Abstract
Aims
It has been demonstrated that inhibition of endothelium derived nitric oxide with NG-monomethyl-l-arginine (l-NMMA) results in a different cardiac and peripheral vascular response. The purpose of this study was to investigate the pharmacokinetic-pharmacodynamic profile of l-NMMA and pharmacokinetic interactions with l-arginine in healthy subjects.
Methods
Plasma pharmacokinetics were analysed from two different studies: In study 1, 3 mg kg−1 l-NMMA was administered i.v. over 5 min and systemic haemodynamics, cardiac output (CO), fundus pulsation amplitude (FPA), and NO-exhalation (exhNO) were measured at baseline and 15, 65, 95, 155, and 305 min after start of drug administration (n=7). In study 2, 17 mg kg−1 min−1 of the physiologic substrate for nitric oxide synthase, l-arginine, was coinfused i.v. over 30 min with a primed constant infusion of 50 μg kg−1 min−1 l-NMMA (n=8).
Results
Bolus infusion of l-NMMA resulted in a maximum plasma concentration of 12.9±3.4 μg ml−1 (mean±s.d.) with elimination half-life of 63.5±14.5 min and clearance of 12.2±3.5 ml min−1 kg−1 and caused a small hypertensive response, decreased CO by 13%, FPA by 26%, exhNO by 46% and increased systemic vascular resistance by 16% (P<0.05 each) 15 min after start of drug administration. Although only limited data points were available in the l-NMMA plasma concentration range between 0 and 4 μg ml−1, drug effects over time were in good agreement with an Emax model (r2>0.98 each), which also suggested that concentrations producing half-maximum effects were higher for FPA than for CO and exhNO. The coinfusion with l-arginine caused a nearly two-fold increase in plasma l-NMMA levels, indicating a pharmacokinetic interaction.
Conclusions
In the absence of a systemic hypertensive response, l-NMMA significantly decreased CO, exhNO, and FPA. The concentration calculated to produce a half maximal effect was equivalent for exhNO and CO, but markedly higher for FPA. Furthermore, measurement of FPA is susceptible to changes in l-NMMA levels at small plasma concentrations.
Keywords: l-arginine, l-NMMA, nitric oxide synthesis, pharmacodynamic, pharmacokinetic
Introduction
Nitric oxide (NO) is synthesized in the vascular endothelium from the guanidino nitrogen of l-arginine (l-Arg) by a constitutive isoform of NO synthase (NOS) [1]. The introduction of NOS inhibitors such as NG-monomethyl-l-arginine (l-NMMA) has enabled the investigation of the physiological role of NO in the regulation of blood flow and blood pressure in animals [2–4] and subsequent experiments have demonstrated the contribution of the NOS system to systemic and regional blood flow in humans [5–7].
The investigation of the physiologic role of NO is limited by the fact that specific inhibitors of the different NOS isoforms are currently not available. Although the key pharmacokinetics of l-NMMA have been studied previously [8], the time course of hypertensive responses to intravenous l-NMMA is inconsistent and its exact pharmacodynamic profile has not been elucidated yet. We have therefore analysed the pharmacokinetic-pharmacodynamic effects of l-NMMA from samples obtained from healthy subjects, in which haemodynamic responses have been reported recently [9]. The relationship of plasma levels of l-NMMA with changes in cardiac output, pulsatile choroidal blood flow, and exhaled NO was calculated. Furthermore, the effect of the physiological precursor and structural antagonist l-Arg on l-NMMA plasma levels in steady state was studied from a different study cohort to assess pharmacokinetic interactions of l-arginine analogs [10].
Methods
Subjects
The study protocols were approved by the Ethics Committee of Vienna University School of Medicine. All volunteers consented to participate in the study after full explanation of what was involved. Sixteen healthy males between 21 and 31 years were studied, eight volunteers participated in study 1 (mean±s.d.: 25±3 years) and eight in study 2 (26±3 years). Each subject passed a screening examination, including physical examination and medical history, haematological status, clinical chemistry, hepatitis and HIV-serology, and urine analysis, to determine health status. Further inclusion criteria were normal ophthalmic findings and ametropy <3 dioptres. Subjects were excluded if any abnormality was found as part of the pretreatment screening unless the investigator considered an abnormality to be clinically irrelevant.
