Abstract
Aims
Sumatriptan is a 5-HT1B/1D-receptor agonist which also has affinity for 5-HT1F-receptors. The vasoconstrictor effects of sumatriptan are thought to be 5-HT1B-receptor mediated and these receptors have been shown to be expressed in human cranial blood vessels. However, in the same tissue mRNA coding for 5-HT1F-receptors has also been identified and this study addresses the possibility of whether 5-HT1F-receptor activation contributes to vasoconstriction.
Methods
The ability of two selective 5-HT1B/1D-receptor antagonists (GR125,743 and GR127,935) with no affinity for 5-HT1F-receptors, to inhibit sumatriptan evoked contractions in human isolated middle meningeal artery was investigated. Using a series of 5-HT1B/1D-receptor agonists (sumatriptan, zolmitriptan, CP122,288, L-741,519 and L-741,604), some with high affinity for 5-HT1F-receptors and the non-selective 5-HT-receptor agonists 5-HT and 5-CT, we compared the vasoconstrictor potency of these drugs in human isolated middle meningeal artery with their affinities at cloned human 5-HT1B-, 5-HT1D-and 5-HT1F-receptors expressed in CHO cell lines.
Results
GR125,743 antagonized sumatriptan evoked contractions in a competitive manner (apparent pA2 9.1) and GR127,935 antagonized sumatriptan-induced responses in a non-competitive manner (reducing the maximum contraction to 27%). There was a significant correlation between vasoconstrictor potency and 5-HT1B-receptor affinity (r = 0.93, P = 0.002) but not with 5-HT1D- or 5-HT1F-receptor affinity (r = 0.74, P = 0.06; r = 0.31, P = 0.49, respectively).
Conclusions
These experiments show that in human middle meningeal artery vasoconstriction to sumatriptan-like agents is 5-HT1B-receptor mediated with little if any contribution from 5-HT1F-receptor activation.
Keywords: 5-HT-receptor subtypes, human middle meningeal artery, vasoconstriction
Introduction
Sumatriptan is often regarded as a selective 5-HT1B/1D-receptor agonist [1, 2]. The clinical effectiveness of this medicine in treating migraine has been attributed to its ability to cause vasoconstriction of excessively dilated cranial blood vessels and to inhibit the release of neuropeptides from trigeminal sensory neurones preventing the development of neurogenic inflammation [3–5]. Sumatriptan and some other 5-HT1B/1D-receptor agonists such as zolmitriptan and naratriptan also have high affinity for 5-HT1F-receptors [6, 7] and the possibility that activation of 5-HT1F-receptors may contribute to the antimigraine action of these drugs cannot be ruled out. Within the trigeminal ganglia mRNAs coding for 5-HT1B-and 5-HT1D-receptors are expressed [8] and selective 5-HT1B/1D-receptor agonists can inhibit trigeminally mediated neurogenic extravasation [9, 10] and neurogenic vasodilation [11]. Recently, it has been demonstrated that mRNA coding for 5-HT1F-receptors is also expressed in trigeminal ganglia [8] and in guinea-pigs LY334370, a selective 5-HT1F-receptor agonist (with 100-fold lower affinity for 5-HT1B/1D-receptors), can inhibit trigeminally mediated dural extravasation [7, 12]. Furthermore, autoradiographic mapping studies have identified using radiolabelled sumatriptan or zolmitriptan, binding sites in human, cat and guinea-pig brains (including trigeminal nucleus caudalis). However, it has not been possible to distinguish between 5-HT1B- and 5-HT1F-receptor binding sites with the use of these drugs as radioligands [13–15]. Similarly, mRNA coding for 5-HT1B-receptors (but not 5-HT1D-receptors) has been detected in human cerebral blood vessels and on this basis it has been generally assumed that the vasoconstrictor effects of sumatriptan are 5-HT1B-receptor mediated [16, 17]. The mRNA coding for 5-HT1F-receptors is also expressed in human vasculature [8] however, little is known about the physiological role of the 5-HT1F-receptor (assuming that the corresponding mRNA is actually translated into a functional receptor protein) although the 5-HT1F-receptor, like the 5-HT1B-receptor, is negatively linked to adenylate cyclase [6] activation of this receptor would be expected to cause vasoconstriction.
