Abstract
Aims
The occurence of serious dysrhythmias, such as torsades de pointes, with terfenadine and astemizole had led to a reexamination of the potential effect of H1 antihistamines on cardiac repolarization. Mizolastine is a potent, selective, nonsedating peripherally acting H1-receptor antagonist which is registered for rhinitis and urticaria at a recommended dose of 10 mg once daily. The present study was carried out to investigate the effects of therapeutic and supratherapeutic doses of mizolastine, on ventricular repolarization in healthy volunteers.
Methods
Twenty-four healthy young volunteers participated in a double-blind, placebo-controlled, randomised study with three parallel groups. Each group consisted of 2 way cross-over 7 day treatment periods where mizolastine (10, 20 or 40 mg) and placebo were randomly administered. On day 1 and day 7, 12-lead ECG recordings were performed prior and 0.5, 1, 2, 3, 4, 6, 8, 12, 16, and 20 h after dosing and from day 2 to day 6, before dosing and 1, 2, 3, and 4 h after.
Results
Whatever the analysis used (raw data, changes from baseline, incidence of individual out-of-range values) no significant differences were observed at any dose level vs placebo, on any of ECG parameters (HR, PR, QRS, QT, and QTc). In particular, no effect of mizolastine vs placebo was shown on QT and QTc although 95% CIs were wide. The only subject who exhibited a QTc≥450 ms received placebo for 7 days.
Conclusions
This study found no evidence of an effect of mizolastine up to 40 mg (four times the therapeutic dose) on ventricular repolarization in healthy volunteers.
Keywords: cardiac repolarization, H1-receptor antagonist, healthy volunteers, mizolastine
Introduction
Since the early 1990s, reports have described the potential for dysrhythmia in patients taking terfenadine with overdose, hepatic impairment or in combination with drugs known to inhibit hepatic oxidative metabolism such as ketoconazole and erythromycin [1]. An European Community pilot project providing up-to-date international data on spontaneous adverse reactions from 17 countries for nonsedating antihistamines recently indicated the presence of increased risk of various types of dysrhythmia, with terfenadine. Similar effects have also been described with astemizole and to a minor degree with loratadine [2].
This has led to reexamination of the potential of all antihistamines to produce torsades de pointes. This life-threatening ventricular dysrhythmia is usually observed in the setting of a prolonged QT interval. Therefore, the prolongation of QT interval, assessed by ECG recording, a sensitive and powerful method, can be used as one of the risk factors for the development of torsades de pointes [3].
Mizolastine, a new benzimidazole derivative, is a potent, selective histamine H1-receptor antagonist [4] devoid of sedative effect in objective measurements [5, 6] or anticholinergic effect [7]. In guinea pig dissociated ventricular myocytes, at concentrations significantly higher than those corresponding to therapeutic free plasma levels, mizolastine does not block potassium channels involved in the lengthening of the action potential [8].
Clinical pharmacology studies did not detect any significant effect on cardiac repolarization up to single doses of 75 mg [9]. During the course of trials carried out in patients suffering from allergic rhinitis [10–12] and urticaria [13], a satisfactory cardiovascular safety has been reported. However, none of these studies was specifically designed to assess the potential cardiac effect of mizolastine, in particular on cardiac repolarization.
The aim of this study, conducted in healthy young volunteers, was to assess the effects on ECG intervals, especially the QT and QTc, of increasing 7 day repeated doses of mizolastine, at the therapeutic dose of 10 mg as well as 2 and 4 times the recommended dose.
Methods
Subjects
Twenty-four healthy male volunteers, aged 24–31 years (25±2 s.d. years), weighing between 57 and 96 kg (75±9 kg) and 167–188 cm (178±6 cm), in height were enrolled in the study. The study was conducted according to the Declaration of Helsinki (Hong Kong Amendment 1989) and French regulations. Written informed consent from subjects and protocol approval from the Boucicaut Hospital Ethic Committee (Paris) were obtained. The subjects were included after a full medical examination, clinical laboratory tests, and a 12-lead ECG (QTc≤420 ms). Strenuous physical activity or working at night were not permitted during the treatment period. Subjects refrained from drinking alcohol for 7 days prior to the study until the end of the trial and abstained from consuming xanthine-containing-beverages throughout the treatment period.