Study protocol
Study 1
After an overnight fast, all subjects rested for at least 20 min in a sitting position to establish a stable baseline. Following baseline measurements of blood pressure, pulse rate, cardiac output (CO), fundus pulsation amplitude (FPA), and exhaled NO (exhNO), 3 mg kg−1 l-NMMA (Clinalfa, Läufelfingen, Switzerland) were administered i.v. over 5 min. Measurements of pharmacodynamic parameters were performed 15, 65, 95, 155, and 305 min after start of l-NMMA infusion. Plasma samples for pharmacokinetic analyses were drawn at baseline and at 2, 4, 5, 6, 8, 10, 15, 25, 35, 50, 65, 95, 125, 155, 185, 245, 305, 365, and 485 min. Altogether, 57 ml blood were taken from each subject.
Study 2
Each subject received a primed constant infusion of l-NMMA with a loading dose of 3 mg kg−1 over an infusion period of 5 min, and a subsequent continuous infusion of 50 μg kg−1 min−1 over 120 min. A coinfusion of l-arginine (Clinalfa; dose: 17 mg kg−1 min−1, infusion period 30 min) was started 65 min after the beginning of l-NMMA infusion. Blood pressure, heart rate, and exhaled NO were measured at regular intervals. Plasma samples were drawn at baseline and at 5, 20, 35, 50, 65, 80, 95, and 125 min.
Methods of evaluation
Noninvasive measurement of systemic haemodynamics
Mean arterial blood pressure (MAP) was measured on the upper arm by an automated oscillometric device and pulse rate was recorded from a finger pulse-oximeter (HP CMS patient monitor, Hewlett Packard, Palo Alto, CA, USA). The reproducibility and sensitivity of this equipment has been reported previously [11].
Doppler ultrasonic measurement of cardiac output
Cardiac output (CO) was assessed by Doppler ultrasound at the aortic valve using a 3.25-MHz probe (CFM 750, Vingmed Sound, Horten, Norway). Cardiac output was calculated by cross sectional area measurement of the aortic valve and determination of the velocity distribution profile. This method has been shown to be primarily suitable for measuring cardiac output changes within a subject rather than comparing absolute values of cardiac output between different subjects [12]. The systemic vascular resistance was calculated as SVR=MAP/CO.
Laserinterferometric measurement of fundus pulsations
The method is based on the interference of the reflected beams from the cornea and retina when the eye is illuminated with coherent light. Using a single mode laser diode the distance changes between cornea and retina during the cardiac cycle can be assessed with high accuracy [13]. These changes depend on the pulsatile inflow of arterial blood into the choroidal vasculature of the eye [14, 15]. The maximum distance change between cornea and retina during the cardiac cycle is called fundus pulsation amplitude (FPA).
Measurement of exhaled NO
Exhaled nitric oxide was measured with a chemiluminescence detector (Nitrogen oxides analyser 8840, Monitor Labs Inc., USA). The instrument was calibrated with certified gases (300 p.p.b. NO in N2, AGA, Vienna, Austria). Subjects were instructed to fully inflate their lungs, hold their breath for 10 s, and exhale for 10 s under nasal occlusion into a Teflon tube [16, 17]. 1000 ml min−1 of the exhaled air was allowed to enter the inlet port. Three consecutive readings were made at each measurement point. The end-expiratory values from the strip recorder readings were used for analysis to assure that inspired NO from the ambient air did not distort the results.
Quantification of l-NMMA plasma levels
Plasma l-NMMA and l-arginine were determined by reversed phase (RP) high performance liquid chromatography (h.p.l.c.) and fluorescence detection [18]. Plasma was diluted with β-(2-thienyl)-(±)-alanine as internal standard and deproteinized with 5-sulfosalicylic acid. Following derivatization with o-phthalaldehyde and 2-mercaptoethanol in borate buffer, the isoindole derivatives were separated on C-18 RP-h.p.l.c. and detected at 330/440 nm [8]. For construction of a calibration curve, plasma samples were spiked with l-NMMA. Detection range was linear from 0.4 to 6 μg ml−1 with a coefficient of correlation of the standard curve of >0.997 and a detection limit of 0.02 μg ml−1. Between-day and within-day variability of l-NMMA standards was <5%.
