Abstract
Aims
We examined the effect of cooling on the response to the endothelium-dependent and -independent dilators, acetylcholine (ACh) and sodium nitroprusside (SNP), respectively, in human microvessels in vitro, and compared the responses between Raynaud’s disease (RD) patients and controls, in order to assess the pathogenic role of the endothelium in RD.
Methods
Subcutaneous resistance arteries were dissected from gluteal fat biopsies taken from patients with RD (n=18) and from age-and sex-matched control subjects (n=17). Vessels were cannulated in a small vessel arteriograph, in which a pressure of 50 mmHg was maintained across the vessel wall. Cumulative concentration-response curves for ACh (10−10–10−4 m) and SNP (10−10–10−3 m) were generated in vessels at either 37° C or 24° C, with endothelium intact for ACh and removed for SNP (n=6 per group).
Results
Neither dilator showed significant differences in sensitivity when comparing responses between vessels from RD patients and controls, at either temperature, but the maximal relaxation to ACh was depressed in vessels from RD patients compared with controls at 37° C (Emax=45±13 in RD vs 89±4 in controls; P=0.004).
Conclusions
These results support the hypothesis that impaired endothelium-dependent vasodilatation is involved in the pathophysiology of RD.
Keywords: cooling, endothelium, human, nitric oxide, Raynaud’s disease, resistance arteries
Introduction
Raynaud’s disease (RD) affects up to 20% of the population and is characterized by intense vasospasm of the extremities, particularly the digits, in response to cold exposure or marked emotion. It causes numbness and pallor, followed by cyanosis, and can produce extreme local discomfort on reperfusion [1]. Despite its first description over 130 years ago [2], the pathophysiology of primary RD remains unresolved. Interestingly, there is a higher incidence of RD in patients with migraine and/or variant angina [3–5], suggesting that RD may be part of a generalized vascular disorder. Thus, investigation into the pathogenesis of RD may clarify the mechanisms underlying these other vasospastic conditions, and point to new directions for the development of drug therapy for vasospasm.
The vascular endothelium generates several vasoactive substances, including the potent vasoconstrictor endothelin-1 (ET-1) [6], and the vasodilator nitric oxide (NO) [7, 8]. A temperature-dependent disorder of the endothelium, involving either underproduction of NO or overproduction of ET-1, might be the critical factor underlying the pathogenesis of the prolonged cold-induced vasospasm of RD. Results from clinical studies examining vasodilatation in RD are conflicting. In dorsal hand veins, endothelium-dependent dilatation to bradykinin is impaired in RD patients [9], and this finding is supported by a study in digital arteries using acetylcholine [10]. Conversely, enhanced fingertip and forearm blood flow responses to the endothelium-dependent dilator methacholine were observed in patients with RD compared with control subjects [11]. However, responses to methacholine are not affected by inhibitors of the NO synthase enzyme [12], implying that methacholine elicits dilatation through an NO-independent mechanism, which perhaps explains the discrepancy between the above studies.
In the present study we investigated the pathogenic role of the vascular endothelium in RD by examining responses to the endothelium-dependent and -independent dilators, acetylcholine (ACh), a stimulator of NO synthase, and sodium nitroprusside (SNP), an NO donor, respectively, in subcutaneous resistance arteries isolated from gluteal fat biopsies taken from RD patients and age- and sex-matched control subjects. As mentioned above, RD may be part of a generalized vasospastic disorder. Thus, we hypothesize that any defect present in the digital circulation will be manifest in arteries taken from the gluteal region. Further support for the use of such arteries comes from a study which demonstrates that endothelial dysfunction is not confined to sites affected by RD [9]. RD is primarily an arterial condition but in this study impaired endothelium-dependent dilatation was present in dorsal hand veins. In the present study vessels were examined at both 37° C and 24° C, temperatures which represent body temperature and moderate cooling, respectively [13]. The effect of cooling on the response to ACh and SNP is an important factor which, until now, has not been addressed in the vessels of patients with RD.