Protocol
This was a double-blind, placebo-controlled, randomised study, with three parallel groups of eight subjects per mizolastine dose level. Each group consisting of a two way cross-over period: mizolastine (10, 20 or 40 mg) vs placebo. All subjects completed two 7 day treatment periods separated by a 7 day wash-out. Mizolastine tablets dosed at 10 mg and indistinguishable placebo tablets were used during the study. The subjects took four tablets once daily in the morning, 30 min before a standardized breakfast.
12-lead ECGs were recorded using a Pagicardette II Cardiograph (Hewlett-Packard, Les Ulis, France) after a 10 min rest. Printouts (25 mm s−1) for each ECG included at least two complexes for each lead and a single lead (D2) run. On days 1 and 7, they were made 15 min before dosing and at 0.5, 1, 2, 3, 4, 6, 8, 12, 16 and 20 h. On day 7, additional ECG recordings were performed at 24 and 36 h postdosing. From day 2 to day 6, ECG measurements were made before dosing and at 1, 2, 3, and 4 h. HR and PR, QRS, QT, and QTc intervals were automatically obtained (Bazett’s formula algorithm). One ECG tracing was identified by the study name, subject number and assessment time.
24 h ECG (Holter) recordings were performed at screening and on day 6 with a Sherpa three channel recorder (Reynolds Medical, Saint-Germain-en-Laye, France) and read using a Holter reading program (386 Bios America Megatrends INC, Plaisir).
Vital signs (SBP, DBP, and HR) were assessed before dosing from day 1 to day 6 and on day 7, before drug administration then 36 h post dosing. They were measured after 10 min in the supine position and after 2 min standing, using an automatic device (cardio CAPtmIICH, Datex, Evry, France). Routine laboratory examination were carried out at screening and at the poststudy visit.
Venous blood samples for the determination of mizolastine levels were taken from the subject’s forearm on day 1 before dosing and at 0.5, 1, 2, 3, 4, 6, 8, 12, 16, and 20 h; on day 2 to day 6 prior drug intake and at 1, 2, 3, 4 h and on day 7 before dosing and at 0.5, 1, 2, 3, 4, 6, 8, 12, 16, 20, 24, and 36 h. Blood samples were centrifuged at 3000 rev min−1 for 10 min at 4° C within 1 h after collection and stored in two nonglass screw-top tubes at −20° C until quantification. Mizolastine concentrations were measured using an h.p.l.c. method in the Clinical Pharmacokinetic Department, Synthélabo Recherche, Chilly Mazarin, France. Linearity was demonstrated between 1.0 and 500 ng ml−1 for a test sample of 1 ml. The limit of quantification was 1.0 ng ml−1, with a coefficient of variation smaller than 8.5% [14].
Statistical analysis
Values outside the following ranges were flagged, i.e. PR≥220 ms, QRS>120 ms, QTc≥450 ms, ΔQTc%≥ 10%, and ΔQTc%≥15%. Analysis of ECG parameters (PR, QRS, QT, QTc), HR, and for QTc max (maximum QTc value) were performed on raw data and changes from baseline separately for day 1 and day 7 using for the repeated measurements a three way anova (subject, treatment, time), repeated option, and Huynh-Feldt probabilities. For QTc, observed, Emax (QTcmax) and time of this maximal value postdose were calculated for day 1 and day 7. If a treatment–time interaction was found (alpha level 5%, two-tailed), a two-way anova analysis (subject, treatment) was used at each time point. Two-way (subject, treatment) anova parametric approaches were used for QTcmax intervals. The power calculation to detect a mean difference of QTc of 10 ms (standard deviation of 15 ms) was 80%. Additionnally, 95% confidence intervals for differences were calculated. Results are expressed as means±s.d. in tables and text.
