Abstract
Aims
To study the mechanisms behind NSAID-associated nephropathy.
Methods
Analysis of published case reports satisfying strict criteria for NSAID nephropathy.
Results
Ninety-seven cases with acute nephritis (AN; 19 patients), minimal change nephropathy (MC; 38 patients), membranous glomerulonephritis (MGN; 19 patients), focal sclerosis (FS; 13 patients) and other glomerulonephritis subgroups (8 patients) were identified. Hypersensitivity reactions were seen in all groups, most often in AN. Proteinuria was more severe in MC and FS than in MGN and unrelated to amount of glomerular deposits. The mean NSAID treatment time was 1.7 months in AN, 8.2 months in MC and 39 months in MGN and associated with amount of glomerular deposits, fusion of podocytes and proteinuria, and inversely associated with hypersensitivity, interstitial damage and renal failure. Rheumatic diseases were common in MGN. At follow-up 68 of 72 patients who had discontinued NSAID treatment had improved, 57 with normal renal function.
Conclusions
NSAID nephropathy may be caused by hypersensitivity. The reaction is milder than in drug-induced acute tubulointerstitial nephritis, probably because the offending drug inhibits the inflammatory reaction it has started itself. Heavy proteinuria is probably due to lymphokines produced as a result of the immunological response. If the allergic reaction is strong, AN is produced rapidly with severe renal failure but little proteinuria; if it is less violent, immunocompetent cells may develop to produce lymphokines and proteinuria. Immune complexes may be formed eventually, secondary to the increased glomerular permeability, more easily in patients with a hyperactive immune system and with little consequence for renal function.
Keywords: non-steroidal antiinflammatory drugs, NSAID nephropathy, tubulointerstitial nephritis, glomerulonephritis, minimal change nephropathy, membranous glomerulonephritis, nephrotic syndrome, side effects
Introduction
Treatment with non-steroidal anti-inflammatory drugs (NSAID) may produce renal side effects. The most common are renal failure with disturbances of salt and water metabolism because NSAIDs inhibit the synthesis of prostaglandins involved in the intrinsic autoregulation of renal function. The effects on renal function are almost always seen in patients with preexisting renal disease or with decreased renal perfusion because NSAIDs have no influence on normal renal function [1–5].
In patients with normal kidneys NSAID treatment may trigger a spectrum of nephritides including tubular, interstitial or tubulointerstitial nephritis, chronic interstitial nephritis with papillary necrosis, and tubulointerstitial nephritis combined with the nephrotic syndrome, often followed by acute or chonic renal failure. In addition, the patients who present with the nephrotic syndrome may have a variety of glomerular changes indistinguishable from those found in minimal change nephropathy, membranous glomerulonephritis, focal sclerosis and other glomerulonephritic subgroups [2, 3, 6–8].
The mechanisms for these nephritides (NSAID nephropathy) have not been explained satisfactorily. Most authors consider that each of them is a separate entity with its own cause and mechanisms. A striking finding in NSAID nephropathy, however, is the absence of a clearcut demarcation between the various subgroups; very often, findings typical for tubular nephritis, interstitial nephritis and glomerulonephritis are found in the same patient. Considering the widespread use of NSAIDs, NSAID nephropathy is a rare complication. It seems highly unlikely that patients with such a rare disease should suffer from two or three rare renal diseases at the same time, each with a different cause. A more likely explanation is that these nephritides have a common cause and that the pathogenetic processes are modulated by secondary factors that may vary from patient to patient. The present review was performed to see if the findings in the major subgroups of NSAID nephropathy were compatible with this assumption.
Methods
To isolate the renal effects of NSAID from other pathogenic conditions the following criteria for inclusion of a case report were used: renal disease should have occurred during treatment with NSAID; treatment with other nephritogenic drugs should have been discontinued at least 3 months before admission; the patient should not suffer from an underlying renal disease; or from diabetes, gout, systemic lupus erythematosus (SLE) or another disease known to predispose to renal disease unless an investigation prior to NSAID treatment, or after its discontinuation, had shown normal urine and normal renal function; the renal tissue should have been studied microscopically, and serum creatinine or creatinine clearance, and degree of proteinuria should be given.
Case reports written in English, German or French were sought in the MEDLINE database using the following search term: (non-steroidal antiinflammatory OR the generic name of each of the NSAIDs in clinical use) AND (nephropathy OR interstitial nephritis OR tubular nephritis OR glomerulonephritis OR glomerulopathy OR nephrotic syndrome OR acute renal failure). Case reports were also sought in reviews of acute renal failure, acute interstitial and tubulointerstitial nephritis and by reviewing the references of the case reports.
The following information was extracted from the case reports: Age and sex, the indication for and the length of NSAID treatment, information about other medical treatments, the peak urinary protein and serum creatinine, the blood pressure, the urine sediment, any symptoms or laboratory data indicating a hypersensitivity reaction, the renal biopsy findings, and glomerular function and degree of proteinuria at follow-up.
Unpublished biopsy findings were sought from the authors of the more recent reports.
