Abstract
Aims
To estimate the incidence of newly diagnosed idiopathic stroke among users of fenfluramine, dexfenfluramine and phentermine compared to obese nonusers.
Methods
We conducted a cohort study with nested case-control analysis utilizing data from the General Practice Research Database in the UK. Eight thousand four hundred and twenty-three subjects aged 69 years or less at the start of follow-up were exposed to at least one of the three study drugs and 17 225 similarly obese subjects were not exposed to any of the study drugs.
Results
We identified 45 incident cases of idiopathic CVA in this cohort of subjects. The incidence of CVA among all current users of a diet drug was 1.3/1000 person-years (95% CI 0.5, 3.5). The incidence for current fenfluramine users (n=2) was 2.6/1000 person-years (95% CI 0.7, 9.6), for current dexfenfluramine users (n=1) 1.1/1000 person-years (95% CI 0.3, 3.8), and for current phentermine users 0/1000 person-years (95% CI 0.0, 12.9). The incidence in obese nonusers was 0.6/1000 person-years (95% CI 0.4, 0.9). The adjusted matched odds ratio (OR) for thrombotic stroke from the case-control analysis comparing current use of a diet drug to nonuse was 2.4 (95% CI 0.6, 9.1). There was only one exposed subject among seven who had haemorrhagic stroke.
Conclusions
The incidence of CVA in generally young obese subjects during use of fenfluramine, dexfenfluramine or phentermine is low. Although we found an OR of 2.4 comparing users of any of the anorexiants with nonusers, this is based on only three exposed cases and the confidence limits are wide. We conclude that our study does not support a substantial increased risk of stroke attributable to the use of fenfluramine, dexfenfluramine or phentermine.
Keywords: CVA, dexfenfluramine, fenfluramine, phentermine
Introduction
Stroke is a leading cause of morbidity and mortality [1]. Obesity has been implicated in the development of stroke, either directly or in relation to other known risk factors, such as diabetes, hypertension and hypercholesterolaemia [2]. In a study of 117 000 nurses, ischaemic and total stroke were found to be associated with both obesity and weight gain [3]. Obesity itself has been shown to be a cardiovascular health risk. More recently certain anorexiant drugs used to treat obesity have been reported to increase the risk for heart valve disorders [4–7]. It is important to know if drugs used to treat obesity directly raise the risk of stroke. A possible association of stroke with the use of phentermine has been reported in one published case report [8], and this possible adverse effect is included on the labelling in Switzerland. Given the risks associated with obesity, it is important that any formal study of anorexiants and stroke be conducted comparing users of anorexiant drugs with similarly obese subjects who did not use such drugs.
We evaluated the risk of a first time diagnosis of stroke (CVA) in users of three diet medications, fenfluramine, dexfenfluramine and phentermine compared to obese nonusers of these drugs, based on information present in the General Practice Research Database (GPRD) derived from experience in the UK.
Methods
The study was based on information derived from the General Practice Research Database (GPRD). Since 1987, over four million residents in the United Kingdom have been enrolled with selected general practitioners (GPs) who use office computers provided by Value Added Medical Products (VAMP Health) and have agreed to provide data for research purposes to the GPRD which is currently owned by the United Kingdom Department of Health. The GPs received 12 months of instruction on the standardized recording of medical information and they agreed to supply anonymized information to academic researchers on an ongoing basis. The information recorded includes patient characteristics, drugs dispensed, clinical diagnoses, notation of referrals to consultants, hospitalizations, certain historical information and other findings (e.g. smoking status, blood pressure, height and weight). Referral letters from consultants and hospitalizations are kept in a manual file and are available (anonymized) to researchers. The general practitioners generate prescriptions directly from the computer, and these are automatically transcribed into the patient’s computer record. The details of each prescription including dose, instructions and quantity are automatically recorded on computer and can be used to determine dose and duration of drug exposure. A modification of the Oxford Medical Information System (OXMIS) classification mapped to ICD codes is used to enter medical diagnoses, and a coded drug dictionary based on the Prescription Pricing Authority’s dictionary is used for the recording of prescriptions. Two large validation studies determined that information on all patient referrals and hospitalizations present in the manual medical records in the general practitioners’ offices was recorded on the computer over 90% of the time [9, 10]. More than 40 drug safety studies have been published based on the GPRD [11].