Therefore, the aim of the current study was to determine whether vasoconstriction in human isolated middle meningeal arteries is mediated through the activation of 5-HT1B-receptors or 5-HT1F-receptors or both. The ability of the selective 5-HT1B/1D-receptor antagonists GR125,743 and GR127,935 [18, 19], which have weaker affinity for 5-HT1F-receptors, to inhibit the vasoconstrictor effects of sumatriptan in this blood vessel were examined. In addition, a series of 5-HT-receptor ligands with various levels of affinity for human 5-HT1B-, 5-HT1D- and 5-HT1F-receptors were tested at each of these receptors expressed in stable CHO cell lines and the affinity values were correlated with measurements of vasoconstrictor potency obtained in human isolated middle meningeal artery.
A preliminary communication of this study was made to the British Pharmacological Society [20].
Methods
The collection of human material for these studies was approved of by the local Ethics Committee.
Functional studies: human middle meningeal arteries
Discarded pieces of dura mater containing segments of middle meningeal arteries were obtained from patients undergoing neurosurgery. The vessels were transported in modified physiological salt solution (4° C) to the laboratory where the arteries were dissected free from the dura mater. Ring segments (2–3 mm in length) were prepared and mounted for isometric tension recording in organ baths containing a standard physiological salt solution aerated with 95%CO2/5% O2, maintained at 37° C, pH 7.4. A resting tension of 4 g was applied. Most patients were prescribed Ca2+-channel antagonists pre-operatively and to remove any residual traces of medication, the segments were washed overnight with physiological salt solution at room temperature. The following day, the temperature was restored to 37° C (1 h equilibration) and the contractile response to the reference agonist KCl (45 mm) was determined. Once the maximum response to KCl was achieved a wash-off period followed (30 min). Cumulative concentration-effect curves to 5-HT and 5-CT and the selective 5-HT1B/1D-receptor agonists zolmitriptan, CP122,288, L-741,519 and L-741,604, were performed (1 nm-30 μm) where increasing concentrations of each drug were added once the previous challenge produced a plateau (2–3 min). Sumatriptan concentration-effect curves (10 nm–100 μm) were also carried out in the absence and presence of the antagonists GR125,743 or GR127,935 (10 nm, equilibration 30 min) where two consequetive concentration-effect curves were performed on a single segment. Vehicle controls were also performed in the same manner.
Binding studies: cell lines
[3H]-5-HT displacement studies were carried out on membranes (approximately 6 mg wet weight per tube) prepared from CHO (chinese hamster ovary) cell lines expressing human 5-HT1B-, 5-HT1D- and 5-HT1F-receptors. 5-HT (10 μm) was used to define non-specific binding. Membranes, radioligand (2 nm) and competing drug were made up in 50 mm Tris HCl buffer (containing 10 μm pargyline, 0.1% ascorbate, 4 mm CaCl2, pH 7.7) and were incubated for 30 min at 37° C. The reaction was terminated by filtration through GF/B filters using a Brandel cell harvester.
Functional studies: cell lines
Membranes from the CHO cells expressing human 5-HT1B-and 5-HT1D-receptors were prepared essentially as described by Lazareno & Birdsall [21]. The final pellets were resuspended in HEPES buffer (20 mm HEPES, 100 mm NaCl, 10 mm MgCl2, 0.1% ascorbate and 10 μm pargyline). Membranes (2.5 mg wet weight per tube) were incubated with 100 μm and 30 μm GDP for 5-HT1B- and 5-HT1D-receptors, respectively, and test drug for 20 min at 30° C, before being transferred to ice for 15 min. [35S]-GTPγS (100 pm) was added to all tubes and the tubes were incubated for a further 30 min at 30° C before being rapidly filtered over GF/B filters using a Brandel cell harvester.
Analysis of data
Human middle meningeal arteries
Concentration-effect curves for all the agonists tested in human middle meningeal arteries were calculated as a percentage of KCl (45 mm) evoked contraction. For the antagonist experiments sumatriptan concentration-effect curves were performed in the absence (control curve) and presence of each antagonist and here the responses were expressed relative to the maximum response obtained in the control curve (=100%). Curves were fitted to the mean data using weighted least squares non-linear regression analysis and the equation:
| 1 |
where the Emax is the maximum contraction evoked by each agonist, EC50 is the molar concentration of an agonist eliciting half maximal response and nH is the Hill coefficient (Grafit version 3.0, Erithacus Software Ltd, Staines, UK.).
Cell lines
In the cell based assay, concentration-effect curves of percentage inhibition for the [3H]-5-HT binding assay were plotted and analysed by non-linear, least squares regression analysis using an iterative curve fitting routine (Marquardt-Leveberg method, RS/1, BBN Sofware Products Corporation, MA, USA). The IC50 values (concentration of drug required to inhibit specific binding by 50%) for all three receptors, 5-HT1B, 5-HT1D and 5-HT1Fwere obtained. For the functional assays, Emax values, for 5-HT1B-and 5-HT1D-receptors were calculated from the concentration-effect curves and analysed by non-linear regression analysis using an iterative curve fitting routine (Marquardt-Levenberg method). Emax values for each drug were expressed as relative to the maximal response to 5-HT (10 μm=100%).