Statistics
As almost all data were dichotomous it was necessary to use non-parametric statistics. For each parameter the patients were divided into two or three appropriate groups as described in Results. Associations between groups were calculated by chi-square using Fisher’s exact test when crosstabs included four squares only and linear-by-linear association when crosstabs included more than four squares. The SPSS statistical program was used for the calculations; P was two-tailed.
Results
Clinical and laboratory findings
Ninety-seven case reports that fulfilled the selected criteria were identified [9–73] with a male/female ratio of 34/63, mean age 54.0 years (median 57). The indication for NSAID treatment was a musculoskeletal disorder in 52 patients, a rheumatic disorder in 30, miscellaneous disorders in eight and unknown in seven. Nineteen different NSAIDs were used; most often fenoprofen which was used in 23 patients, followed by sulindac (eight patients), ibuprofen and diclofenac (seven patients each). Thirteen patients had been treated with more than one NSAID, in most cases the other NSAID was aspirin. The total treatment time was given for 91 patients and varied between 24 h and 22 years (median 6 months) (Table 1). Thirty-three patients had other medical treatments. Blood pressure at admission was given for 67 patients and signs or symptoms of hypersensitivity was noted in 32 patients (Table 2). The glomerular function was determined in 63 patients by serum creatinine, in 19 by creatinine clearance and 15 had dialysis. Degree of proteinuria was quantified in 88 patients, in nine it was graded by albustix only (Table 1). A urine sediment was studied in 78 patients.
Table 1.
Grouping of the patients according to various parameters.
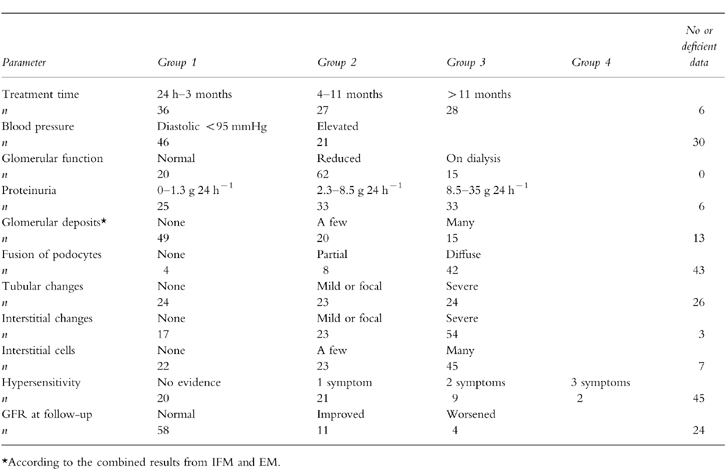
Table 2.
Number of patients with signs or symptoms of systemic hypersensitivity.

Biopsy findings
The renal biopsy was studied by light microscopy (LM) in all patients, by immunofluorescence microscopy (IM) in 75 patients, and by electron microscopy (EM) in 64 patients.
Light microscopy
In 67 patients the glomeruli were normal. Nineteen of these patients had minimal proteinuria (<1 g 24 h−1; median 0.4 g 24 h−1); all except two had varying degrees of interstitial or tubulointerstitial damage, all had renal failure and a short treatment time (median 1 month). These nineteen patients were classified as acute nephritis (AN). Thirty-seven of the patients with normal glomeruli had nephrotic range proteinuria and no deposits by IM and/or EM and were therefore classified as minimal change nephropathy (MC). Nine of the patients with normal glomeruli by light microscopy had evidence of membranous glomerulonephritis (MGN) on immunofluorescence and/or electron microscopy and were grouped together with ten patients with typical MGN on light microscopy. Twelve patients with 10 or more % sclerotic glomeruli and normal non-sclerotic glomeruli by LM and one patient with glomerular tip lesion were classified as focal sclerosis. Eight patients had other types of glomerular changes (Table 3).
Table 3.
Number of patients by type of nephritis.

Immunofluorescence microscopy
(IFM). IFM was performed in 71 cases, immunoperoxidase microscopy in one. In 34 patients no deposits were seen. Twenty-one had granular deposits with various localizations in the glomeruli; four had also tubular, interstitial or vascular deposits. Ten patients had tubular, interstitial or vascular deposits but no glomerular deposits, two had linear glomerular deposits.
Electron microscopy
This was performed in 63 patients. In 19 patients deposits were noted in the glomeruli; 12 had subepithelial and/or intramembranous deposits, three had subendothelial deposits, nine had mesangial deposits and 33 had none. The condition of the foot processes of the glomerular epithelial cells were recorded in 54 patients (Table 1).
Follow-up
The glomerular function at follow-up, a few days to 10 years after discontinuation of NSAID (median 4 months), was reported in 86 patients. Patients with an abnormal glomerular filtration rate (GFR) and followed for less than 1 month or whose follow-up time was unknown, and patients with insufficient information about possible NSAID treatment during follow-up were excluded from the statistical calculations. Four patients, who had continued taking NSAID, were also excluded. In one GFR had worsened; the other three were on dialysis treatment. Most of the rest had recovered completely and even those whose GFR was still abnormal at follow-up had improved considerably. Four patients had worsened, three of them had had severe renal failure at admission (Table 1).