Study design
This study was conducted in two parts. First, using a matched cohort design we estimated the incidence of first-time CVAs (see Table 1) in a cohort of 8423 subjects who had ever received at least one prescription for any of the study drugs, and a cohort of 17 225 nonusers. The cohorts included subjects aged 69 years or younger at start of follow-up. The cohort of ‘nonusers’ of the study drugs was matched two to one to the user groups by age (within 2 years), sex, general practice (when possible) and a diagnosis of obesity. A random date was assigned to each person in the nonuser cohort and considered to be the ‘proxy’ exposure date. Subjects with a diagnosis of any cerebrovascular or cardiovascular disease prior to the exposure date were excluded with the exception of those who had a diagnosis of hypertension and diabetes.
Table 1.
ICD codes used to identify subjects with a diagnosis of CVA.
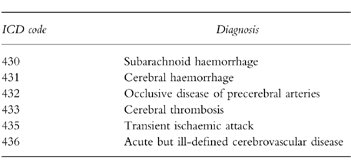
For both cohorts, we then ascertained all first-time computer-recorded CVAs occurring at some time after the date of first relevant drug exposure for the exposed cohort, and the random date for the unexposed cohort and sent for all available clinical records including referral letters and hospitalizations for subjects with a first-time CVA. These clinical records were reviewed without knowledge of exposure status to validate the diagnosis of CVA and to distinguish between thrombotic and haemorrhagic strokes. Subjects were excluded if they had a prior CVA/cerebrovascular disease recorded in the case history or if the diagnosis of CVA could not be confirmed.
In the cohort analysis, the risk for newly diagnosed ‘idiopathic’ cerebrovascular disease was calculated by dividing the number of cases by the person-time at risk for the study cohorts according to current, recent or past use of diet drugs. Current exposure was defined as a prescription within 60 days prior to the date of the CVA, recent exposure as 61–180 days, past as >180 days. Since the cohorts were matched for age, sex, general practice and obesity, we did not anticipate important confounding by these variables.
In order to more closely control for possible confounding, we conducted a nested case-control analysis wherein exposure in all cases was compared with a group of noncases derived at random from the base population of the identified cohorts. Six controls, where possible, were matched to each case by age, sex and general practice. Additional variables (body mass index, history of diabetes, hypertension, hypercholesterolaemia, smoking status) were identified from computer and controlled for with conditional logistic regression using SAS statistical programming. In the nested case-control study, we evaluated risks associated with current, recent and past use of the different types of diet drugs. Fenfluramine and dexfenfluramine were combined in this analysis because they have the same chemical formulation and effect on appetite suppression. As in the cohort analysis, current exposure was defined as a prescription within 60 days prior to the index date, recent exposure as 61–180 days, past as>180 days.
Results
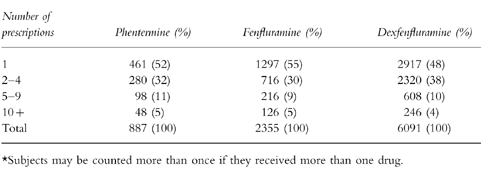
We identified 6091 subjects exposed to dexfenfluramine, 2355 exposed to fenfluramine, 887 exposed to phentermine and 17 225 unexposed obese subjects. The age and sex distribution of the three cohorts was similar. Most users of each study drug were women (dexfenfluramine 86%, fenfluramine 87%, phentermine 90%) and were aged 49 years or younger (dexfenfluramine and fenfluramine 72%, phentermine 78%). The total number of prescriptions received by exposed subjects is presented in Table 2. Ten percent of subjects who were users of diet medicines received more than one diet medication at some time but less than 0.5% received any two diet drugs simultaneously.
Table 2.
The distribution of number of study drug prescriptions*.
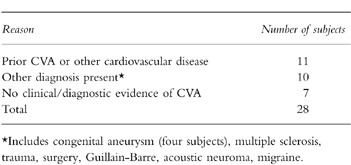
From the computer record, we identified 80 possible cases of CVA for whom we needed clinical records. We received 73 (91%) clinical records. We made decisions on case status for the seven subjects (two of whom had died from the CVA) for whom we did not receive clinical records based on review of the computer record. After review of the records, 28 were excluded (see Table 3) and an additional seven cases with a diagnosis of transient ischaemic attack (TIA), none of whom was currently exposed to a study drug were also excluded in further analyses. The remaining 45 were included as cases of first-time CVA.
Table 3.
Reasons for exclusions of potential cases of incident idiopathic CVA.