Methods
The protocol of this study was approved by the Lothian Research Ethics Committee, and written, witnessed, informed consent was obtained from each subject. Seventeen control subjects (29–64 years; 14 females and 3 males) and 18 RD patients (28–60 years; 16 females and 2 males) were recruited to this study [Table 1]. Patients with primary RD were classified according to the criteria of Allen & Brown [14]: (i) colour changes occurring bilaterally in the hands evoked by cold or emotional stimuli; (ii) absence of gangrene; (iii) absence of any known causative disease; and (iv) history of symptoms for a minimum of 2 years. The mean duration of the RD was 18±3 years (mean±s.e. mean). One patient had Raynaud’s phenomenon associated with Sjögren’s syndrome. All subjects had an alcohol intake <14 (females) and <21 (males) units per week, and were normotensive (systolic blood pressure <140 mmHg; diastolic blood pressure <90 mmHg). Three control subjects, and one RD patient, were smokers. Except for one control subject and one RD patient who were taking hormone replacement therapy, none of the volunteers was receiving any medication at the time of the study, and all were in general good health. All subjects abstained from aspirin-containing drugs for 1 week, and from caffeine-containing drinks or alcohol for 12 h before the biopsy was taken. A 50 ml blood sample was taken from each subject for immunological screening and for the measurement of haemoglobin, erythrocyte sedimentation rate, urea and electrolytes, glucose and creatinine.
Table 1.
Subject and patient details, and baseline data for vessels studied at 37° C and 24 deg; C.
Skin biopsies, ≈2 cm long, 0.75 cm wide and 0.75 cm deep, were taken from the gluteal region under local anaesthesia (1% lignocaine, Astra Pharmaceuticals Ltd, UK) [15] and were placed directly into physiological salt solution (PSS) (mm: 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3, 0.026 EDTA, and 5.5 glucose). Small arteries with a mean luminal diameter of 323±92 μm (n=48) were excised under a dissecting microscope and cannulated in a perfusion myograph (Living Systems Instrumentation Inc., Burlington, VT, USA). Usually, the biopsy yielded more than one artery. If there were two arteries, the first was mounted in the myograph, whilst the second was kept in a refrigerator overnight to use the following day. Although this does not appear to have any adverse effects on the functional responses of the vessel [16], the order of experiments performed on the arteries was randomised to prevent any influence of overnight storage on the results. A pressure servo unit maintained intraluminal pressure, without flow, at 50 mmHg. Luminal diameter was measured using a video dimension analyser. After a 60–90 min equilibration period, during which the vessel chamber was superfused with PSS, continuously gassed with 95% O2/5% CO2, and the temperature raised to 37° C, the contractility of the microvessels was assessed separately using noradrenaline 10−5 m (NA) and potassium chloride 60 mm (KCl). Acetylcholine 10−6 m (ACh) was given during maximal contraction to NA to assess endothelial integrity. Removal of the endothelium was achieved by passing air through the lumen of the vessel [17] and confirmed by the loss of ACh-induced relaxation during constriction to NA. Cooling was achieved by passing the superfusate through a rapid heat-exchange Peltier element (Moor Instruments Ltd, Millwey, Axminster, Devon, UK) before it entered the arteriograph. Cumulative concentration-response curves to the endothelium-dependent dilator ACh (10−10–10−4 m) and the endothelium-independent dilator sodium nitroprusside (SNP; 10−10–10−3 m) were generated in the presence of NA (10−5 m) in intact and denuded vessels, respectively, at either 37° C or 24° C (n=6 for each group).
Compounds
Acetylcholine HCl, noradrenaline HCl, potassium chloride and sodium nitroprusside dihydrate were obtained from Sigma Chemical Co., UK. All drugs were dissolved in PSS.
Data analysis
Responses are expressed as percentage (%) relaxation towards resting lumen diameter in vessels preconstricted with NA (10−5 m). EC50 values (the concentration producing 50% relaxation) were calculated from individual log concentration-response curves via Graphpad Prism 2.1. EC50 and Emax (the maximal dilatation relative to resting lumen diameter) values are shown as mean±s.e. mean, and n=the number of vessels studied. One-way analysis of variance (anova) was performed to compare the responses between the 37° C and 24° C groups, and the control and RD groups, followed by Bonferroni posthoc tests if significant. The nonparametric Kruskal–Wallis one-way anova on ranks test was performed when the variances of the groups differed significantly. Statistical significance was accepted at P<0.05.