Pharmacokinetic-pharmacodynamic analysis
Pharmacokinetics
The plasma concentrations of l-NMMA were subjected to noncompartmental analysis. The area under the plasma concentration vs time curve (AUC) was calculated using the trapezoidal rule. The area under the first moment curve (AUMC) was determined using a plot of plasma concentration times time (C·t) vs time and calculation of its area under the curve calculated by the trapezoidal rule. The elimination half-life (t1/2) was calculated from 0.693/λz where λz is the negative slope of the terminal linear phase of a plot of ln Cvs time. The initial volume of distribution (Vc) after the infusion was calculated as D/C0, where C0 is the initial concentration immediately after the infusion. The volume of distribution at steady state (Vdss) was determined as the product of total body clearance and mean residence time (CL. MRT).
Pharmacodynamics
The pharmacodynamic endpoints observed were exhaled nitric oxide, cardiac output and FPA. The mean drug effect over time was calculated as percentage reduction from their respective baseline values recorded at time t=0. The pharmacodynamic model used was the Emax model (eqn 1), in which the effect (E) and plasma concentration (C) were related as
 |
1 |
where Emax is the maximum percentage reduction from the baseline value and EC50 is the concentration that causes 50% of Emax. Emax refers to the intrinsic activity of the drug, EC50 to its potency. The evaluation of goodness of fit was done by the respective model selection criteria (MSC) and correlation coefficient values using the software Scientist (MicroMath, Salt Lake City, UT, USA). The Model Selection Criterion (MSC) is a parameter that allows comparison of the goodness of a fit of the same data set to different models and relates the coefficient of determination to the number of parameters that are required to obtain the fit. When comparing two models with different numbers of parameters, this criterion places a burden on the model with more parameters to not only have a better coefficient of determination, but quantifies how much better it must be for the model to be deemed more appropriate. The MSC has been normalized so that it is independent of the scaling of the data points. Furthermore, the most appropriate model will be that with largest MSC. Usually, MSC values of more than 2–3 are considered good.
Statistical analysis
Statistical analysis was done with the CSS Statistica® software package (StatSoft Inc., Tulsa, OK, USA). Drug concentrations and changes in haemodynamic parameters over baseline were analysed with Friedman anova. A Pvalue of <0.05 was considered significant. Results are presented as means±s.d..
Results
Plasma samples were available from seven subjects in study 1 and eight subjects in study 2. The pharmacokinetic parameters calculated from study 1 are given in Table 1, plasma concentrations are illustrated in Figure 1. In the primed constant infusion experiments in study 2, plasma levels of l-NMMA increased to 9.5±5.2 μg ml−1 at the end of the 5 min bolus administration and to steady state plasma concentrations of ≈4.0±1.6 μg ml−1. During coinfusion with l-Arg, which resulted in a 66±6 fold increase in plasma l-Arg levels to 932±206 μg ml−1, a significant increase in mean plasma l-NMMA to 7.1±1.6 μg ml−1 (P<0.001, anova) was noted. Plasma concentrations of l-NMMA remained elevated 25 min after the end of l-Arg infusion at 7.5±1.7 μg ml−1.
Table 1.
Pharmacokinetic parameters of 3 mg kg −1 l-NMMA i.v. over 5 min obtained from noncompartmental analysis. Results are presented as means±s.d., n=7.
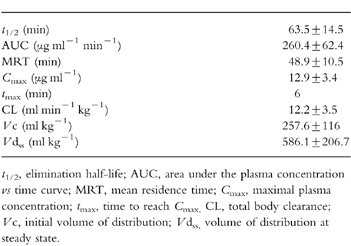
Figure 1.
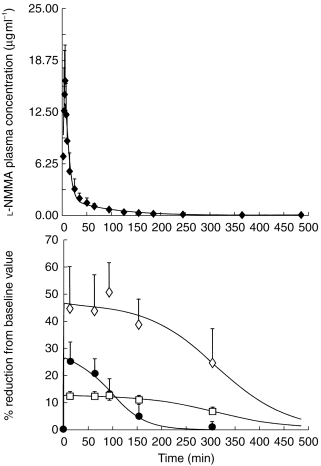
Plasma concentrations of l-NMMA following a single i.v. dose of 3 mg kg−1 over 5 min and pharmacodynamic effects on cardiac output (CO, □), fundus pulsation amplitude (FPA, •), and exhaled NO (exhNO, ◊), which are presented as percentage reduction from the baseline values. Data are from study 1 and are presented as means±s.d., n=7.