Cohort analysis
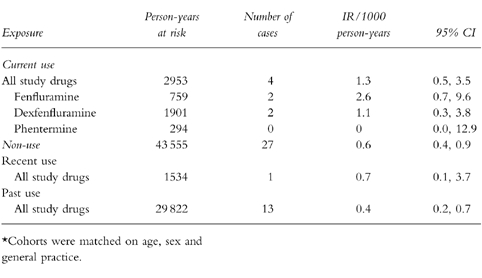
Table 4 presents the incidence rates for CVA including both thrombotic and haemorrhagic for the cohort study. There were four cases of CVA currently exposed to one of the diet drugs, yielding a crude incidence rate (IR) of 1.3/1000 person-years (95% CI 0.5, 3.5). There were 27 cases of CVA among nonusers (IR 0.6, 95% CI 0.4, 0.9), one case among recent users (IR 0.7, 95% CI 0.1, 3.7), and 13 cases among past users (IR 0.4, 95% CI 0.2, 0.7). We estimated the incidence rates for each drug separately. The incidence for current fenfluramine use was 2.6/1000 person-years (95% CI 0.7, 9.6) and for current dexfenfluramine 1.1/1000 person-years (95% CI 0.3, 3.8). The incidence rate for current phentermine use was 0/1000 person-years with a 95% upper confidence limit of 12.9.
Table 4.
Crude incidence rates* of CVA (thrombotic and haemorrhagic) according to exposure to diet medications.
Case-control analysis
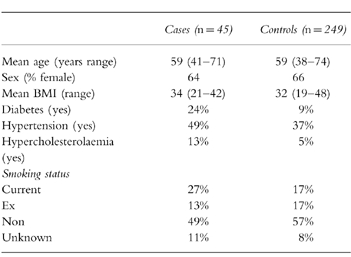
The cases and controls are described in Table 5 according to age, sex, body mass index (BMI), hypertension, smoking status, hypercholesterolaemia and diabetes. The cases were divided into types of stroke, thrombotic (n=38) or haemorrhagic (n=7). Three of the subjects with thrombotic stroke and one with haemorrhagic were currently exposed to a study drug.
Table 5.
Description of subjects in the nested case-control analysis according to case status.
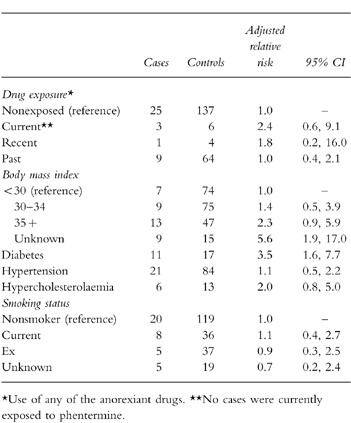
The matched odds ratio (OR) estimate for thrombotic stroke adjusted for body mass index, smoking status, hypertension, diabetes and hypercholesterolaemia comparing current users of any of the anorexiants with nonusers is 2.4 (95% CI 0.6, 9.1) (see Table 6). The OR for recent use is 1.8 (95% CI 0.2, 16.0) and for past use 1.0 (95% CI 0.4, 2.1). The adjusted risks for relevant covariates are also presented in Table 6. Subjects with a body mass index of 35 or more compared with subjects with a BMI less than 30 had an OR of 2.3 (95% CI 0.9, 5.9). Subjects with diabetes had the highest independent OR when compared with nondiabetics (RR 3.5, 95% CI 1.6, 7.7). We found a modest elevated risk of thrombotic stroke among subjects with hypertension and hypercholesterolaemia, as well as current smokers. Among subjects with haemorrhagic stroke, one was currently exposed to a study drug.
Table 6.
Results of adjusted matched case-control analysis (thrombotic stroke).
Discussion
The current study encompassed 8423 relatively young subjects who received at least one prescription for fenfluramine, dexfenfluramine or phentermine and 17 225 subjects closely similar to the users of diet medications according to age, sex, general practice and a diagnosis of obesity. Potential subjects with a known past history of myocardial infarction (MI), stroke and angina were excluded from the cohorts in order to avoid selection bias in such subjects. From the base study population, that encompassed some 75 000 person-years, we identified, after review of case records, 45 cases of first-time idiopathic stroke, 38 with thrombotic stroke and 7 with haemorrhagic stroke. This resulted in an overall incidence of CVA in this study of 0.6/1000 person-years. The incidence rate for currently exposed subjects was 1.3/1000 person-years. None of the cases was noted to have a pre-existing heart valve disorder and there was no suggestion in any of the case histories that the stroke was secondary to thrombotic emboli.