Results
The mean arterial pressure in control subjects and patients with RD did not differ significantly (92±2 mmHg for controls vs 94±2 mmHg for RD; P=0.48). Haemoglobin and erythrocyte sedimentation rate (ESR) were normal for each group, there being no significant difference in values between the groups (haemoglobin: 128±3 g l−1for controls vs131±3 g l−1 for RD; P=0.47; ESR: 5±1 mm h−1 controls vs 5±1 mm h−1 for RD: P=0.94). Normal urea, electrolytes, glucose and creatinine were confirmed in all volunteers. The results from the immunological screening showed fluorescence antinuclear antibodies (FANA) were positive (titre greater than 40) in one control subject and four RD patients: two of the RD patients had a low titre (>40 but<160), one had a moderate titre (>160 but<640), all with speckled pattern, and the control subject and one RD patient had a high titre (>640; rheumatoid factor was present in the control subject and anticentromere antibody in the RD patient).
Mean resting lumen diameter, % contraction to KCl, and percentage relaxation to ACh (for intact artery studies) did not differ significantly between the control subjects when studied at 37° C and 24° C, and the RD patient group at 37° C and 24° C (Table 1). The addition of ACh (10−6 m) did not result in an increase in diameter beyond that of the resting lumen diameter in any of the groups, suggesting myogenic tone was not present in any of the vessels studied.
Responses to acetylcholine
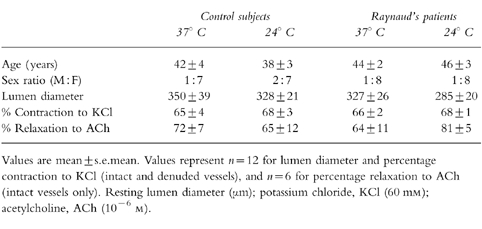
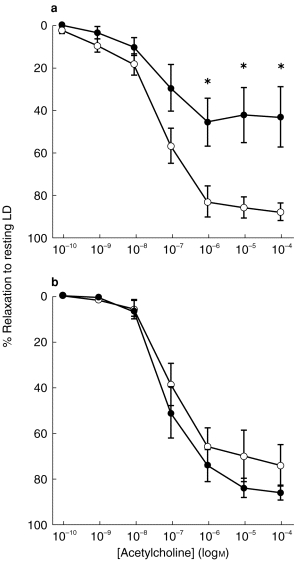
ACh produced a concentration-dependent relaxation in all vessels (Figure 1). Cooling had no significant effect on the sensitivity to ACh in arteries from control and RD patients (P=0.10, Kruskal–Wallis; Table 2), although there was a marked enhancement in the maximal relaxation attainable at 24° C in RD vessels (Emax=45±13 at 37° C vs 86±3 at 24° C in RD; P=0.006, anova-Bonferroni; Figure 3).
Figure 1.
Comparison between the relaxant response to ACh in subcutaneous resistance arteries, isolated from control subjects and RD patients preconstricted with NA (10−5 m): concentration-relaxation curves to ACh (expressed as percentage relaxation towards resting lumen diameter (LD)) at (a) 37° C and (b) at 24° C in vessels from control subjects (○; n=6) and RD patients (•; n=6). All values are mean±s.e.mean. *P<0.05 (Kruskal–Wallis).
Table 2.
EC50 values for acetylcholine (ACh) and sodium nitroprusside (SNP) concentration-response curves in human resistance arteries obtained from gluteal biopsies.

Figure 3.
Maximum relaxation (expressed as a percentage of the resting lumen diameter) in subcutaneous resistance arteries isolated from control subjects (open bars; n=6) and RD patients (hatched bars; n=6) preconstricted with NA (10−5 m) to (a) acetylcholine and (b) sodium nitroprusside at 37° C and 24° C. All values are mean±s.e.mean. **P<0.01 (one-way anova; Bonferroni).
Comparison of the relaxant responses to ACh obtained in arteries from control subjects with those from RD patients showed that sensitivity did not differ (P=0.10, Kruskal–Wallis; Table 2 and Figure 1) but the maximal relaxation was significantly depressed in RD patients at 37° C (Emax=89±4 in controls vs 45±13 in RD; P=0.004, anova-Bonferroni; Figure 1a and Figure 3). At 24° C relaxation to ACh was not significantly different between controls and RD patients (P=0.26, anova-Bonferroni; Figure 1b, Figure 3 and Table 2).