Associations
Associations between all parameters in Table 1 and the major subgroups were calculated, as were associations between each parameter. In the following the most important associations are mentioned only.
The lowest GFR was seen in patients with AN (AN vs all others; P < 0.01). The GFR was also lower in patients with MC than in patients with MGN (P < 0.01). A strong, positive correlation was found between amount of glomerular deposits and the GFR (P < 0.00001). Except for MGN, interstitial damage was frequent in all groups and associated with a low GFR (P < 0.0001). Similar findings were noted for tubular damage.
As a consequence of the criteria chosen for AN, degree of proteinuria was less severe in AN than in MC and MGN. Proteinuria was associated with foot process fusion (P < 0.001). Less predictable was that proteinuria was more pronounced in MC and FS than in MGN (P < 0.05). No association was found to number of glomerular deposits.
Treatment time was strongly associated with diagnoses. Almost all cases of AN occurred during the first 3 months of treatment (P < 0.0001; AN vs all others) whereas most patients with MGN had been treated for more than 11 months (P < 0.01; MGN vs all others). The mean treatment time for AN was 1.7 months (median 1 month; range 24 h to 3 months); for MC it was 8.2 months (median 6 months; range a few days to 24 months) and for MGN it was 39.0 months (median 12 months; range 5 weeks to 22 years). Treatment time was also associated with glomerular deposits (P = 0.01), proteinuria (P < 0.02) and foot process fusion (P < 0.001), and inversely associated with interstitial damage and hypersensitivity (P < 0.02 in both cases). Signs or symptoms of hypersensitivity occurred in all types of nephritis, but most often in AN (P = 0.01; AN vs all others). Patients with MGN and patients with glomerular deposits had more often a rheumatic disease than had other patients (P < 0.02 in both calculations). No associations were found with sediment findings, use of diuretics or antihypertensive drugs, or with blood pressure.
Patients who had been treated with more than one NSAID had more often an abnormal GFR at follow-up than patients treated with one only. Although the number treated with more than one NSAID was small the difference was statistically significant. Treatment of the nephritis with corticosteroids was associated with a more serious outcome, but those who were treated with steroids had also a more serious disease at admission.
Discussion
The peculiar syndrome of acute or subacute tubulointerstitial nephritis associated with the nephrotic syndrome seen after NSAID treatment has given rise to much speculation about the underlying mechanisms. In a previous review [7] NSAID nephropathy was considered as a clinical entity distinct from drug-associated acute tubular interstitial nephritis (ATIN), not only because of the frequent occurrence of heavy proteinuria, but also because in contrast to ATIN it was preferentially seen in old age, and the onset occurred after much longer exposure to the drug and was followed by signs and symptoms of systemic hypersensitivity in a few cases only. It could also be added that the course in NSAID nephropathy most often is milder and more protracted.
Since then many more cases have been reported allowing a more detailed description of the syndrome. First, systemic hypersensitivity seems to be more frequent than reported previously. Many case reports were incomplete on that point, probably because the relevant analyses were not performed. After exclusion of the cases with insufficient information it appeared that features of hypersensitivity were just as frequent in AN as has been reported in drug-induced ATIN, and about 50% of the rest presented with evidence of hypersensitivity also.
The high frequency of allergic features indicates that drug hypersensitivity may also trigger NSAID nephropathy. Also suggestive is analyses of the mononuclear, interstitial cells. In one study they were entirely T-cells [26]; in another study, 80% were T-cells and of the 20% B-lymphocytes the great majority were IgE-bearing [62] and treatment after discontinuation of the drug with another NSAID did not result in relapse [64, 69].
The lower frequency of allergic symptoms in patients with heavy proteinuria could be explained by a time-factor. As these patients had a milder and more protracted course they may have sought medical help at a time where the allergic reactions had disappeared.
The higher mean age in NSAID nephropathy compared with ATIN does not necessarily mean different mechanisms, but rather that the indication for NSAID treatment most often is a disease of middle and old age, whereas antibiotics, the commonest cause of drug-associated ATIN, are used in patients of all ages.
Heavy proteinuria with the nephrotic syndrome is not unique for NSAID nephropathy either, but is also known from drug-induced ATIN [74–76]. Linton et al. [77] found 0.4 to to 1.7 g urine protein 24 h−1 in nine cases; Pusey et al. [78] noted close to nephrotic range proteinuria in three of seven cases; and Galpin et al. [79] found similar and even higher degrees of proteinuria in three of 14 cases.
Also suggestive of common mechanisms is that fusion of the epithelial foot processes, a constant finding in patients with heavy proteinuria, is common in patients with drug-induced ATIN also, as well as in NSAID-induced AN. In the study by Pusey et al. [78] for instance, a consistent finding on EM was partial foot process fusion; and in the present study this was seen also in five of the eight patients with AN, whose biopsy was studied by EM.
Thus, it is not self-evident that drug-associated ATIN and NSAID nephropathy are different entities with different mechanisms; both diseases may be effects of immunologic reactions against the offending drug. But why had some patients heavy proteinuria?
