The Randomised Aldosterone Evaluation Study (RALES) was recently terminated prematurely because it demonstrated that spironolactone was superior to placebo at reducing death in patients with moderate to severe chronic heart failure who were already being treated with standard therapy including an ACE inhibitor. This is a very important finding as it should directly influence the clinical management of patients with moderate to severe CHF. Its other major impact is that it should be possible to use the RALES results to provide important mechanistic clues as to why the CHF disease process progresses towards death. This article will therefore address the question of what are the possible mechanisms whereby spironolactone improves mortality in CHF?
Diuretic effect
Before postulating complex mechanisms, it must be remembered that spironolactone is a very effective diuretic and it is possible that spironolactone acted merely by improving natriuresis so that cardiac preload and afterload were reduced. Furthermore, spironolactone could have done so without producing the neuroendocrine activation that an equivalent dose of frusemide would produce i.e. the benefit of spironolactone could simply be that it is a diuretic which uniquely also causes neuroendocrine inhibition rather than the neuroendocrine activation seen with frusemide. Indeed, its profile of action is very reminiscent of the profile of action of natriuretic peptides i.e. diuresis plus neuroendocrine inhibition. It is also reminiscent of our current optimum treatment for CHF i.e. frusemide plus ACE inhibition to produce neuroendocrine inhibition. In that sense, spironolactone may cause more of the same in terms of more diuresis and more inhibition of the renin angiotensin aldosterone system than is got from traditional frusemide/ACE inhibitor therapy.
Aldosterone escape
However, the above may be an oversimplification and it is quite likely that aldosterone exerts pharmacological effects which have not received as much prominence as they should have done.
Indeed, the traditional view is that it is angiotensin II which is the principal culprit in the renin angiotensin aldosterone system (RAAS). However, it is now appreciated that aldosterone is a second culprit and that the harmful effects of aldosterone are additional to the harmful effects of angiotensin II.
This is important because the chronic suppressive effects of ACE inhibitors on aldosterone levels in heart failure are weak, variable and unsustained. Indeed we have recently shown that both aldosterone and plasma angiotensin II are elevated in many CHF patients who are being treated with chronic ACE inhibitor therapy [1]. In our patients, 40% had an elevated aldosterone while only 15% had an elevated plasma AII level. The important clinical point is that there is plenty of residual aldosterone around for this to be a potential problem even in the presence of an ACE inhibitor.
What therefore are the harmful effects of aldosterone in CHF? Interestingly, nearly all of the harmful effects of angiotensin II with which we are familiar also occur with aldosterone.
Magnesium/potassium loss
Firstly, aldosterone independently causes magnesium loss from the body by increasing urine magnesium output. The increase in serum magnesium after an ACE inhibitor in CHF is of the order of only 2% while spironolactone produces a 13% increase [2].
This importance of retaining magnesium can be illustrated by the fact that magnesium replacement by either the intravenous route or by oral supplementation causes a fall in ventricular arrhythmias in CHF [3]. In addition, potassium supplementation can produce a dramatic reduction in cardiovascular events, even suggesting perhaps, that atherosclerotic events may be reduced by increasing total body K/Mg levels [4]. The data on what Mg does in post MI patients is not relevant to CHF patients because the latter ingest chronic diuretic therapy which depletes the body of Mg while the former do not.
Sympathetic activation
The second harmful effect of aldosterone relates to its ability to potentiate the effects of catecholamines. One mechanism contributing to this is that aldosterone blocks catecholamine uptake in tissues. Corticosterone is the classical inhibitor of noradrenaline uptake2 and since the chemical structure of corticosterone and aldosterone are similar, it is not too surprising that aldosterone acts similarly to block the uptake2 process.
In a recent animal experiment, we found that aldosterone did indeed block noradrenaline uptake in the heart in vivo [2]. We went on to show that spironolactone increased myocardial noradrenaline uptake in patients with CHF, as shown by mIBG scanning [2]. It is tempting to speculate even further and suggest that because catecholamines are arrhythmogenic, their potentiation by aldosterone may increase arrhythmias and hasten death.
Parasympathetic/baroreflex inhibition
The third harmful effect of aldosterone is that it may also reduce parasympathetic activity. The evidence for this is threefold. Firstly, in a very elegant series of animal studies, Wang et al. (1992) showed conclusively that aldosterone not only directly reduces baroreceptor discharge from the carotid sinus but also that aldosterone reduces the heart rate response to changes in blood pressure [5]. These effects were seen with both acute and chronic administration of aldosterone [6]. Secondly, we found confirmatory evidence in man in that aldosterone halved the reflex bradycardic response to an equivalent pressor stimuli, an effect which was not due to changes in sympathetic activity or angiotensin II [7]. Thirdly in our own study of CHF patients spironolactone reduced heart rate and increased heart rate variability despite also reducing blood pressure [8]. Our observation that spironolactone increased heart rate variability in CHF patients is strong evidence for aldosterone having parasympatholytic effects [8].