Pharmacokinetic analysis
The following parameters were calculated: maximum concentration (Cmax), time of maximum concentration (tmax), apparent elimination half-life (t1/2,z). Area under the curve (AUC) was calculated by the linear trapezoidal method between 0 and 24 h and extrapolated to infinity on day 1. The apparent elimination half-lives were estimated with a loglinear regression on the terminal points of the concentration-time profiles down to the last analytically reliable point, using the Pharm-NCA® release 1.3 software (SIMED, Créteil, France).
Results
12-lead ECG
Figures 1, 2, 3 show the evolution of mean (±s.e.mean) QTc interval for each mizolastine dosage vs corresponding placebo on day 1 and day 7.
Figure 1.
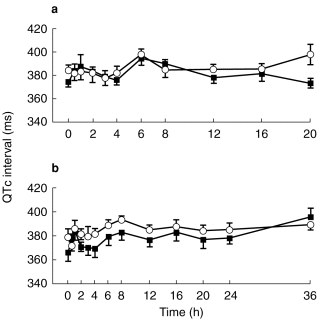
Mean (±s.e.mean) QTc interval (ms) on day 1 (a) and day 7 (b) after (▪) mizolastine 10 mg (n=8) vs (○) placebo (n=8) in healthy volunteers.
Figure 2.
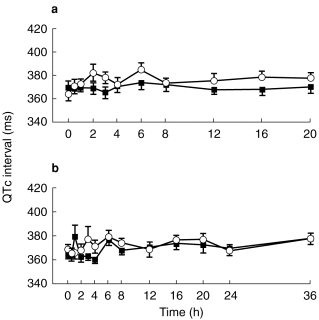
Mean (±s.e.mean) QTc interval (ms) on day 1 (a) and day 7 (b) after (▪) mizolastine 20 mg (n=8) vs (○) placebo (n=8) in healthy volunteers.
Figure 3.
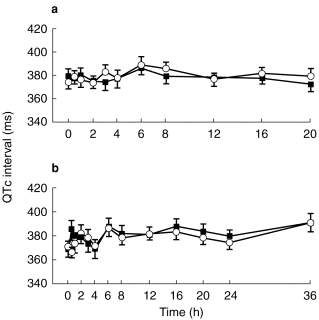
Mean (±s.e.mean) QTc interval (ms) on day 1 (a) and day 7 (b) after (▪) mizolastine 40 mg (n=8) vs (○) placebo (n=8) in healthy volunteers.
At baseline, no statistically significant difference in ECG parameters was demonstrated between the placebo and active groups at each dose level. Whatever the analysis methodology used, raw data, changes from baseline, incidence of individual out-of-range values, no significant difference was observed at any dose level vs placebo, on any of the ECG parameters (HR, PR, QRS, QT, and QTc). A trend toward a decrease was observed in QTc interval for the mizolastine group. However, no dose effect was found and 95% CIs were wide.
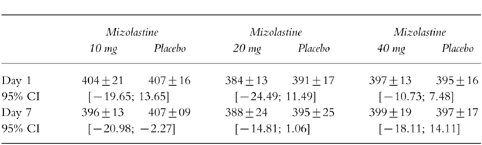
The results observed with each mizolastine dosage vs corresponding placebo on the QTcmax interval on day 1 and day 7 are summarized in Table 1. On day 7, a significantly (P<0.05) shorter QTcmax was observed after 10 mg mizolastine when compared with placebo group (396 ms and 407 ms, respectively).
Table 1.
QTcmax (ms) interval (mean±s.d.) and 95% confidence intervals for differences on day 1 and day 7 after mizolastine 10 mg, 20 mg and 40 mg (n=8) vs placebo (n=8) in healthy volunteers.
Only one subject presented with a QTc value ≥450 ms. The value was exactly 450 ms and it was observed on day 7 after placebo treatment. There was no evidence of any change in the morphology of the T wave.
24-h ECG (Holter) recordings
No relevant modifications were reported in the 24 h ECG Holter recordings.
Clinical safety
The overall clinical safety of mizolastine during the study was satisfactory. No serious adverse events were recorded. Sixteen emergent adverse events were observed during the study. Four were reported during mizolastine 10 mg treatment, 6 during mizolastine 40 mg treatment, and 6 during placebo period. The most frequent adverse events were headache and somnolence. An increase in the frequency of occurrence of somnolence (7 episodes) was observed in the 40 mg treatment group when compared with mizolastine 10 mg and placebo, 3 episodes and 1 episode, respectively.