A toxic effect on the podocyte is unlikely because in other patients, NSAID treatment is not followed by proteinuria; on the contrary, NSAIDs have been used to ameliorate proteinuria in the nephrotic syndrome [80]. Neither could immune complex formation be responsible, at least not in patients with MC and focal sclerosis. Such formation may participate in MGN, but proteinuria was not associated with amount of glomerular deposits, and patients with MGN had less proteinuria and also a milder course than patients with MC. Hence, a humoral mechanism of proteinuria seems likely, as suggested by others also [26]. Cells of the immune system are said to produce one or more intrinsic, humoral factors, probably lymphokines, which change the permeability of the podocyte [81], a mechanism considered likely in childhood MC [82].
That an allergic reaction is responsible is supported by the absence of major clinical, laboratory or pathologic differences between drug-induced ATIN and NSAID-induced AN. The differences between drug-induced ATIN and NSAID nephropathy with heavy proteinuria may be quantitative rather than qualitative. In drug-associated ATIN and in NSAID-associated AN the immune system reacts abruptly; the patient seeks medical advice at an early stage of the disease where most features of hypersensitivity are still present. Treatment with the offending drug is therefore discontinued early in the course before a more substantial production of lymphokines has started. In patients with heavy proteinuria the symptoms appeared after months or years. The torpid course may allow the acute allergic symptoms to abate and give more time for the lymphokines to induce podocyte disturbances and thus pronounced proteinuria.
A crucial question remains: if NSAID nephropathy is due to hypersensitivity, why are the reactions much slower and milder after NSAIDs than after other drugs? The answer may be that the offending NSAID holds back the reactions it has started itself. NSAIDs are thought to exert their effect by suppressing a variety of inflammatory mediators, many of which participate in anaphylactic and cell-mediated hypersensitivity [83, 84]. As a consequence, the very reactions that are responsible for the tissue damage in NSAID nephropathy may be weakened or delayed by the same drug that started the reactions.
Most likely, the formation of immune complexes in NSAID nephropathy is secondary to the increased permeability to macromolecules and does not necessarily contribute to the renal damage. More complexes may be formed the longer time the increased permeability persists and more easily in patients with a hyperreactive or dysregulatory immune system. That proteinuria was associated with treatment time, but not with amount of glomerular deposits, although the two latter were associated with each other, also indicates that lymphokine production is more important for the creation of proteinuria than the formation of immune complexes. In accordance, a large number of clinical and experimental observations have shown that even pronounced MGN may appear without inducing proteinuria or renal failure [85].
Most important in the management of NSAID nephropathy is to discontinue treatment with the offending drug. A worsening of renal function was seen in all patients who continued NSAID treatment, and with few exceptions all patients improved after discontinuation of the drug, in most cases with total remission.
Conclusions
The nephritides seen after treatment with NSAID are not necessarily separate entities distinctly demarcated from each other and with separate mechanisms, but rather a continuous spectrum of renal responses to a state of hypersensitivity against the drug. The clinical, laboratory and pathologic variations are most likely determined by the type and strength of the immunologic response and the length and magnitude of the drug exposure. The milder and more protracted course in NSAID-induced nephropathy compared with drug-induced ATIN may be due to a delay of the immunologic reactions induced by the offending NSAID itself causing the immune system to work ineffectively and in slow-motion.
Acknowledgments
I thank Drs R. Cahen, F. Ducret, K. Farrington, J. Gilly, A. J. Howie, D. Pagniez, and F. Schillinger for providing supplementary data.