Any aldosterone induced reduction in parasympathetic activity could contribute to cardiac death. The evidence linking parasympathetic activity with survival is compelling. For example, in myocardial ischaemia, vagal stimulation reduced the frequency of reperfusion induced VF from 60% to 7% while abolishing VT altogether [10]. Also in myocardial ischaemia, vagal stimulation increased survival from 12% to 57% [9]. Indeed, for all cardioactive drugs studied so far the effect of these drugs on survival is paralleled by the effect of those same drugs on the parasympathetic component of heart rate variability [11].
Malignant autonomic profile of aldosterone
In view of the above, it can be seen that aldosterone has a particularly malignant profile of action as far as the autonomic nervous system is concerned. Not only does aldosterone increase cardiac sympathetic activity but it also decreases parasympathetic activity. This is a particularly poignant since the parasympathetic nervous system is thought to oppose the arrhythmogenic effects of the sympathetic nervous system. Indeed, the recent UK Heart trial has shown clearly that autonomic dysfunction as measured by heart rate variability as here is a strong independent predictor of mortality in heart failure [12].
Myocardial fibrosis
The fourth adverse effect of aldosterone is its ability to stimulate fibrosis in the myocardium. This is one of the adverse effects of aldosterone which appears to be attributable not only to aldosterone but also to angiotensin II. Nevertheless a separate effect of aldosterone does appear to exist [13]. Brilla et al showed that aldosterone induces biventricular fibrosis in the rat and that myocardial fibrosis could be prevented by spironolactone at a dose which was too low to alter the blood pressure itself [14].
Studying myocardial fibrosis in man is difficult but the idea has recently been proposed that plasma levels of procollagen type III amino terminal peptide (PIIINP) may be a useful index of myocardial collagen turnover [15, 16]. Interestingly, we recently found that spironolactone reduced PIIINP levels in CHF patients, which is the first clinical evidence that aldosterone promotes myocardial collagen formation [8].
From the above, it is increasingly likely that aldosterone causes patchy myocardial fibrosis, which could lower the threshold for malignant ventricular arrhythmias in CHF.
Induction of ventricular arrhythmias
Aldosterone could induce ventricular arrhythmias by a combination of the effects described above: magnesium depletion, sympathetic activation, parasympathetic inhibition, myocardial ischaemia and myocardial fibrosis. Indeed, it would be hard to imagine a more pro-arrhythmogenic profile of action for any substance. The most striking data with regard to aldosterone and arrhythmias come from Arora & Somari, who performed coronary artery ligation in dogs and then studied the ventricular arrhythmias produced when adrenaline or aldosterone was infused [17]. Adrenaline was arrhythmogenic as expected, but aldosterone displayed an even morestriking, prolonged arrhythmogenic effect that followed a distinct dose-response relationship.
Aldosterone and QT dispersion
Interestingly, the above effects of aldosterone produce the kind of cardiac abnormalities which are thought to underlie QT dispersion (Figure 2). The reason why this is important is because there are a lot of data to suggest that QT dispersion is a marker for those who are at high risk of cardiac death [18–22]. The cardiac abnormalities associated with QT dispersion are ischaemia, LV dilatation/dysfunction, autonomic abnormalities, low K/Mg and myocardial fibrosis i.e. all except ischaemia are common to both QT dispersion and aldosterone. To confirm this, we recently found that spironolactone markedly reduced QT dispersion in patients with CHF [23[.
Figure 2.
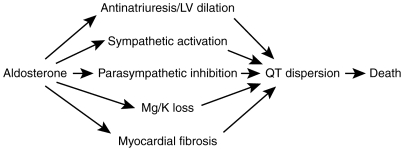
Relationship between aldosterone and QT dispersion and cardiac death.
Figure 1.
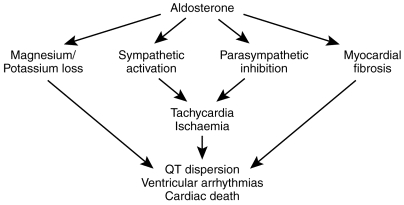
Potentially adverse effects of aldosterone in chronic heart failure.
Diurnal profile of aldosterone (Figure 3)
Figure 3.