Responses to sodium nitroprusside
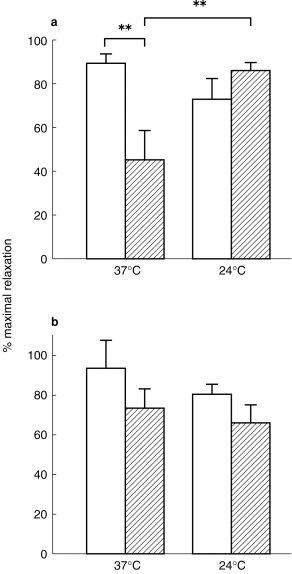
SNP produced a concentration-dependent relaxation in all arteries (Figure 2). Neither sensitivity nor maximal relaxation to SNP differed significantly after cooling in either control or RD vessels (P=0.13, Kruskal–Wallis for EC50; Table 2; P=0.45, anova for Emax; Figure 3).
Figure 2.
Comparison between the relaxant response to SNP in subcutaneous resistance arteries, isolated from control subjects and RD patients preconstricted with NA (10−5 m): concentration-relaxation curves to SNP (expressed as percentage relaxation towards resting lumen diameter (LD)) at (a) 37° C and (b) at 24° C in vessels from control subjects (○; n=6) and RD patients (•; n=6). All values are mean±s.e.mean.
Although there were no statistically significant differences in the relaxation to SNP between arteries from RD patients and controls, at 37° C there was trend towards a rightward-shift in the concentration-relaxation curve generated in RD vessels (Figure 2a). The maximal relaxation to SNP tended to be reduced in vessels from RD patients compared with that in controls at 37° C, but this failed to reach statistical significance (Emax=95±14 in controls vs 74±10 in RD, P=0.45, anova; Figure 3). When calculating the power of the study to detect differences between patients and controls it was found that the power of the performed test (with alpha)=0.05 for both EC50 (Kruskal–Wallis) and Emax (anova), hence the negative findings should be interpreted with caution.
Discussion
In the present study, using arteries obtained from gluteal fat biopsies, cooling was found to have no significant effect on the sensitivity to the endothelium-dependent vasodilator, ACh, in either vessels from RD patients or controls, however, the maximal relaxation to ACh was attenuated in RD arteries at 37° C compared with 24° C, but not in control arteries. When comparing responses to ACh between arteries from RD patients and control subjects, there was a significantly reduced maximal relaxation attainable in RD vs controls. This difference was only apparent at 37° C and not during cooling. Sensitivity to ACh was not significantly different between the groups. Neither sensitivity nor maximal relaxation to the endothelium-independent vasodilator, SNP, was significantly affected by cooling in control and RD vessels, nor when comparing responses between the RD and control groups. The results from recent clinical studies examining endothelium-dependent dilatation in dorsal hand-veins [9] and digital arteries [10] of patients with RD are in agreement with the present data, in that the cutaneous resistance arteries of patients with RD show impaired responsiveness to an endothelium-dependent dilator at 37° C, without a significant difference in endothelium-independent dilatation and, thus, support the hypothesis that gluteal subcutaneous resistance arteries manifest a defect present in the digital vascular bed.
The present finding that endothelium-dependent relaxation was impaired at 37° C in RD vessels implies there is a dysfunction of the endothelium in RD. Unexpectedly, however, no such impairment was found during cooling. Relaxation to SNP was similar at both temperatures, suggesting that smooth muscle sensitivity to NO is not temperature-dependent in RD patients and that any dysfunction in the NO pathway lies at a site proximal to the smooth muscle. The finding that endothelium-dependent dilator activity is relatively depressed at 37° C in RD, but is normal at 24° C would appear contradictory in the context of cold-induced vasospasm, but can perhaps be explained by the fact that we studied agonist-stimulated generation of endothelium-dependent vasodilators, which may not necessarily reflect basal production in vivo. There is evidence to suggest that cooling increases the affinity of a wide range of agonists for their receptors. This may reflect changes in the fluidity of the cell membrane which reveal binding sites on the receptor surface more easily [18]. In addition, increased formation of high affinity receptor-G-protein complexes, which are sensitive to temperature, has been shown to occur for a number of receptor types, including muscarinic receptors [19]. Studies using canine saphenous arteries and veins support such an increase in the affinity of muscarinic receptors during cooling, since both the constrictor [20] and dilator responses [21] to ACh were found to be augmented by cooling. Perhaps, then, a depressed response to the endothelium-dependent dilator at 24° C is being masked by a concomitant increase in muscarinic receptor number and/or affinity, though the lack of potentiation of responses in control vessels during cooling would argue against such an explanation.