Tables with more comprehensive information can be acquired from the author.
References
- 1.Dunn MJ, Zambraski EJ. Renal effects of drugs that inhibit prostaglandin synthesis. Kidney Int. 1980;18:609–622. doi: 10.1038/ki.1980.179. [DOI] [PubMed] [Google Scholar]
- 2.Garella S, Matarese RA. Renal effects of prostaglandins and clinical adverse effects of nonsteroidal anti-inflammatory agents. Medicine. 1984;63:165–181. doi: 10.1097/00005792-198405000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal antiinflammatory drugs. N Engl J Med. 1984;310:563–572. doi: 10.1056/NEJM198403013100905. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael J, Shankel SW. Effects of nonsteroidal anti-inflammatory drugs on prostaglandins and renal function. Am J Med. 1985;78:992–1000. doi: 10.1016/0002-9343(85)90223-2. [DOI] [PubMed] [Google Scholar]
- 5.Pirson Y, van Ypersele de Strihou C. Renal side effects of nonsteroidal antiinflammatory drugs: Clinical relevance. Am J Kidney Dis. 1986;8:338–344. doi: 10.1016/s0272-6386(86)80108-1. [DOI] [PubMed] [Google Scholar]
- 6.Abraham PA, Keane WF. Glomerular and interstitial disease induced by nonsteroidal anti-inflammatory drugs. Am J Nephrol. 1984;4:1–6. doi: 10.1159/000166764. [DOI] [PubMed] [Google Scholar]
- 7.Porile JL, Bakris GL, Garella S. Acute interstitial nephritis with glomerulopathy due to nonsteroidal anti-inflammatory agents: a review of its clinical spectrum and effects of steroid therapy. J Clin Pharmacol. 1990;30:468–475. doi: 10.1002/j.1552-4604.1990.tb03487.x. [DOI] [PubMed] [Google Scholar]
- 8.Radford MG, Holley KE, Grande JP, et al. Reversible membranous nephropathy associated with the use of nonsteroidal anti-inflammatory drugs. JAMA. 1996;276:466–469. [PubMed] [Google Scholar]
- 9.Adams DH, Howie AJ, Michael J, McConkey B, Bacon PA, Adu D. Non-steroidal anti-inflammatory drugs and renal failure. Lancet. 1986;i:57–60. doi: 10.1016/s0140-6736(86)90714-2. [DOI] [PubMed] [Google Scholar]
- 10.Artinano M, Etheridge WB, Stroehlein KB, Barcenas CG. Progression of minimal-change glomerulopathy to focal glomerulosclerosis in a patient with fenoprofen nephropathy. Am J Nephrol. 1986;6:353–357. doi: 10.1159/000167189. [DOI] [PubMed] [Google Scholar]
- 11.Bander SJ. Reversible renal failure and nephrotic syndrome without interstitial nephritis from zomepirac. Am J Kidney Dis. 1985;6:236. doi: 10.1016/s0272-6386(85)80178-5. [DOI] [PubMed] [Google Scholar]
- 12.Bender WL, Whelton A, Beschorner WE, Darwish MO, Hall-Craggs M, Solez K. Interstitial nephritis, proteinuria, and renal failure caused by nonsteroidal anti-inflammatory drugs. Am J Med. 1984;76:1006–1012. doi: 10.1016/0002-9343(84)90849-0. [DOI] [PubMed] [Google Scholar]
- 13.Billings RA, Burry HC, Emslie FS, Kerr GD. Vasculitis with alclofenac therapy. Br Med J. 1974;4:263–265. doi: 10.1136/bmj.4.5939.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brezin JH, Katz SM, Schwartz AB, Chinitz JL. Reversible renal failure and nephrotic syndrome associated with nonsteroidal anti-inflammatory drugs. N Engl J Med. 1979;301:1271–1273. doi: 10.1056/NEJM197912063012306. [DOI] [PubMed] [Google Scholar]
- 15.Brunner K, Burger HR, Greminger P, Streuli R. Mefenaminsäure (Ponstan)-induzierte akute interstitielle Nephritis mit reversiblem, schwerem, nicht-oligurischem Nierenversagen. Schweiz Med Wschr. 1985;115:1730–1734. [PubMed] [Google Scholar]
- 16.Cahen R, Trolliet P, Francçois B, Chazot C. Fenoprofen-induced membranous glomerulonephritis. Nephrol Dial Transplant. 1988;3:705–706. doi: 10.1093/oxfordjournals.ndt.a091734. [DOI] [PubMed] [Google Scholar]
- 17.Campistol JM, Galofre J, Botey A, Torras A, Revert L. Reversible membranous nephritis associated with diclofenac. Nephrol Dial Transpl. 1989;4:393–395. doi: 10.1093/oxfordjournals.ndt.a091897. [DOI] [PubMed] [Google Scholar]
- 18.Cartwright KC, Trotter TL, Cohen ML. Naproxen nephrotoxicity. Arizona Med. 1979;36:124–126. [PubMed] [Google Scholar]
- 19.Chatterjee GP. Nephrotic syndrome induced by tolmetin. JAMA. 1981;246:1589. [PubMed] [Google Scholar]
- 20.Champion de Crespigny PJ, Becker GJ, Ihle BU, Walter NMA, Wright CA, Kincaid-Smith P. Renal failure and nephrotic syndrome associated with sulindac. Clin Nephrol. 1988;30:52–55. [PubMed] [Google Scholar]