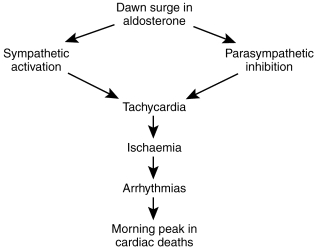
Hypothesis concerning dawn surge in aldosterone and early morning cardiac death.
It must also be remembered that aldosterone, like cortisol, displays a marked diurnal pattern [24]. Aldosterone peaks during the early morning even while lying supine in bed.
What could be the consequences of such a dawn surge in aldosterone? Interestingly it is well known that adverse cardiovascular events also display a marked diurnal profile with a breakfast time peak in their frequency. Sudden cardiac death and myocardial infarction are both commoner at this time of day [25]. The reason for this diurnal profile is unknown but an increase in sympathetic activity is thought to contribute, since β-adrenoceptor blockers reduce the morning peak of cardiovascular events. Since aldosterone itself increases sympathetic activity, we have hypothesised that the dawn increase in aldosterone could contribute to the morning peak of cardiovascular events by way of increasing sympathetic activity inhibiting parasympathetic activity, increasing heart rate and hence ischaemia. In fact, we have been able to confirm part of this hypothesis by showing that spironolactone does reduce the normal 06.00–10.00 h increase in heart rate in CHF patients [8]. More recently in unpublished data, we found that spironolactone increased parasympathetic activity and decreased sympathetic activity in CHF patients in the 06.00–10.00 h time period [23].
β-adrenoceptor blocker therapy
Three recent trials show clearly that β-adrenoceptor blockade reduces mortality in CHF (Carvedilol programme, CIBIS 2, MERIT). In the RALES trial very few patients were on beta blockade. Progress in CHF research is so rapid that the results of trials are often inevitably out of date once they are published. Whether spironolactone will still exert the same mortality benefit in the presence of β-adrenoceptor blockers is unknown although the effects of spironolactone on Mg/K, on the parasympathetic and on fibrosis should persist even in the presence of β-adrenoceptor blockade which gives us hope that the effect of spironolactone will be additive to that of β-adrenoceptor blockade.
Conclusion
Despite ACE inhibitor therapy, a substantial amount of residual aldosterone exists in patients with CHF. Potentially harmful effects of this residual aldosterone include magnesium loss, sympathetic activation, parasympathetic inhibition, myocardial ischaemia, myocardial fibrosis and the production of ventricular arrhythmias. We have now produced clinical data to show that aldosterone does exert all of these harmful effects, even in ACEI treated patients [2, 8]. The autonomic effects of aldosterone appear to develop in the short term and with a notable circadian pattern while the effects of aldosterone on myocardial fibrosis develop over a longer term. All of these effects either alone or together could contribute to the reduced mortality seen in the RALES study when spironolactone was added to standard diuretic/ACE inhibitor in CHF.
References
- 1.Struthers AD. Aldosterone escape during ACE inhibitor therapy in chronic heart failure. J Cardiac Failure. 2:47–54. doi: 10.1016/s1071-9164(96)80009-1. [DOI] [PubMed] [Google Scholar]
- 2.Barr CS, Lang CC, Hanson J, Arnott M, Kennedy N, Struthers AD. Effects of adding spironolactone to an ACE inhibitor in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1995;76:1259–1265. doi: 10.1016/s0002-9149(99)80353-1. [DOI] [PubMed] [Google Scholar]
- 3.Bashir Y, Sneddon JF, Staunton A, et al. Effects of oral magnesium chloride replacement in CHF secondary to coronary artery disease. Am J Cardiol. 1993;72:1156–1162. doi: 10.1016/0002-9149(93)90986-m. [DOI] [PubMed] [Google Scholar]
- 4.Ascherio A, Rimm EB, Hernan MA, et al. Intake of potassium, magnesium, calcium and fiber and risk of stroke among US men. Circulation. 1998;98:1198–1204. doi: 10.1161/01.cir.98.12.1198. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, McClaim JM, Zucker IH. Aldosterone reduces baroreceptor discharge in the dog. Hypertension. 1992;19:270–277. doi: 10.1161/01.hyp.19.3.270. [DOI] [PubMed] [Google Scholar]