No significant change, in blood pressure or heart rate, was found between the two treatments.
Pharmacokinetics
The mizolastine pharmacokinetic parameters obtained on day 1 and day 7 after a repeated once daily oral administration of 10 mg, 20 mg, or 40 mg mizolastine in three groups of eight healthy volunteers are presented in Table 2.
Table 2.
Mizolastine pharmacokinetic parameters (mean±s.d.) on day 1 and day 7 after repeated once-daily oral administration of 10 mg, 20 mg, or 40 mg mizolastine in three groups of eight healthy volunteers.
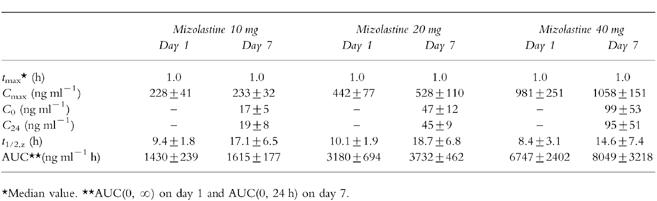
Mizolastine trough levels were stable from day 3 to day 7 showing steady state was reached from day 3. Cmax and AUC increased in proportion to the dose: the dose-related linearity within 10–40 mg range was confirmed. No unexpected accumulation was observed between day 1 and day 7.
Discussion
The present study shows that mizolastine is devoid of any effect on the ventricular repolarization in healthy volunteers. No changes in T-wave morphology or prolongation QTc interval were detected after mizolastine compared with placebo whatever the dose of mizolastine although 95% CIs were wide and a minor effect cannot be excluded with certainty. This lack of effect was obtained despite of high plasma concentration of mizolastine. At 40 mg mizolastine, the concentration reached four fold the concentration obtained at therapeutic dosage.
This study was especially designed to detect small changes in the QT and QTc interval related to mizolastine. Furthermore, in order to detect any possible dose-related effect of mizolastine on QT interval, doses of two and four times the therapeutic 10 mg dose were used in this study. Although a quantitative relationship between QT interval prolongation and torsade de pointes has not yet clearly established, prolonged QT intervals associated with a dose-related change is recognized as one of the important risk factors for the development of this serious arrhythmia [3].
Evidence of serious dysrhythmias, such as torsades de pointes, with terfenadine and astemizole has raised questions about the cardiotoxic potential of other second generation histamine H1-receptor antagonists. The recent adverse drug reaction report [2] of the five widely used nonsedating antihistamines confirmed the increased risk of cardiac problems with terfenadine and astemizole either on overdosage or when prescribed to at risk patients. Cytochromes P450 3A4 are extensively involved in the metabolism of terfenadine and astemizole. The inhibition of CYP3A4 by coadministration of ketoconazole or erythromycin alters the metabolism of terfenadine. This effect leads to the cardiac accumulation of the unmetabolized parent compound, a potent blocker of myocardial potassium channel and thus, capable of altering cardiac repolarization [15, 16]. The main metabolic pathway of mizolastine is glucuronidation (about 70%) which is independent of cytochromes P450 3A. A small extent is metabolized via CYP3A by oxydation of the benzimidazole ring, N-dealkylation and hydroxylation followed by methylation of the pyrimidinone moiety. Moreover, CYP1A2 and CYP2A6 subfamilies can also contribute to mizolastine hydroxylation. Therefore, only limited interactions due to metabolic inhibition of cytochromes P450 by compounds such as azote antifungals and macrolides are likely to occur.