- 21.Curt GA, Kaldany A, Whitley LG, et al. Reversible rapidly progressive renal failure with nephrotic syndrome due to fenoprofen calcium. Ann Intern Med. 1980;92:72–73. doi: 10.7326/0003-4819-92-1-72. [DOI] [PubMed] [Google Scholar]
- 22.Dhar SK, Yum M. Acute tubular necrosis and minimal-change glomerulopathy associated with fenoprofen therapy. Indiana Med. 1984;77:956–957. [PubMed] [Google Scholar]
- 23.Ducret F, Pointet P, Pichot C. Glomérulonéphrite extra-membraneuse. Toxicité probable du diclofénac au cours d’une polyarthrite rhumatoïde. Néphrologie. 1980;1:143–144. [PubMed] [Google Scholar]
- 24.Estenne M, Naeije R, Ketelbant-Balasse P, Gausset P, Demanet JC. Toxicite aigue de la glafenine (glifanan) Acta Clin Belg. 1979;34:208–213. doi: 10.1080/22953337.1979.11718688. [DOI] [PubMed] [Google Scholar]
- 25.Feinfeld DA, Olesnicky L, Pirani CL, Appel GB. Nephrotic syndrome associated with use of the nonsteroidal anti-inflammatory drugs. Case report and review of the literature. Nephron. 1984;37:174–179. doi: 10.1159/000183239. [DOI] [PubMed] [Google Scholar]
- 26.Finkelstein A, Fraley DS, Stachura I, et al. Fenoprofen nephropathy: lipoid nephrosis and interstitial nephritis. A possible T-lymphocyte disorder. Am J Med. 1982;72:81–87. doi: 10.1016/0002-9343(82)90591-5. [DOI] [PubMed] [Google Scholar]
- 27.Gary NE, Dodelson R, Eisinger RP. Indomethacin-associated acute renal failure. Am J Med. 1980;69:135–136. doi: 10.1016/0002-9343(80)90511-2. [DOI] [PubMed] [Google Scholar]
- 28.Green J, Yoffe B, Barzilai D, Better OS. Reversible acute interstitial nephritis associated with indomethacin. Israel J Med Sci. 1985;21:142–145. [PubMed] [Google Scholar]
- 29.Greenstone M, Hartley B, Gabriel R, Bevan G. Acute nephrotic syndrome with reversible renal failure after phenylbutazone. Br Med J. 1981;282:950–951. doi: 10.1136/bmj.282.6268.950-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffiths ML. End-stage renal failure caused by regular use of anti-inflammatory analgesic medication for minor sports injuries. S Afr Med J. 1992;81:377–378. [PubMed] [Google Scholar]
- 31.Hamilton DV, Pryor JS, Cardoe N. Fenclofenac-induced nephrotic syndrome. Br Med J. 1979;3:391. doi: 10.1136/bmj.2.6186.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handa SP. Renal effects of fenoprofen. Ann Intern Med. 1980;93:508. doi: 10.7326/0003-4819-93-3-508. [DOI] [PubMed] [Google Scholar]
- 33.Honkanen E, Törnroth T, Pettersson E, Skrifvars B. Membranous glomerulonephritis in rheumatoid arthritis not related to gold or D-penicillamine therapy: a report of four cases and review of the literature. Clin Nephrol. 1987;27:87–93. [PubMed] [Google Scholar]
- 34.Hurault de Ligny B, Faure G, Béné M, Kessler M, Huriet C. Glomérulonéphrite extra-membraneuse au cours du traitement d′une polyarthrite rhumatoïde par diclofénac. Néphrologie. 1984;5:135–136. [PubMed] [Google Scholar]
- 35.Katz SM, Capaldo R, Everts EA, DiGregorio JG. Tolmetin. Association with reversible renal failure and acute interstitial nephritis. JAMA. 1981;246:243–245. doi: 10.1001/jama.246.3.243. [DOI] [PubMed] [Google Scholar]
- 36.Kaufhold J, Wilkowski M, McCabe K. Flurbiprofen-associated acute tubulointerstitial nephritis. Am J Nephrol. 1991;11:144–146. doi: 10.1159/000168291. [DOI] [PubMed] [Google Scholar]
- 37.Kuhlmann U, Fontana A, Briner J, Steinemann U, Siegenthaler W. Akute interstitielle Nephritis mit oligurischem Nierenversagen nach Phenylbutazon-Medikation. Schweiz Med Wschr. 1978;108:494–499. [PubMed] [Google Scholar]
- 38.Lang P, Santelli G, Benarbia S, Moritz S, Charpentier B, Fries D. Etude immunopathologique d’une néphropathie interstitielle aiguë induite par la clométacine. Presse Med. 1986;15:915–918. [PubMed] [Google Scholar]
- 39.Lantz B, Cochat P, Bouchet JL, Fischbach M. Short-term niflumic-acid-induced acute renal failure in children. Nephrol Dial Transpl. 1994;9:1234–1239. [PubMed] [Google Scholar]
- 40.Laxer RM, Silverman ED, Williamson Balfe J, Poucell S, Baumal R. Naproxen-associated renal failure in a child with arthritis and inflammatory bowel disease. Pediatrics. 1987;80:904–908. [PubMed] [Google Scholar]
- 41.Ling BN, Bourke E, Campbell WG, Delaney VB. Naproxen-induced nephropathy in systemic lupus erythematosus. Nephron. 1990;54:249–255. doi: 10.1159/000185864. [DOI] [PubMed] [Google Scholar]
