoxetine or paroxetine), drug dose, other possible explanations for hyponatraemia, concomitant medications, age, gender, weight, plasma creatinine concentration, calculated creatinine clearance [11] and plasma albumin concentration. To ensure that the hyponatraemia was of significant degree, cases were included only if the lowest plasma sodium concentration was less than 130 mmol l−1 (reference range 134–146 mmol l−1). The time from starting the drug to development of hyponatraemia was approximated by noting the date of the first recorded low plasma sodium concentration. The time to resolution of hyponatraemia after stopping the drug was noted.
Each case identified as ‘definite’ or ‘probable’ was matched with four randomly selected control patients drawn from the same denominator population. Controls had to satisfy the following criteria:
They must have been taking a SSRI antidepressant for more than 3 months. This was to ensure a reasonable time to develop the complication.
The lowest measured plasma sodium concentration had to be 135 mmol l−1 or greater. This was to ensure there was a clear distinction between cases and controls.
The following information was obtained for each control patient: drug (fluoxetine or paroxetine), drug dose, concomitant medications, age, gender, weight, plasma creatinine concentration, calculated creatinine clearance [11] and plasma albumin concentration. The patient’s General Practitioner was contacted to obtain missing information where necessary.
Paroxetine was first used in our patients 12 months after the beginning of the study so the duration of use for this drug was 2.5 years.
Differences between cases and controls in continuous variables were compared using Student’s unpaired t-test. Categorical variables were compared using the Chi-square test. Multiple logistic regression was used to compare cases and controls to determine if there was an interaction between any of the demographic characteristics such as weight or gender.
Results
Over the study period, 845 people received an SSRI antidepressant, including 501 people taking fluoxetine over 3.5 years and 344 people taking paroxetine over 2.5 years.
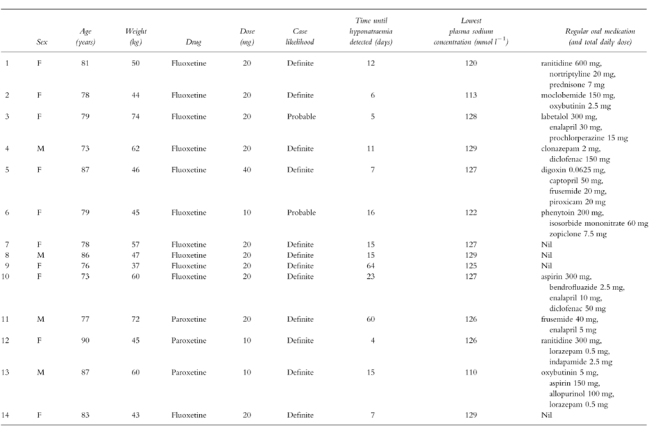
Within this group, 42 people were identified as having developed hyponatraemia. In 14 cases this was considered to be related to the SSRI use (Table 1). In 12 cases the relationship between the SSRI antidepressant and hyponatraemia was considered ‘definite’ as it followed a reasonable time course after administration of the drug, resolved after stopping the drug and could not be explained by other means. Two cases were classified as ‘probable’ because there were other possible explanations for the hyponatraemia—in case 3, the patient was also taking prochlorperazine (a drug known to cause hyponatraemia) and, in case 6, the patient had mild hyponatraemia prior to commencing the medication (128–129 mmol l−1) which worsened whilst on fluoxetine and partially resolved on discontinuation of the drug. All patients recovered from the complication after stopping the medication. No patients were rechallenged with the drug.
Table 1.
Demographic, medication and plasma sodium details of the cases.
The incidence of hyponatraemia due to SSRI antidepressant use in the study population was 4.7 cases/1000 people treated/year. The incidence with fluoxetine alone was 6.3/1000/year. The three cases of hyponatraemia in people taking paroxetine over the shorter study period for this drug resulted in an incidence of 3.5 cases/1000/year.
Hyponatraemia was detected at a mean of 18.6 days, a median of 13.5 days and a range of 4–64 days after commencing the drug. Seventy-nine percent of cases occurred within the first 3 weeks of treatment.
Differences between cases and controls are shown in Table 2. Cases had lower body weights compared with controls (P < 0.01). The dose of drug was not significantly different between cases and controls but the dose per kilogram of body weight tended to be higher in cases (P = 0.07). There were trends for cases to be female (P = 0.07) and to be to older than controls (P = 0.09).
Table 2.
Univariate comparison of cases with controls.