Despite there being a tendency for RD arteries to have a reduced relaxant response to the endothelium-independent dilator, SNP, at 37° C compared with controls, this did not reach statistical significance. Interestingly, in their study of dorsal hand veins, Bedarida et al. [9] reported that the EC50 values for SNP were highly variable in the RD group. The same is true of the present data and, as mentioned in the results section, the power of our study is likely to be insufficient to detect small differences between the groups. Thus, we cannot exclude the possibility that a decreased smooth muscle sensitivity to NO exists in RD patients. The fact that we were able to detect a significant attenuation of the relaxation induced by the endothelium-dependent dilator ACh, but not to the NO donor SNP, is suggestive that the response to ACh is not mediated solely through NO release. The generation of additional vasodilators, such as prostacyclin (PGI2) [22] and endothelium-derived hyperpolarising factor (EDHF) [23] may contribute to the response. Indeed, Deng and colleagues [24] found only 30% of the relaxation response to ACh in subcutaneous resistance arteries isolated from gluteal biopsies, as used in the present study, could be blocked by inhibition of NOS, with a significant proportion of the remaining response being mediated by EDHF. The mediators of the response to ACh would appear to depend upon the age of the subject from whom the resistance artery is taken because the NOS inhibitor NG-nitro-l-arginine (l-NOARG) was shown to completely abolish the ACh-induced relaxation in gluteal resistance arteries taken from patients of the mean age range 68–70 years [25], indicating little or no contribution from other dilator factors in these vessels. In the study by Deng et al. [24] the mean age range of the subjects was 38–45 years, which is very similar to that in the present study (38–46 years). One would therefore anticipate that a similar profile of mediators of the ACh-induced relaxation would be found for the vessels used in the present study, i.e. about a third being due to NO. Interestingly, vasodilatation to ACh has been shown to be less dependent on NO in both the finger skin microcirculation and the forearm vasculature in patients with RD compared with controls [26]. These results are further corroborated by a recent study by Khan and coworkers [27] who reported that the attenuated vasodilator responses to ACh in the cutaneous digital circulation were not improved by oral supplementation of l-arginine, the substrate for NOS. The authors concluded that the reduced vasodilatation in RD is unlikely to be due to an impairment in the l-arginine/NO pathway, but it could also be because this pathway has little involvement in the response to ACh in RD. Further experiments could be carried out using antagonists and inhibitors of the NO, EDHF, and PGI2 pathways, to elucidate mediators of the ACh response, both at 37° C and during cooling, in resistance arteries isolated from patients with RD.
The attenuated vasodilatation to ACh found in arteries from RD patients at 37° C is thought to represent an impaired responsiveness to endothelium-dependent vasodilator factors. An alternative explanation would be that there is an increase in vascular smooth muscle responsiveness to ACh which counteracts the dilator response directly, through muscarinic receptor-mediated contraction or indirectly, through the release of vasoconstrictor prostanoids. However, this would seem unlikely given that there was no contraction observed at higher doses of ACh (10−6–10−4 m), merely a blunting of the relaxation-response curve. To confirm this, experiments would need to be repeated in endothelial-denuded vessels, and in the presence of the cyclooxygenase inhibitor, indomethacin.