- 6.Wang W. Chronic administration of aldosterone depresses baroreceptor reflex in the dog. Hypertension. 1994;24:571–575. doi: 10.1161/01.hyp.24.5.571. [DOI] [PubMed] [Google Scholar]
- 7.Yee KM, Struthers AD. Aldosterone blunts the baroreflex response in man. Clin Sci. 1998;95:687–692. doi: 10.1042/cs0950687. [DOI] [PubMed] [Google Scholar]
- 8.MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res. 1997;35:30–34. doi: 10.1016/s0008-6363(97)00091-6. [DOI] [PubMed] [Google Scholar]
- 9.Myers RW, Pearlman AS, Hayman RM, et al. Beneficial effects of vagal stimulation and bradycardia during experimental acute myocardial ischaemia. Circulation. 1974;49:943–947. doi: 10.1161/01.cir.49.5.943. [DOI] [PubMed] [Google Scholar]
- 10.Zuanetti G, Ferrari GM, Proiri SG, Schwartz PJ. Protective effect of vagal stimulation on reperfusion arrhythmias in cats. Circulation Res. 1987;61:429–435. doi: 10.1161/01.res.61.3.429. [DOI] [PubMed] [Google Scholar]
- 11.Tuininga YS, van Veldhuisen DJ, Brouwer J, et al. Heart rate variability in LV dysfunction and heart failure: effects and implications of drug treatment. Br Heart J. 1994;72:509–513. doi: 10.1136/hrt.72.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolan J, Batin PD, Andrew R, et al. Prospective Study of Heart Rate Variability and Mortality in Chronic Heart Failure. Results of the UK Heart Failure Evaulation and Assessment of Risk Trial UK Heart. Circulation. 1998;98:1510–1516. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- 13.Brilla CG, Matsubara LS, Weber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronisin. J Mol Cell Cardiol. 1993;25:563–575. doi: 10.1006/jmcc.1993.1066. [DOI] [PubMed] [Google Scholar]
- 14.Brilla CG, Matsubara LS, Weber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronisin. J Mol Cell Cardiol. 1993;25:563–575. doi: 10.1006/jmcc.1993.1066. [DOI] [PubMed] [Google Scholar]
- 15.Klappacher G, Franzen P, Haab D, et al. Measuring extracellular matrix tyrnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol. 1995;75:913–918. doi: 10.1016/s0002-9149(99)80686-9. [DOI] [PubMed] [Google Scholar]
- 16.Diez J, Laviades C, Mayor G, Gil MJ, Monreal I. Increased serum concentrations of procollagen peptides in essential hypertension. Circulation. 1995;91:1450–56. doi: 10.1161/01.cir.91.5.1450. [DOI] [PubMed] [Google Scholar]
- 17.Arora RB, Somari P. Ectopic arrhythmia provoking action of aldosterone. Life Sci. 1962;5:215–218. doi: 10.1016/0024-3205(62)90021-8. [DOI] [PubMed] [Google Scholar]
- 18.Fu GS, Meissner A, Simon R. Repolarisation dispersion and sudden cardiac death inpatients with left ventricular dysfunction. Eur Heart J. 1997;18:281–289. doi: 10.1093/oxfordjournals.eurheartj.a015232. [DOI] [PubMed] [Google Scholar]
- 19.Barr CS, Naas A, Freeman M, Lang CC, Struthers AD. QT dispersion and sudden unexpected death in CHF. Lancet. 1994;343:327–329. doi: 10.1016/s0140-6736(94)91164-9. [DOI] [PubMed] [Google Scholar]
- 20.Naas A, Davidson NC, Thompson C, et al. QT and QTc dispersion are accurate predictors of cardiac death in newly diagnosed NIDDM patients. Br Med J. 1998;316:745–746. doi: 10.1136/bmj.316.7133.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choy AM, Lang CC, Chomsky DM, Rayos GH, Wilson JR, Roden DM. Normalisation of acquired QT prologation in humans by intravenous potassium. Circulation. 1997;96:2149–2154. doi: 10.1161/01.cir.96.7.2149. [DOI] [PubMed] [Google Scholar]
- 22.Darbar D, Luck J, Davidson NC, et al. Sensitivity and specificity of QTc dispersion for identification of risk of cardiac death in patients with PVD. Br Med J. 1996;312:874–879. doi: 10.1136/bmj.312.7035.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yee KM, Pringle SD, Struthers AD. Aldosterone blockade improves QT dispersion in CHF. Eur Heart J. 1998;19(suppl: 601) (abstract) [Google Scholar]
- 24.Armbruster H, Vetter W, Beckerhoff R, Nussberger J, Vetter H, Siegenthaler W. Diurnal variations of plasma aldosterone in supine man: relationship to plasma renin activity and plasma cortisol. Acta Endocrinolgica. 1975;80:95–103. doi: 10.1530/acta.0.0800095. [DOI] [PubMed] [Google Scholar]
- 25.Muller JE, Stone PG, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]