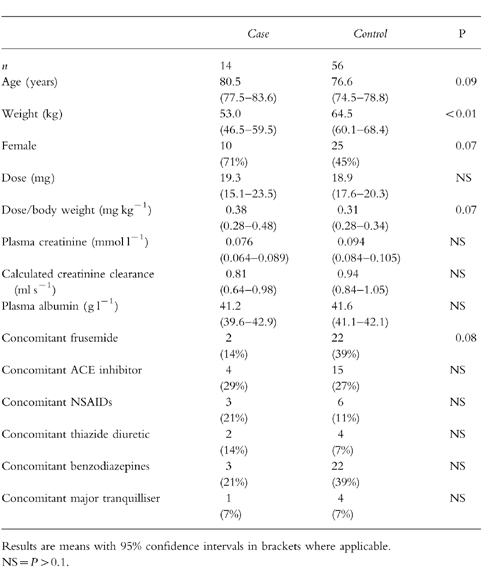
Serum creatinine was slightly, but not significantly, lower in cases than controls. Because of lower body weights in the cases, the calculated creatinine clearance was slightly, but not significantly, lower in cases than controls. There was no difference between cases and controls in serum albumin or concomitant medications although there was a trend towards cases to be less likely to take frusemide (P = 0.08).
There was significant co-variation between gender and weight with women being lighter than men. Following multiple logistic regression, weight, rather than gender, remained the significant factor which distinguished the cases from the controls (Table 3).
Table 3.
Multivariate comparison of cases with controls.
Discussion
These results indicate that hyponatraemia is a significant complication of treatment with SSRI antidepressants in elderly people occurring in this population at an incidence of 4.7 cases/1000/year. It occurred in the majority of cases (79%) within the first 3 weeks of treatment and in all cases within 10 weeks.
The incidence of 4.7/1000/year applies to a group of elderly people with active medical problems. Our calculated incidence rate was derived by systematically identifying all possible cases within a defined population. This contrasts with a published rate in people aged 65 years or over of 1.35/1000/year which was derived from spontaneous reports to an intensive medicines monitoring scheme over a 4 year period [3]. We would suggest that the higher rate of 4.7/1000/year seen in our series is more accurate as most of the cases in our series were not notified to a central monitoring agency. We are not aware of any other estimates of the incidence rate of this complication. Our calculated incidence rate may be an underestimate because some people included in the denominator population may have been taking an SSRI antidepressant for a short time. They may not, therefore, have had sufficient time to develop complicating hyponatraemia. In addition, some people within the denominator population may not have had their plasma sodium concentration closely monitored. These limitations were overcome in the case-control part of our study which looked for risk factors, but a case-control method does not allow for an estimation of incidence.
The difference in incidence rate between fluoxetine (6.3/1000/year) and paroxetine (3.5 cases/1000/year) should be interpreted cautiously as there was greater use of fluoxetine overall and the paroxetine rate was based on only three cases.
The largest review of reported cases of hyponatraemia complicating use of SSRI antidepressants recorded 736 cases. These were derived from spontaneous reports, published cases and post-marketing surveillance. Fluoxetine accounted for 75.3% of cases and paroxetine for 12.4% [4]. The median time to onset was 13 days (range 3 to 120 days). In the 546 cases where age was given, 75.1% occurred in people aged 65 years and over. In the 588 cases where gender was stated, 75.5% occurred in women [4]. Our case series is remarkably similar: 78% involved fluoxetine, the median time to onset was 13.5 days and 71% were women. All people in our denominator population, and therefore numerator population, were aged 65 years or over. Thus, despite our cases being identified retrospectively, it is reassuring that they seem typical of other reported cases.
Unlike previous case series, our study is the first to have made comparisons with controls. Ideally, a large prospective study should be undertaken, but we believe this study is an important first step in identifying risk factors more objectively.
Uncontrolled case series have postulated that women are at greater risk of hyponatraemia [3–5, 12]. Our data also suggested this but, following multiple logistic regression, we found this to be confounded by low body weight. Low body weight therefore, rather than female gender, is the greater risk factor for hyponatraemia complicating SSRI antidepressant use. There was a limited range of daily doses used in our series (10 mg, 20 mg or 40 mg) so the difference between cases and controls in dose/body weight was not pronounced. The findings would support a dose-response component to the relationship between drug use and hyponatraemia although there is also an idiosyncratic component. The observation that advanced age may be a risk factor is likely to be underestimated in our series as it is difficult to detect trends with age within a population already defined by age.