- 42.Lofgren RP, Nelson AE, Ehlers SM. Fenoprofen-induced acute interstitial nephritis presenting with nephrotic syndrome. Minn Med. 1981;64:287–290. [PubMed] [Google Scholar]
- 43.Lomvardias S, Pinn VW, Wadhwa ML, Koshy KM, Heller M. Nephrotic syndrome associated with sulindac. N Engl J Med. 1981;304:424. doi: 10.1056/NEJM198102123040715. [DOI] [PubMed] [Google Scholar]
- 44.Lorch J, Lefavour G, Davidson H, Cortell S. Renal effects of fenoprofen. Ann Intern Med. 1980;93:508. doi: 10.7326/0003-4819-93-3-508. [DOI] [PubMed] [Google Scholar]
- 45.Lourie SH, Denman SJ, Schroeder ET. Association of renal papillary necrosis and ankylosing spondylitis. Arthrit Rheum. 1977;20:917–921. doi: 10.1002/art.1780200403. [DOI] [PubMed] [Google Scholar]
- 46.Marasco WA, Gikas PW, Azziz-Baumgartner R, Hyzy R, Eldredge CJ, Stross J. Ibuprofen-associated renal dysfunction. Pathophysiologic mechanisms of acute renal failure, hyperkalemia, tubular nbecrosis, and proteinuria. Arch Int Med. 1987;147:2107–2116. doi: 10.1001/archinte.147.12.2107. [DOI] [PubMed] [Google Scholar]
- 47.McLeish KR, Senitzer D, Gohara AF. Acute interstitial nephritis in a patient with aspirin hypersensitivity. Clin Immunol Immunopathol. 1979;14:64–69. doi: 10.1016/0090-1229(79)90126-0. [DOI] [PubMed] [Google Scholar]
- 48.Mease PJ, Ellsworth AJ, Killen PD, Willkens RF. Zomepirac, interstitial nephritis, and renal failure. Ann Intern Med. 1982;97:454. doi: 10.7326/0003-4819-97-3-454_1. [DOI] [PubMed] [Google Scholar]
- 49.Mitnick PD, Klein WJ. Piroxicam-induced renal disease. Arch Intern Med. 1984;144:63–64. [PubMed] [Google Scholar]
- 50.Morgenstern SJ, Bruns FJ, Fraley DS, Kirsch M, Borochovitz D. Ibuprofen-associated lipoid nephrosis without interstitial nephritis. Am J Kidney Dis. 1989;14:50–52. doi: 10.1016/s0272-6386(89)80093-9. [DOI] [PubMed] [Google Scholar]
- 51.Mourad G, Mimran A, Baldet P, Barjon P. Insuffisance rénale aiguë réversible et syndrome néphrotique induits par le fénoprofène. Néphrologie. 1982;3:65–68. [PubMed] [Google Scholar]
- 52.Noël C, Dracon M, Dhondt JL, et al. Néphrite interstitielle aiguë avec uvéite chez l’adulte: à propos de trois observations. Néphrologie. 1986;5:195–197. [PubMed] [Google Scholar]
- 53.O′Callaghan CA, Andrews PA, Ogg CS. Renal disease and use of topical non-steroidal anti-inflammatory drugs. Br Med J. 1994;308:110–111. doi: 10.1136/bmj.308.6921.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pagniez D, Gosset D, Hardouin P, Noël C, Delvallez L, Dequiedt P. Évolution vers la hyalinose segmentaire et focale d’un syndrome néphrotique á lésions glomérulaires minimes chez une patiente traitée par le sulindac. Nephrologie. 1988;9:90–91. [PubMed] [Google Scholar]
- 55.Pascoe MD, Gordon GD, Temple-Camp CRE. Tolmetin-induced acute renal failure. A case report. S Afr Med J. 1986;70:232–233. [PubMed] [Google Scholar]
- 56.Ray PE, Rigolizzo D, Wara DR, Piel CF. Naproxen nephrotoxicity in a 2-year-old child. Am J Dis Child. 1988;142:524–525. doi: 10.1001/archpedi.1988.02150050062032. [DOI] [PubMed] [Google Scholar]
- 57.Reeves WB, Foley RJ, Weinman EJ. Nephrotoxicity from nonsteroidal anti-inflammatory drugs. Southern Med J. 1985;78:318–321. doi: 10.1097/00007611-198503000-00021. [DOI] [PubMed] [Google Scholar]
- 58.Robinson J, Malleson P, Lirenman D, Carter J. Nephrotic syndrome associated with nonsteroidal anti-inflammatory drug use in two children. Pediatrics. 1990;85:844–847. [PubMed] [Google Scholar]
- 59.Schillinger F, Montagnac R, Milcent T. Glomérulonéphrite extra-membraneuse réversible secondaire à un traitement par diclofénac. Sem Hôp Paris. 1987;63:1831–1832. [Google Scholar]
- 60.Schwarz A, Krause PH, Keller F, Offermann G, Mihatsch MJ. Granulomatous interstitial nephritis after nonsteroidal anti-inflammatory drugs. Am J Nephrol. 1988;8:410–416. doi: 10.1159/000167627. [DOI] [PubMed] [Google Scholar]
- 61.Sennesael J, van den Houte K, Verbeelen D. Reversible membranous glomerulonephritis associated with ketoprofen. Clin Nephrol. 1986;26:213–215. [PubMed] [Google Scholar]
- 62.Stachura I, Jayakumar S, Bourke E. T and B lymphocyte subsets in fenoprofen nephropathy. Am J Med. 1983;75:9–16. doi: 10.1016/0002-9343(83)91161-0. [DOI] [PubMed] [Google Scholar]