In study 1, l-NMMA had a transient and subtle effect on systemic haemodynamics: Mean arterial pressure increased slightly from 85.0±6.5–87.5±7.0 mmHg, pulse rate decreased from 69.5±9.6–65.2±10.6 beats min−1, and systemic vascular resistance significantly increased from 1013±295 dyn s cm−5 by 16% (P<0.05) 15 min after start of drug administration. l-NMMA caused a maximum percentage reduction from the baseline value (Emax) in CO of 13%, in FPA of 26%, and in exhaled NO of 46% (P<0.01 each, Figure 1). The presented PD model allowed very good fits of the experimentally measured data although only limited points were available and an EC50 for CO, FPA, and exhaled NO of 0.02 μg ml−1, 0.66 μg ml−1 and 0.02 μg ml−1 was calculated, respectively. The continuous infusion of l-NMMA in study 2 reduced exhaled NO by 69% (P<0.005), but no change in blood pressure and pulse rate was noted [10].
Figure 1 illustrates the effect of l-NMMA on the three dynamic parameters with respect to time. FPA and CO returned to near baseline values after 300 min. The relationship between the percentage reduction from the baseline values and the total plasma concentrations of l-NMMA is presented in Figure 2. The effect predicted by model was close to experimental measures as indicated by the good fit for the effect of the drug (model selection criteria >2.00 each).
Figure 2.
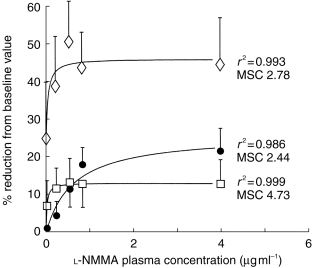
Relationship between the percentage reduction from the baseline values of cardiac output (CO, □), fundus pulsation amplitude (FPA, •), and exhaled NO (exhNO, ◊), and the plasma concentrations of l-NMMA from study 1. Results are presented as means±s.d., n=7. The Emax model (solid lines) provided a good fit for the effect of the drug on all the three dynamic parameters as indicated by the high coefficients of correlation (r2) and model selection criteria (MSC).
Discussion
Our results demonstrate that l-NMMA quickly disappears from plasma but exerts prolonged pharmacodynamic effects. Although the calculated half-life and clearance of l-NMMA is compatible with previous observations [8], the Cmax was substantially higher in our subjects, which is due to the fact that the peak concentrations of l-NMMA were missed in other experiments as pharmacokinetics were based on a limited number of plasma samples. l-NMMA levels at the end of the five minute bolus infusion period in our primed constant infusion studies were in the range of those observed previously.
After completion of infusion, l-Arg plasma concentration rapidly decreased within 30 min to ≈47% of its maximum level, which is in agreement with previous data [19]. Interestingly, levels of l-NMMA increased during l-Arg administration. The reduced l-NMMA clearance can be attributed to a decreased excretion, inhibition of metabolising enzymes, or redistribution. We consider it unlikely that this results from competitive renal excretion mechanisms because the reabsorption of l-Arg in the kidney is much more specific than for acidic or neutral amino acids [20]. Furthermore, given the fact that l-Arg counteracted the reduction in renal blood flow of l-NMMA [10], an increased renal flow should have been paralleled by an increased l-NMMA excretion. Secondly, l-NMMA is metabolized by dimethylarginine demethylaminohydrolase (DDAH) [21] to citrulline, whereas l-Arg is not a substrate of this enzyme. It is therefore unlikely that competition for DDAH could have been responsible for the pharmacokinetic interaction. However, l-Arg and l-NMMA are transported into endothelial and red blood cells by the same amino acid transport system [22, 23]. As this transport follows saturable kinetics, it is possible that excess doses of l-Arg have caused a redistribution of l-NMMA. Our study does not provide direct evidence whether a change in clearance and/or volume of distribution is responsible for the increased l-NMMA levels. Nevertheless, the observed pharmacokinetic interaction is compatible with the antagonistic pharmacodynamic effects of l-Arg [10].
Our data support the concept of structural interaction between l-NMMA and l-Arg in vivo and provide a rationale that l-Arg may be used to compete for an antagonistic NOS substrate. However, under physiological circumstances l-Arg is present at very high intracellular concentrations, even in excess of the membrane transport capacity. While NO synthesis is not limited by substrate availability in healthy subjects, it has been demonstrated that substrate deprivation alters the kinetics of l-Arg transport [22]. It is unclear if this phenomenon may provide a mechanism for enhanced l-Arg supply to sustain NO formation and if the antagonism of l-Arg on inhibition of NOS could be used therapeutically in diseases.