Hyponatraemia complicating diuretic use has been well described 13, 14]. This includes some cases occurring in people taking SSRI antidepressants [15]. In the vast majority of cases, the hyponatraemia is associated with thiazide diuretics [13]. Frusemide rarely causes this complication [16] and, indeed, has been used successfully to raise the serum sodium in SIADH [17]. The trend seen in our study where cases were less likely than controls to be taking frusemide could indicate that loop diuretics may have protected some of our cases from this complication. Frusemide was taken as treatment for heart failure. It is therefore impossible to separate an effect of frusemide from an effect of controlled heart failure. Patients with uncontrolled heart failure were excluded as cases or controls.
Angiotensin converting enzyme inhibitors [18] and non-steroidal inflammatory drugs [19] are also risk factors for hyponatraemia, especially in the elderly. We were unable to detect any relationship between use of these drugs and SSRI antidepressant-induced hyponatraemia, and no trends were evident.
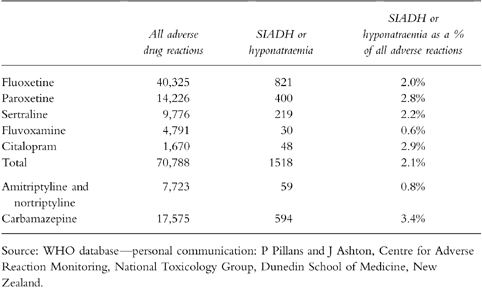
Reports to the World Health Organisation (WHO) of adverse drug reactions for SSRI antidepressants (fluoxetine, paroxetine, sertraline, fluvoxamine, citalopram), amitriptyline, nortriptyline and carbamazepine are shown in Table 4. Compared with amitriptyline or nortriptyline, SSRI antidepressants have a higher proportion of all adverse reactions due to SIADH or hyponatraemia. The rate is closer to that of carbamazepine, perhaps the drug most well known for being associated with SIADH. This could mean SSRI antidepressants are more likely to cause SIADH than tricyclic antidepressants or that tricyclic antidepressants are more likely to cause side effects other than hyponatraemia.
Table 4.
Adverse drug reactions reported to the World Health Organisation to July 1998.
Comparisons by age and sex, where these were reported to the WHO, are shown in Table 5. It can be seen that, for people aged 70 years and over, hyponatraemia or SIADH as an adverse reaction were vastly over-represented compared with younger people. Similarly hyponatraemia or SIADH were disproportionately present in women. These findings do not incorporate a denominator population of people who did not experience an adverse event and therefore cannot be used to estimate risk.
Table 5.
Adverse drug reactions from SSRI antidepressants* reported to the World Health Organisation to July 1998 where age and sex were recorded.
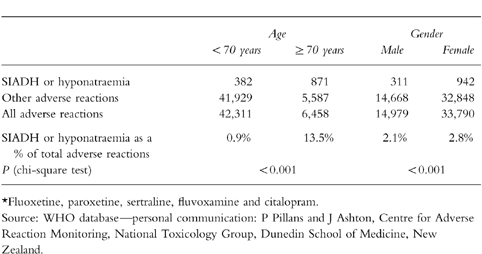
Our findings, and the WHO data, are consistent with reduced weight and/or increased age as being important risk factors. These risk factors could both act by causing a reduction in drug clearance which, for a given dose, will result in higher concentrations. There may also be a pharmacodynamic effect as the predominance of the complication occurring in older people is consistent with the known increased risk of SIADH in this population.
Although hyponatraemia is a well recognised complication of the use of SSRI antidepressants, at an incidence rate of around 4.7/1000/year it is difficult to justify routine monitoring of plasma sodium concentrations in all people taking this class of drug. However, if monitoring were contemplated, doing so at 3–4 weeks after initiation of therapy would detect the majority of cases. There should remain a high degree of suspicion if an elderly person of low body weight develops symptoms consistent with hyponatraemia whilst taking a SSRI antidepressant.
Acknowledgments
This study was undertaken as a summer student project and received financial assistance from the Canterbury Health Care for the Elderly Education Trust. We are grateful to Dr Chris Frampton for statistical advice and to Dr Peter Pillans and Ms Janelle Ashton for their assistance in providing the WHO data.