However, the cardiotoxic action of antihistamines might also be observed when the drug is administered alone in the absence of any potent metabolic modifiers. In a four period cross-over study, terfenadine administered in healthy volunteers at doses of 60 mg and 180 mg twice daily (i.e. three times the recommended dose), was associated with dose-related increase in QTc interval: the mean increase in QTc from baseline was 6 ms and 19 ms, respectively [17]. In addition, a retrospective analysis of ECG data from eight clinical studies confirmed the dose effect of terfenadine on QTc interval. A mean of 6 ms increase in the QTc interval at 60 mg, 7 ms at 120 mg and 42 ms at 300 mg (5 times the recommended dose) terfenadine twice daily were described [19]. Ebastine administered for 10 days at therapeutic dose (10 mg day−1) induced a QTc increase from baseline of 20 ms in healthy young volunteers (compared with 2 ms for placebo) and 24 ms in elderly healthy volunteers (compared with 6 ms for placebo) [18]. Sale et al. found that cetirizine does not prolong the mean QTc interval at doses of up to six times the usual recommended dose [20]. During that study, one subject showed an increase in QTc from 404 to 470 ms at 6 h after the 5th day of dosing 60 mg. Morover, the US data sheet indicates that cetirizine 20 mg caused a mean increase in QTc of 9.1 ms from baseline after 10 days of therapy. Finally, in normal volunteers receiving 40 mg loratadine (four times the therapeutic dose) once daily for 90 days, no statistically significant changes for any ECG parameter were observed [21]. The lipophilicity of the drug must also be considered. This factor might contribute to the cardiotoxicity. Compared with loratadine and cetirizine [22], mizolastine has a low lipophilicity that could translate into a reduced cardiac distribution.
This study also investigated pharmacokinetics and overall clinical safety of increasing repeated doses of mizolastine. Good clinical and laboratory safety were reported for each mizolastine dosage and the present pharmacokinetic results corroborated the findings of the previous studies [8, 23].
In conclusion, the study showed the lack of cardiovascular effect of mizolastine up to 40 mg (four times the therapeutic dose) and in particular the absence of any effect on ventricular repolarization in healthy volunteers.
References
- 1.Simons FER. H1-receptor antagonists. Comparative tolerability and safety. Drug Safety. 1994;10:350–380. doi: 10.2165/00002018-199410050-00002. [DOI] [PubMed] [Google Scholar]
- 2.Lindquist M, Edwards IR. Risks of non-sedating antihistamines. Lancet. 1997;349:1322. doi: 10.1016/S0140-6736(97)26018-6. [DOI] [PubMed] [Google Scholar]
- 3.Benedict CR. The QT interval and drug-associated Torsades de Pointes. Drug Invest. 1993;5:69–79. [Google Scholar]
- 4.Arbilla S, Schoemaker H, Scatton B. SL85.0324: a novel benzimidazole derivative with selective histamine H1-receptor antagonist properties. Eur J Clin Pharmacol. 1990;183:218. [Google Scholar]
- 5.Patat A, Perault MC, Vandel B, Ulliac N, Zieleniuk I, Rosenzweig P. Lack of interaction between a new antihistamine, mizolastine, and lorazepam on psychomotor performance and memory in healthy volunteers. Br J Clin Pharmacol. 1995;39:31–38. doi: 10.1111/j.1365-2125.1995.tb04406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vuurman E, Uiterwijk M, Rosenzweig P, O’Hanlon JF. Effects of mizolastine and clemastine on actual driving and psychomotor performance in healthy volunteers. Eur J Clin Pharmacol. 1994;47:253–259. doi: 10.1007/BF02570505. [DOI] [PubMed] [Google Scholar]