The presented pharmacokinetic-pharmacodynamic model allowed excellent fit of the l-NMMA plasma concentrations and experimentally measured data over the time period studied. The design of the study was not optimal with regard to the time of measurement of CO and exhNO, and the applied model is only valid in the concentration range studied. It is possible that with a further increase of l-NMMA plasma concentrations higher than those studied, the effect on outcome variables may further increase. Future studies should be done at various doses and with adequate sampling times to confirm the calculated EC50 values. Interestingly, the concentration calculated to produce half of the maximum effect was markedly higher for FPA than for the effects on exhaled NO and cardiac output. It is obvious that the reliability of these calculations strongly depend on the accuracy of l-NMMA plasma concentration and pharmacodynamic effect measurements and extrapolation of the pharmacodynamic curve beyond 155 min from our data may not be very accurate. However, the sensitivity and time course of the response of FPA indicates that this parameter could be particularly useful to study pharmacodynamic effects of l-NMMA. On the other hand, this also suggests that plasma levels of l-NMMA have to be taken into account when effects of subtle doses of l-NMMA on FPA are studied, whereas small differences in l-NMMA concentrations are less likely to confound measures of cardiac output or exhaled NO.
The time course of changes in FPA was different from those in cardiac output or exhaled NO (Figure 1). While we have not studied a dose–response relationship to l-NMMA and can therefore only speculate that the sensitivity of NO synthesis inhibition may be different in these vascular beds, it is well known that perfusion rate is particularly high in the choroidal vasculature [24], which could have affected the local kinetics of l-NMMA. It is also possible that counteracting pharmacodynamic mechanisms are more pronounced in the eye as compared with the other vascular beds studied. l-NMMA slightly increased systemic vascular resistance but did not exert major systemic hypertensive effects in our experiments. This is compatible with other studies in healthy subjects, where the pressor response was not consistent at similar time points after bolus administration [25] and effects on heart rate and mean arterial pressure were observed only with higher systemic doses during continuous drug infusion [6]. The dose of l-NMMA, however, was chosen to cause subpressor effects and not to significantly alter regional perfusion pressure or confound measurements through systemic reflex responses.
In conclusion, l-NMMA resulted in a significant reduction of cardiac output, FPA, and exhaled NO. The pharmacokinetic interaction between l-NMMA and l-Arg was demonstrated in vivo and the pharmacokinetic-pharmacodynamic model described the effect of l-NMMA on all three dynamic outcome parameters with high accuracy. The concentration predicted to produce a half maximal effect is higher for FPA than for cardiac output and exhaled NO.
Acknowledgments
We thank Andreas Punz and Fritz Garo (Department of Surgery, Allgemeines Krankenhaus Wien) for technical advices.
References
- 1.Palmer RMJ, Rees DD, Ashton DS, Moncada S. l-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988;153:1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- 2.Palmer RMJ, Moncada S. A novel citrulline forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989;158:348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- 3.Gardiner SM, Compton AM, Bennet T, Palmer RMJ, Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990;15:486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- 4.Rees DD, Palmer MJ, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci USA. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haynes WG, Noon JP, Walker BR, Webb DJ. L-NMMA increases blood pressure in man. Lancet. 1993;342:931–932. doi: 10.1016/0140-6736(93)91981-q. [DOI] [PubMed] [Google Scholar]
- 6.Stamler JS, Loh E, Roddy M, Currie KE, Creager MA. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation. 1994;89:2035–2040. doi: 10.1161/01.cir.89.5.2035. [DOI] [PubMed] [Google Scholar]