References
- 1.Abbott R. Hyponatremia due to antidepressant medications. Ann Emerg Med. 1983;12:708–710. doi: 10.1016/s0196-0644(83)80423-5. [DOI] [PubMed] [Google Scholar]
- 2.Cohen BJ, Mahelsky M, Adler L. More cases of SIADH with fluoxetine. Am J Psychiatry. 1990;147:948–949. doi: 10.1176/ajp.147.7.948b. [DOI] [PubMed] [Google Scholar]
- 3.Pillans PI, Coulter DM. Fluoxetine and hyponatraemia—a potential hazard in the elderly. NZ Med J. 1994;107:85–86. [PubMed] [Google Scholar]
- 4.Liu BA, Mittmann N, Knowles SR, Shear NH. Hyponatraemia and the syndrome of inappropriate secretion of antidiuretic hormone associated with the use of selective serotonin reuptake inhibitors: a review of spontaneous reports. Can Med Assoc J. 1996;155:519–527. [PMC free article] [PubMed] [Google Scholar]
- 5.Adverse Drug Reactions Advisory Committee. Selective serotonin reuptake inhibitors and SIADH. Med J Aust. 1996;164:562. [PubMed] [Google Scholar]
- 6.Spigset O, Hedenmalm K. Hyponatraemia and the syndrome of inappropriate antidiuretic hormone secretion (SIADH) induced by psychotropic drugs. Drug Safety. 1995;12:209–225. doi: 10.2165/00002018-199512030-00006. [DOI] [PubMed] [Google Scholar]
- 7.Girault C, Richard JC, Chevron V, Goulle JP, Droy JM, Bonmarchand G, Leroy J. Syndrome of inappropriate secretion of antidiuretic hormone in two elderly women with elevated serum fluoxetine. J Toxicol Clin Toxicol. 1997;35:93–95. doi: 10.3109/15563659709001172. [DOI] [PubMed] [Google Scholar]
- 8.Chan TYK. Drug-induced syndrome of inappropriate antidiuretic hormone secretion. Drugs & Aging. 1997;11:27–44. doi: 10.2165/00002512-199711010-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kramer MS, Leventhal JM, Hutchinson TA, Feinstein AR. An algorithm for the operational assessment of adverse drug reactions. I. Background, Description and Instructions for Use. JAMA. 1979;242:623–632. [PubMed] [Google Scholar]
- 10.Hutchinson TA, Leventhal JM, Kramer MS, Karch FE, Lipman AG, Feinstein AR. An algorithm for the operational assessment of adverse drug reactions. II. Demonstration of reproducibility and validity. JAMA. 1979;242:633–638. [PubMed] [Google Scholar]
- 11.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 12.Burke D, Fanker S. Fluoxetine and the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) Aust N Z J Psychiatry. 1996;30:295–298. doi: 10.3109/00048679609076109. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenblick M, Friedlander Y, Rosin AJ. Diuretic-induced severe hyponatraemia. Review and analysis of 129 reported patients. Chest. 1993;103:601–606. doi: 10.1378/chest.103.2.601. [DOI] [PubMed] [Google Scholar]
- 14.Siegler EL, Tamres D, Berlin JA, Allen-Taylor L, Strom BL. Risk factors for the development of hyponatremia in psychiatric inpatients. Arch Intern Med. 1995;155:953–957. [PubMed] [Google Scholar]
- 15.ten Holt WL, van Iperen CE, Schrijver G, Bartelink AK. Severe hyponatremia during therapy with fluoxetine. Arch Int Med. 1996;156:681–682. doi: 10.1001/archinte.156.6.681. [DOI] [PubMed] [Google Scholar]
- 16.Rose BD. New approach to disturbances in the plasma sodium concentration. Am J Med. 1986;81:1033–1040. doi: 10.1016/0002-9343(86)90401-8. [DOI] [PubMed] [Google Scholar]
- 17.Decaux G, Waterlot Y, Genette F, Mockel J. Treatment of the syndrome of inappropriate secretion of antidiuretic hormone with furosemide. N Engl J Med. 1981;304:329–330. doi: 10.1056/NEJM198102053040605. [DOI] [PubMed] [Google Scholar]
- 18.Nicholls MG, Espiner EA, Ikram H, Maslowski AH. Hyponatraemia in congestive heart failure during treatment with captopril. Br Med J. 1980;281:909. doi: 10.1136/bmj.281.6245.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zawada ET., Jr Renal consequences of nonsteroidal anti inflammatory drugs. Postgrad Med. 1982;71:223–230. doi: 10.1080/00325481.1982.11716077. [DOI] [PubMed] [Google Scholar]