- 7.Danjou P, Molinier P, Berlin I, Patat A, Rosenzweig P, Morselli PL. Assessment of the anticholinergic effect of the new antihistamine mizolastine in healthy subjects. Br J Clin Pharmacol. 1992;34:328–331. doi: 10.1111/j.1365-2125.1992.tb05638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biton B, Maitre S, Depoortere H, et al. Effect of the novel non sedative H1 receptor antagonist mizolastine on cardiac potassium and sodium currents. Allergy. 1998;43(Suppl):160. [Google Scholar]
- 9.Rosenzweig P, Thébaut JJ, Caplain H, et al. Pharmacodynamics and pharmacokinetics of mizolastine (SL 85. 0324), a new nonsedative H1 antihistamine. Ann Allergy. 1992;2:135–139. [PubMed] [Google Scholar]
- 10.Stern MA, Darnell R, Tudor D. Can an antihistamine delay appearance of hay fever symptoms when given prior to pollen season ? Allergy. 1997;52:440–444. doi: 10.1111/j.1398-9995.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 11.Leynadier F, Bousquet J, Murrieta M, Attali P. Efficacy and safety of mizolastine in seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 1996;76:163–168. doi: 10.1016/s1081-1206(10)63417-5. [DOI] [PubMed] [Google Scholar]
- 12.Tasman AJ, Weber F. A double blind placebo controlled multicentered study on mizolastine, a new nonsedative H1-receptor antagonist, in patients suffering from perennial allergic rhinoconjunctivitis. Allergy. 1995;50:79. [Google Scholar]
- 13.Brostoff J, Fitzharris P, Dunmore C, Theron M, Blondin P. Efficacy of mizolastine, a new antihistamine, compared with placebo in the treatment of chronic idiopathic urticaria. Allergy. 1996;51:320–325. [PubMed] [Google Scholar]
- 14.Ascalone V, Guinebault P, Rouchouse A. Determination of mizolastine, a new antihistaminic drug, in human plasma by liquid-liquid extraction, solid-phase extraction and column switching techniques in combination with high performance liquid chromatography. J Chromatogr. 1993;619:275–284. doi: 10.1016/0378-4347(93)80117-m. [DOI] [PubMed] [Google Scholar]
- 15.Honig PK, Worthman DC, Zamani K, Conner DP, Mulin JC, Cantilena LR. Terfenadine–Ketoconazole interaction. Pharmacokinetic and electrocardiographic consequences. JAMA. 1993;269:1513–1518. [PubMed] [Google Scholar]
- 16.Honig PK, Woosley RL, Zamani K, Conner DP, Cantilena LR. Changes in the pharmacokinetics and electrocardiographic pharmacodynamics of terfenadine with concomitant administration of erythromycin. Clin Pharmacol Ther. 1992;52:231–238. doi: 10.1038/clpt.1992.135. [DOI] [PubMed] [Google Scholar]
- 17.Pratt CM, Ruberg S, Morganroth J, et al. Dose–response relation between terfenadine (Seldane) and the QTc interval on the scalar electrocardiogram: distinguishing a drug effect from spontaneous variability. Am Heart J. 1995;131:472–480. doi: 10.1016/s0002-8703(96)90525-6. [DOI] [PubMed] [Google Scholar]
- 18.Geary WJ, Garcia JD, Dockhorn RJ. Electrocardiographic safety of 10 mg of ebastine in healthy elderly and young adult volunteers. Allergy. 1995;50(Suppl 26):199. [Google Scholar]
- 19.Morganroth J. QTc interval prolongation: is it beneficial or harmful? Am J Cardiology. 1993;72:1B–59B. [PubMed] [Google Scholar]
- 20.Sale ME, Barbey JT, Woosley RL, et al. The electrocardiographic effects of cetirizine in normal subjects. Clin Pharmacol Ther. 1994;56:295–301. doi: 10.1038/clpt.1994.140. [DOI] [PubMed] [Google Scholar]
- 21.Affrime MB, Lorber R, Danzig M, Cuss F, Brannan MD, Kenilworth NJ. Three month evaluation of electrocardiographic effects of loratadine in humans. Allergy. 1993;48(Suppl 16):29. [Google Scholar]
- 22.Spencer MC, Faulds D, Peters DH. Ceterizine, a reappraisal of its pharmacological properties and therapeutic use in allergic disorders. Drugs. 1993;46:1055–1080. doi: 10.2165/00003495-199346060-00008. [DOI] [PubMed] [Google Scholar]
- 23.Pinquier JL, Caplain H, Cabanis MJ, Dubruc C, Stalla-Bourdillon A, Rosenzweig P. Inhibition of histamine-induced skin wheal and flare after 5 days of mizolastine. J Clin Pharmacol. 1996;36:72–78. doi: 10.1002/j.1552-4604.1996.tb04154.x. [DOI] [PubMed] [Google Scholar]