- 7.Bech JN, Nielsen CB, Pedersen EB. Effects of systemic NO synthesis inhibition on RPF, GFR, UNa, and vasoactive hormones in healthy humans. Am J Physiol. 1996;270:F845–F851. doi: 10.1152/ajprenal.1996.270.5.F845. [DOI] [PubMed] [Google Scholar]
- 8.Haynes WG, Hand MF, Dockrell ME, et al. Physiological role of nitric oxide in regulation of renal function in humans. Am J Physiol. 1997;272:F364–F371. doi: 10.1152/ajprenal.1997.272.3.F364. [DOI] [PubMed] [Google Scholar]
- 9.Schmetterer L, Krejcy K, Kastner J, et al. The effect of systemic nitric oxide-synthase inhibition on ocular fundus pulsations in man. Exp Eye Res. 1997;64:305–312. doi: 10.1006/exer.1996.0213. [DOI] [PubMed] [Google Scholar]
- 10.Wolzt M, Schmetterer L, Ferber W, et al. Effect of nitric oxide synthase inhibition on renal hemodynamics in humans: reversal by l-arginine. Am J Physiol. 1997;272:F178–F182. doi: 10.1152/ajprenal.1997.272.2.F178. [DOI] [PubMed] [Google Scholar]
- 11.Wolzt M, Schmetterer L, Rheinberger A, et al. Comparison of non-invasive methods for the assessment of haemodynamic drug effects in healthy male and female volunteers: sex differences in cardiovascular responsiveness. Br J Clin Pharmacol. 1995;39:347–359. doi: 10.1111/j.1365-2125.1995.tb04462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coates AJS. Doppler ultrasonic measurement of cardiac output: reproducibility and validation. Eur Heart J. 1990;11(Suppl I):49–61. doi: 10.1093/eurheartj/11.suppl_i.49. [DOI] [PubMed] [Google Scholar]
- 13.Schmetterer L, Lexer F, Unfried C, Sattmann H, Fercher A. Topical measurement of fundus pulsations. Opt Eng. 1995;34:711–716. [Google Scholar]
- 14.Schmetterer L, Wolzt M, Salomon A, et al. Effect of isoproterenol, phenylephrine, and sodium nitroprusside on fundus pulsations in healthy volunteers. Br J Ophthalmol. 1996;80:217–223. doi: 10.1136/bjo.80.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmetterer L, Lexer F, Findl O, et al. Noninvasive investigations on the normal human circulation. Invest Opht Vis Sci. 1998;39:1210–1220. [PubMed] [Google Scholar]
- 16.Jilma B, Kastner J, Mensik C, et al. Sex differences in nitric oxide production. Life Sci. 1996;58:469–476. doi: 10.1016/0024-3205(95)02311-9. [DOI] [PubMed] [Google Scholar]
- 17.Schmetterer L, Strenn K, Kastner J, Eichler HG, Wolzt M. Exhaled NO during graded changes in inhaled oxygen in man. Thorax. 1997;52:736–738. doi: 10.1136/thx.52.8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth M. Fluorescence reaction for amino acids. Anal Chem. 1971;43:880–882. doi: 10.1021/ac60302a020. [DOI] [PubMed] [Google Scholar]
- 19.Bode-Böger SM, Böger RH, Galland A, Tsikas D, Frölich JC. l-arginine-induced vasodilation in healthy humans: Pharmacokinetic-pharmacodynamic relationship. Br J Clin Pharmacol. 1998;46:489–497. doi: 10.1046/j.1365-2125.1998.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dantzler WH, Silbernagl S. Basic amino acid transport in renal papilla: microinfusion of Henle’s loop and vasa recta. Am J Physiol. 1993;265:F830–F838. doi: 10.1152/ajprenal.1993.265.6.F830. [DOI] [PubMed] [Google Scholar]
- 21.MacAllister RJ, Parry H, Kimoto M, et al. Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br J Pharmacol. 1996;119:1533–1540. doi: 10.1111/j.1476-5381.1996.tb16069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogle RG, Baydoun AR, Pearson JD, Mann GE. Regulation of l-arginine transport and nitric oxide release in superperfused porcine aortic endothelial cells. J Physiol (Lond) 1996;490:229–241. doi: 10.1113/jphysiol.1996.sp021138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendes Ribeiro AC, Hanssen H, Kiessling K, Roberts NB, Mann GE, Ellory JC. Transport of l-arginine and the nitric oxide inhibitor NG-monomethyl-l-arginine in human erythrocytes in chronic renal failure. Clin Sci. 1997;93:57–64. doi: 10.1042/cs0930057. [DOI] [PubMed] [Google Scholar]
- 24.Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15:15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- 25.Haynes WG, Noon JP, Walker BR, Webb DJ. Inhibition of nitric oxide synthesis increases blood pressure in healthy subjects. J Hypertens. 1993;11:1375–1380. doi: 10.1097/00004872-199312000-00009. [DOI] [PubMed] [Google Scholar]