Impaired endothelium-dependent dilatation in RD patients is likely to lead to an enhanced response to vasoconstrictor factors such as ET-1 through a direct reduction in counteracting relaxation and also as a result of a decrease in the inhibition of ET-1 synthesis [28]. In recent parallel studies in our laboratory examining the role of ET-1 in the pathogenesis of RD, using arteries from the same group of patients and controls as in this study, we found that neither the responsiveness of gluteal resistance arteries to ET-1, nor plasma levels of ET-1, differed significantly between RD patients and control subjects [29]. We concluded that ET-1 was unlikely to play a primary pathogenic role in RD, but may be involved in mediating vasospasm secondary to impaired endothelium-dependent vasodilatation, as demonstrated in the present report. Under physiological conditions, cold-induced vasoconstriction is likely to be balanced by local vasodilator mechanisms, whereas, in the pathophysiological setting of RD, our results suggest a dysfunctional endothelium-dependent dilator system leads to vasospasm, the prolonged nature of which favours the involvement of a sustained vasoconstrictor such as ET-1. Clearly, the mechanism(s) involved in the impaired cholinergic dilator response in RD requires further investigation.
In summary, the results obtained at 37° C support the hypothesis that vascular endothelial function is abnormal in RD, in that resistance arteries from RD patients displayed an attenuated response to the endothelium-dependent dilator, ACh, compared with control vessels. Whilst the involvement of abnormal vascular smooth muscle responsiveness cannot be excluded, these results implicate the vascular endothelium in the pathophysiology of RD.
Acknowledgments
This work was supported by the British Heart Foundation and the Scottish Home and Health Department. PJWS was the recipient of an AJ Clark Studentship from the British Pharmacological Society and is currently a Wellcome Trust International Prize Travelling Research Fellow.
References
- 1.Dowd P. Raynaud’s phenomenon. Lancet. 1995;346:283–290. [PubMed] [Google Scholar]
- 2.Raynaud M. In: De l’asphyxie locale et de la gangrene symetrique des extremities. Leclerc L, editor. These de Paris: Library Editor; 1862. [Google Scholar]
- 3.Miller D, Waters DD, Warnica W, Stachcic J, Kreeft J, Theroux P. Is variant angina the coronary manifestation of a generalized vasospastic disorder? N Engl J Med. 1981;30:736–738. doi: 10.1056/NEJM198103263041306. [DOI] [PubMed] [Google Scholar]
- 4.O’Keeffe ST, Tsapatsaris NP, Beetham WP. Increased prevalence of migraine and chest pain in patients with primary Raynaud’s disease. Ann Int Med. 1992;116:985–989. doi: 10.7326/0003-4819-116-12-985. [DOI] [PubMed] [Google Scholar]
- 5.Zahavi I, Chagnac A, Mering R, Davidovich S, Kuritzky A. Prevalence of Raynaud’s phenomenon in patients with migraine. Arch Intern Med. 1984;144:742–744. [PubMed] [Google Scholar]
- 6.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;33:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 7.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle to acetylcholine. Nature. 1980;228:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 8.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 9.Bedarida GV, Kim D, Blaschke TF, Hoffman BB. Venodilation in Raynaud’s disease. Lancet. 1993;342:1451–1454. doi: 10.1016/0140-6736(93)92932-j. [DOI] [PubMed] [Google Scholar]
- 10.Singh S, De Trafford JC, Baskerville PA, Martin JF. Response of digital arteries to endothelium dependent and independent vasodilators in patients with Raynaud’s phenomenon. Eur J Clin Invest. 1995;25:182–185. doi: 10.1111/j.1365-2362.1995.tb01546.x. [DOI] [PubMed] [Google Scholar]
- 11.Khan F, Coffman JD. Enhanced cholinergic cutaneous vasodilatation in Raynaud’s phenomenon. Circulation. 1994;89:1183–1188. doi: 10.1161/01.cir.89.3.1183. [DOI] [PubMed] [Google Scholar]