- 63.Tattersall J, Greenwood R, Farrington K. Membranous nephropathy associated with diclofenac. Postgrad Med J. 1992;68:392–393. doi: 10.1136/pgmj.68.799.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tietjen DP. Recurrence and specificity of nephrotic syndrome due to tolmetin. Am J Med. 1989;87:354–355. doi: 10.1016/s0002-9343(89)80167-6. [DOI] [PubMed] [Google Scholar]
- 65.Tolins JP, Seel P. Ibuprofen-induced interstitial nephritis and the nephrotic syndrome. Minn Med. 1987;70:509–511. [PubMed] [Google Scholar]
- 66.Turner GAL, Walker RJ, Bailey RR, Lynn KL, Swainson CP. Sulindac-induced acute interstitial nephritis. N Z Med J. 1984;97:239–240. [PubMed] [Google Scholar]
- 67.Vallès M, Tovar JL. Salsalate and minimal-change nephrotic syndrome. Ann Int Med. 1987;107:116. doi: 10.7326/0003-4819-107-1-116_2. [DOI] [PubMed] [Google Scholar]
- 68.Venning V, Dixon AJ, Oliver DO. Mefenamic acid nephropathy. Lancet. 1980;ii:745–746. [Google Scholar]
- 69.Warren GV, Korbet SM, Schwartz MM, Lewis EJ. Minimal change glomerulonephropathy associated with nonsteroidal antiinflammatory drugs. Am J Kidney Dis. 1989;13:127–130. doi: 10.1016/s0272-6386(89)80130-1. [DOI] [PubMed] [Google Scholar]
- 70.Wasser WG, Goldstein MH, Barba F, Churg J. Persistent renal failure following administration of naproxen. Mount Sinai J Med. 1982;49:127–129. [PubMed] [Google Scholar]
- 71.Wendland ML, Wagoner RD, Holley KE. Renal failure associated with fenoprofen. Mayo Clin Proc. 1980;55:103–107. [PubMed] [Google Scholar]
- 72.Whelton A, Bender W, Vaghaiwalla F, Hall-Craggs M, Solez K. Sulindac and renal impairment. JAMA. 1983;249:2892–2893. [PubMed] [Google Scholar]
- 73.Wolters J, van Breda Vriesman PJC. Minimal change nephropathy and interstitial nephritis associated with diclofenac. Neth J Med. 1985;28:311–314. [PubMed] [Google Scholar]
- 74.Rennke HG, Roos PC, Wall SG. Drug-induced interstitial nephritis with heavy glomerular proteinuria. N Engl J Med. 1980;302:691–692. [PubMed] [Google Scholar]
- 75.Neugarten J, Gallo GR, Baldwin DS. Rifampin-induced nephrotic syndrome and acute interstitial nephritis. Am J Nephrol. 1983;3:38–42. doi: 10.1159/000166685. [DOI] [PubMed] [Google Scholar]
- 76.Averbuch SD, Austin HA, Sherwin SA, Antonovych T, Bunn PA, Longo DL. Acute interstitial nephritis with the nephrotic syndrome following recombinant leucocyte A interferon therapy for mycosis fungoides. N Engl J Med. 1984;310:32–35. doi: 10.1056/NEJM198401053100107. [DOI] [PubMed] [Google Scholar]
- 77.Linton AL, Clark WF, Driedger AA, Turnbull DI, Lindsay RM. Acute interstitial nephritis due to drugs. Review of the literature with a report of nine cases. Ann Int Med. 1980;93:735–741. doi: 10.7326/0003-4819-93-5-735. [DOI] [PubMed] [Google Scholar]
- 78.Pusey CD, Saltissi D, Bloodworth L, Rainford DJ, Christie JL. Drug associated acute interstitial nephritis: clinical and pathologic features and the response to high dose steroid therapy. Q J Med. 1983;52:194–211. [PubMed] [Google Scholar]
- 79.Galpin JE, Shinaberger JH, Stanley TM, et al. Acute interstitial nephritis due to methicillin. Am J Med. 1978;65:756–765. doi: 10.1016/0002-9343(78)90793-3. [DOI] [PubMed] [Google Scholar]
- 80.Tiggeler RGWL, Hulme B, Wijdeveld PGAB. Effect of indomethacin on glomerular permeability in the nephrotic syndrome. Kidney Int. 1979;16:312–321. doi: 10.1038/ki.1979.133. [DOI] [PubMed] [Google Scholar]
- 81.Sewell RF, Short CD. Minimal-change nephropathy: how does the immune system affect the glomerulus? Nephrol Dial Transplant. 1993;8:108–112. [PubMed] [Google Scholar]
- 82.Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 1991;40:453–460. doi: 10.1038/ki.1991.232. [DOI] [PubMed] [Google Scholar]
- 83.Abramson S, Edelson H, Kaplan H, Ludewig R, Weissmann G. Inhibition of neutrophil activation by nonsteroidal anti-inflammatory drugs. Am J Med. 1984;77:3–6. doi: 10.1016/s0002-9343(84)80085-6. [DOI] [PubMed] [Google Scholar]
- 84.Goodwin JS. Immunologic effects of nonsteroidal anti-inflammatory drugs. Am J Med. 1984;77:7–15. doi: 10.1016/s0002-9343(84)80086-8. [DOI] [PubMed] [Google Scholar]
- 85.Ravnskov U. The subepithelial formation of immune complexes in membranous glomerulonephritis may be harmless and secondary to toxic or allergic factors. Scand J Immunol. 1998;48:469–474. [PubMed] [Google Scholar]


