Abstract
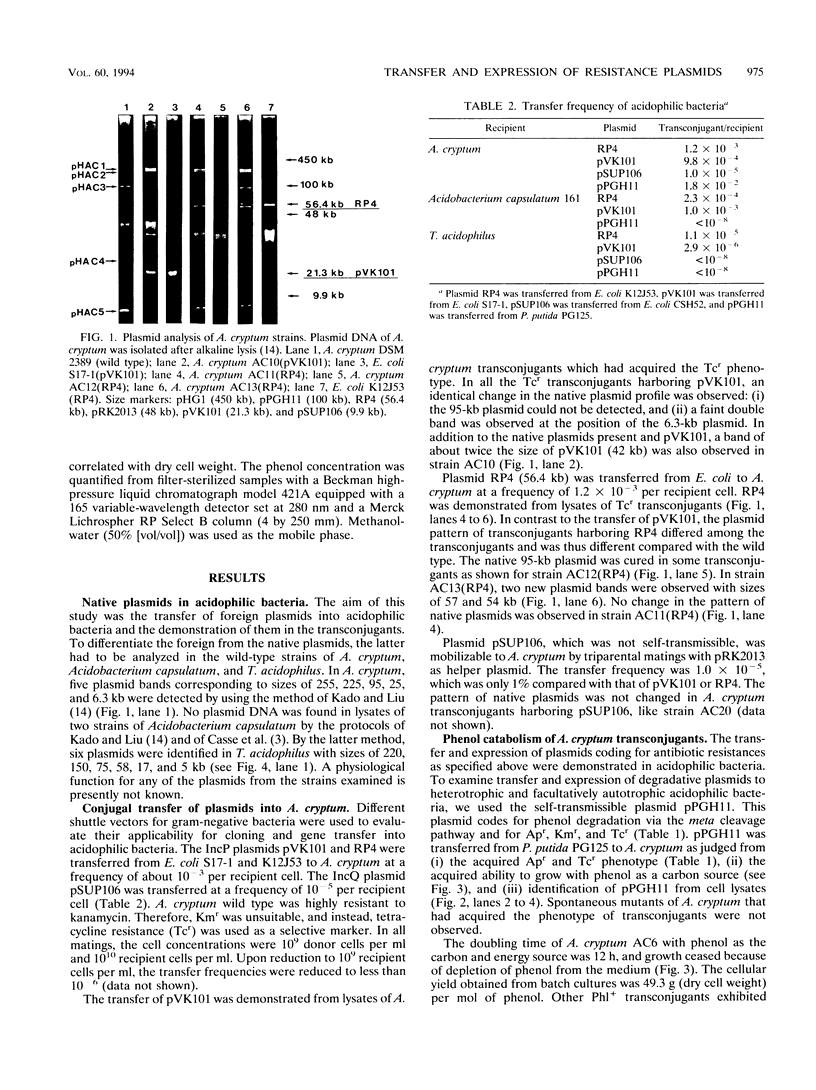
The genetic accessibility of selected acidophilic bacteria was investigated to evaluate their applicability to degrading pollutants in acidic environments. The IncP1 antibiotic resistance plasmids RP4 and pVK101 and the phenol degradation-encoding plasmid pPGH11 were transferred from neutrophilic bacteria into the extreme acidophilic eubacterium Acidiphilium cryptum at frequencies of 1.8 x 10(-2) to 9.8 x 10(-4) transconjugants per recipient cell. The IncQ antibiotic resistance plasmid pSUP106 was mobilizable to A. cryptum by triparental matings at a frequency of 10(-5) transconjugants per recipient cell. In the transconjugants, antibiotic resistances and the ability to degrade phenol were expressed. A. cryptum AC6 (pPGH11) grew with 2.5 mM phenol at a doubling time of 12 h and a yield of 0.52 g (dry cell weight) per g of phenol. A. cryptum harbored five native plasmids of 255 to 6.3 kb in size. Plasmids RP4 and pVK101 were transferred from Escherichia coli into Acidobacterium capsulatum at frequencies of 10(-3) and 2.3 x 10(-4) and to the facultative autotroph Thiobacillus acidophilus at frequencies of 1.1 x 10(-5) and 2.9 x 10(-6) transconjugants per recipient cell, respectively. Plasmid pPGH11 could not be transferred into the latter strains. T. acidophilus wild type contained six so far cryptic plasmids of 220 to 5 kb.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley C. L. Bacterial leaching. CRC Crit Rev Microbiol. 1978;6(3):207–26I. doi: 10.3109/10408417809090623. [DOI] [PubMed] [Google Scholar]
- Chandra T. S., Friedrich C. G. Tn5-induced mutations affecting sulfur-oxidizing ability (Sox) of Thiosphaera pantotropha. J Bacteriol. 1986 May;166(2):446–452. doi: 10.1128/jb.166.2.446-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. S., Summers A. O. Wide-host-range plasmids function in the genus thiobacillus. Appl Environ Microbiol. 1983 Sep;46(3):565–572. doi: 10.1128/aem.46.3.565-572.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay R., Silver M. Thiobacillus acidophilus sp. nov.; isolation and some physiological characteristics. Can J Microbiol. 1975 Mar;21(3):281–288. doi: 10.1139/m75-040. [DOI] [PubMed] [Google Scholar]
- Herrmann H., Janke D., Krejsa S., Roy M. In vivo generation of R68.45-pPGH1 hybrid plasmids conferring a Phl+ (meta pathway) phenotype. Mol Gen Genet. 1988 Sep;214(1):173–176. doi: 10.1007/BF00340199. [DOI] [PubMed] [Google Scholar]
- Jin S. M., Yan W. M., Wang Z. N. Transfer of IncP Plasmids to Extremely Acidophilic Thiobacillus thiooxidans. Appl Environ Microbiol. 1992 Jan;58(1):429–430. doi: 10.1128/aem.58.1.429-430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Kusano T., Sugawara K., Inoue C., Takeshima T., Numata M., Shiratori T. Electrotransformation of Thiobacillus ferrooxidans with plasmids containing a mer determinant. J Bacteriol. 1992 Oct;174(20):6617–6623. doi: 10.1128/jb.174.20.6617-6623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. A., Dugan P. R., Tuovinen O. H. Plasmid DNA in acidophilic, chemolithotrophic thiobacilli. Can J Microbiol. 1981 Aug;27(8):850–853. doi: 10.1139/m81-133. [DOI] [PubMed] [Google Scholar]
- Priefer U. B., Simon R., Pühler A. Extension of the host range of Escherichia coli vectors by incorporation of RSF1010 replication and mobilization functions. J Bacteriol. 1985 Jul;163(1):324–330. doi: 10.1128/jb.163.1.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]