- 12.Chowienczyk PJ, Cockcroft JR, Ritter JM. Differential inhibition by NG-monomethyl-l-arginine of vasodilator effects of acetylcholine and methacholine in human forearm. Br J Pharmacol. 1993;110:736–738. doi: 10.1111/j.1476-5381.1993.tb13873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monge L, Garcia-Villalon AL, Montoya JJ, Garcia JL, Gomez B, Dieguez G. Response of rabbit ear artery to endothelin-1 during cooling. Br J Pharmacol. 1991;104:609–612. doi: 10.1111/j.1476-5381.1991.tb12477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen EV, Brown GE. Raynaud’s disease: a critical review of the minimal requisites for diagnosis. Am J Med. 1932;183:187–200. [Google Scholar]
- 15.Aalkjaer C, Heagerty AM, Petersen KK, Swales JD, Mulvany MJ. Evidence for increased media thickness, increased neural amine uptake, and depressed excitation-contraction coupling in isolated resistance vessels from essential hypertensives. Circ Res. 1987;61:181–186. doi: 10.1161/01.res.61.2.181. [DOI] [PubMed] [Google Scholar]
- 16.Laing RJ, Jakubowski J, Morice AH. An in vitro study of the pharmacological responses of rat middle cerebral artery: effects of overnight storage. J Vasc Res. 1995;32:230–236. doi: 10.1159/000159097. [DOI] [PubMed] [Google Scholar]
- 17.Falloon BJ, Bund SJ, Tulip JR, Heagerty AM. In vitro perfusion studies of resistance artery function in genetic hypertension. Hypertension. 1993;22:486–495. doi: 10.1161/01.hyp.22.4.486. [DOI] [PubMed] [Google Scholar]
- 18.Vanhoutte PM. Physical factors of regulation. In: Bohr DF, Somlyo AP, Sparks HV, editors. Handbook of Physiology, Section 2, Circulation, Vascular Smooth Muscle. II. Bethesda, Maryland: Am Physiol Soc.; 1980. pp. 443–474. [Google Scholar]
- 19.Aronstam RS, Narayanan TK. Temperature effect on the detection of muscarinic receptor–G protein interactions in ligand binding assays. Biochem Pharmacol. 1988;37:1045–1049. doi: 10.1016/0006-2952(88)90508-4. [DOI] [PubMed] [Google Scholar]
- 20.Vanhoutte PM, Shepherd JT. Effect of temperature on reactivity of isolated cutaneous veins of the dog. Am J Physiol. 1970;218:187–190. doi: 10.1152/ajplegacy.1970.218.1.187. [DOI] [PubMed] [Google Scholar]
- 21.Soares de Moura R, Vanhoutte PM. Effect of cooling and warming on the vasodilator response of the isolated dog saphenous artery to acetylcholine. Brazilian J Med Biol Res. 1988;21:157–159. [PubMed] [Google Scholar]
- 22.Moncada S, Gryglewski RJ, Bunting S, et al. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–667. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- 23.Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- 24.Deng L-Y, Li J-S, Shiffrin EL. Endothelium-dependent relaxation of small arteries from essential hypertensive patients: mechanisms and comparison with normotensive subjects and with responses of vessels from spontaneously hypertensive rats. Clin Sci. 1995;88:611–622. doi: 10.1042/cs0880611. [DOI] [PubMed] [Google Scholar]
- 25.James MA, Watt PAC, Potter JF, Thurston H, Swales JD. Endothelial function in subcutaneous resistance arteries from elderly hypertensive and normotensive subjects. Clin Sci. 1997;92:139–145. doi: 10.1042/cs0920139. [DOI] [PubMed] [Google Scholar]
- 26.Gardner-Medwin JM, Cockcroft JR, Macdonald IA, Rowell RJ. Reduced nitric oxide-dependent vasodilatation in primary Raynaud’s phenomenon. Br J Clin Pharmacol. 1996;42:650P–651P. [Google Scholar]
- 27.Khan F, Litchfield SJ, McLaren M, Veale DJ, Littleford RC, Belch JJF. Oral l-arginine supplementation and cutaneous vascular responses in patients with primary Raynaud’s phenomenon. Arth Rheum. 1997;40:352–357. doi: 10.1002/art.1780400220. [DOI] [PubMed] [Google Scholar]
- 28.Boulanger C, Luscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith PJW, Ferro CJ, McQueen DS, Webb DJ. Functional studies in small arteries do not support a primary role for endothelin in the pathogenesis of Raynaud’s disease. J Cardiovasc Pharmacol. 1998;31(Suppl. 1):S473–S476. doi: 10.1097/00005344-199800001-00135. [DOI] [PubMed] [Google Scholar]