Among the 38 subjects with thrombotic stroke, three were currently exposed to a study diet medicine, one was recently exposed, 9 were exposed in the past, and 25 occurred in never users. In the nested case-control analysis the odds ratio for stroke adjusting for BMI, diabetes, hypertension, hypercholesterolaemia and smoking was 2.4 (95% CI 0.6, 9.1) comparing current users of diet medications with nonusers.
While the OR for thrombotic stroke was slightly elevated for current users of diet medicines, the estimate is based on only three exposed cases and the 95% confidence intervals are wide. Thus, the results are most consistent with the proposition that there is no strong positive association between the use of the study diet medications and thrombotic stroke, although a higher risk cannot be ruled out. Idiopathic haemorrhagic stroke was uncommon in this study population and only one of the seven cases was a ‘current user’ of a study medicine.
One of the strengths of this study is that we compared the risk of CVA in users of the anorexiant drugs with the risk of CVA in other obese subjects. Since obesity itself is associated with an increased risk of CVA, the use of a nonexposed obese comparison group enabled us to at least partially control for the background risk of CVA in the analysis.
A limitation of the study is that the outcome of CVA is relatively uncommon in this young relatively healthy population of obese subjects who did or did not use certain diet medications. Thus among the 8423 women who used diet medicines there were only three who developed a thrombotic stroke while taking the medicines. While the estimated risk for current users is slightly higher than that for nonusers or past users, the confidence intervals are very wide and therefore the point estimate of risk is unstable. Nevertheless we consider these results to be of substantive interest since no other formal studies of this issue have been published.
We conclude that there is unlikely to be a substantial increased risk of first-time stroke attributable to the use of fenfluramine, dexfenfluramine or phentermine.
Acknowledgments
We thank the participating general practitioners for their excellent cooperation and Dr Alan Dean and his team for their generous help.
The Boston Collaborative Drug Surveillance Program is supported in part by grants from: Astra AB, Bayer AG, Berlex Laboratories, Boots Healthcare International, Glaxo Wellcome Inc., Hoffmann-La Roche, RW Johnson Pharmaceutical Research Institute, Medeva PLC and Novartis Pharmaceuticals. This study was supported by Medeva PLC.
References
- 1.Bronner LL, Kanter DS, Manson JE. Primary prevention of stroke. N Engl J Med. 1995;333:1392–1400. doi: 10.1056/NEJM199511233332106. [DOI] [PubMed] [Google Scholar]
- 2.Prospective Studies Collaboration. Cholesterol, diastolic blood pressure and stroke: 13 000 strokes in 450 000 people in 45 prospective cohorts. Lancet. 1995;346:1647–1653. [PubMed] [Google Scholar]
- 3.Rexrode KM, Hennekens CH, Willett WC, et al. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA. 1997;277:1539–1545. doi: 10.1001/jama.1997.03540430051032. [DOI] [PubMed] [Google Scholar]
- 4.Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 5.Graham DJ, Green L. Further cases of valvular heart disease associated with fenfluramine-phentermine (letter) N Engl J Med. 1997;337:635. doi: 10.1056/NEJM199708283370911. [DOI] [PubMed] [Google Scholar]
- 6.Kurz X, Van Ermen A. Valvular heart disease associated with fenfluramine-phentermine (letter) N Engl J Med. 1997;337:1772–1773. [PubMed] [Google Scholar]
- 7.Rasmussen S, Corya BC, Glassman RD. Valvular heart disease associated with fenfluramine-phentermine (letter) N Engl J Med. 1997;337:1773. [PubMed] [Google Scholar]
- 8.Kokkinos J, Levine SR. Possible association of ischemic stroke with phentermine. Stroke. 1993;24:310–313. doi: 10.1161/01.str.24.2.310. [DOI] [PubMed] [Google Scholar]
- 9.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. Br Med J. 1991;302:766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jick H, Terris BZ, Derby LE, Jick SS. Further validation of information recorded on a general practitioner based computerized data resource in the United Kingdom. Pharmacoepidemiology and Drug Safety. 1992;1:347–349. [Google Scholar]
- 11.Jick H. A database worth saving (commentary) Lancet. 1997;350:1045–1046. doi: 10.1016/S0140-6736(05)70451-7. [DOI] [PubMed] [Google Scholar]