Correlations between agonist vasoconstrictor potency and receptor affinity
Correlations were made using linear regression analysis between the EC50 values obtained for vasoconstrictor potency and IC50 values obtained at 5-HT1B-, 5-HT1D- and 5-HT1F-receptors (logarithmic transformations) using BMDP software (University of California Press, Los Angeles).
Drugs and solutions
Modified physiological salt solution used for transport of arteries to the laboratories, had the following composition (mm): NaCl 90, KCl 5, MgSO4 0.5, Na2HPO4 1, NaHCO3 29, CaCl2 1.25, Na pyruvate 5, l-glutamic acid 5, fumaric acid 5 and EDTA (40 μm). The standard physiological salt solution used for the functional studies had the following composition (mm): NaCl 118, KCl 4.7, NaHCO3 25.3, KH2PO4 1.19, MgSO4 1.19, CaCl2 1.25 and glucose 11.1.
5-HT (5-hydroxytryptamine), 5-CT (5-carboxytryptamine) and guanosine 5′ diphosphate (GDP) were obtained from Sigma. Sumatriptan, zolmitriptan, CP122,288, L-741,519, L-741,604, GR125,743 and GR127,935 were synthesised by the Medicinal Chemistry Department at Merck Sharp & Dohme Research Laboratories, Harlow, UK. 5-hydroxy[3H]tryptamine creatinine sulphate (80–120 Ci mmol−1) and guanosine 5′[γ-thio] triphosphate, [35S] ([35S]GTPγS) (1000–1500 Ci mmol−1) were purchased from Amersham International.
Results
Human middle meningeal arteries: effect of 5-HT-receptor agonists
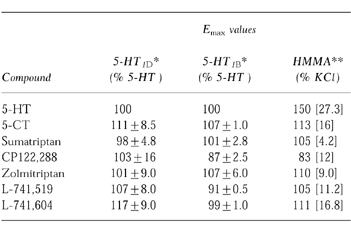
All the agonists caused concentration-dependent tonic contractions of human isolated middle meningeal arteries and the EC50 values are shown in Table 1 (n = 4–6). The rank order of potency was 5-CT > L-741,604 > L-741, 519 > zolmitriptan > 5-HT > sumatriptan > CP122 288. Similar maximal contractions (Emax) were observed for all the above agonists (83–113% relative to 45 mm KCl), with the exception of 5-HT which produced a greater maximal contraction (150% relative to 45 mm KCl, see Table 2, n = 4–6).
Table 1.
Measurements (nm) of the vasoconstrictor potency (EC50) in human middle meningeal arteries (HMMA) and affinity for cloned human 5-HT1-receptors (IC50). EC50 values represent asymtotic mean [asymtotic error] derived from non-linear regression analysis. Curves were fitted to the overalll mean data (n = 4–6). IC50 values represent mean values (±s.e.mean) derived from n = 3–4 experiments. Concentration-effect curves of percentage inhibition for [3H]-5-HT binding assays were plotted and analysed by non-linear, least squares regression analysis using an iterative curve fitting routine (Marquardt-Levenberg method). IC50 values were calculate from these curves.
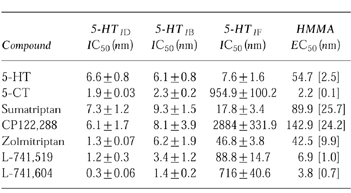
Table 2.
Measurements of the Emax values obtained for [35S]GTPγS studies for 5-HT1B/1D-receptors and human middle meningeal arteries. *For the cell line data values represent means derived from n = 2–3 experiments (±s.e.mean). Concentration-effect curves of percentage inhibition in binding above the basal for the [35S]GTPγS assay were plotted and analysed by non-linear, least squares regression analysis using an iterative curve fitting routine (Marquardt-Levenberg method). Emax values were expressed as a percentage of the maximal 5-HT (10 μm) response. **For human middle meningeal arteries values represent mean [asymptotic error] expressed as a percentage of the KCl (45 mm) evoked response derived from non-linear regression analysis. Curves were fitted to the overall mean data (n = 4–6).
Human middle meningeal arteries: effect of selective 5-HT1B/1D-receptor antagonists
The selective 5-HT1B/1D-receptor antagonists GR125,743 and GR127,935 antagonised sumatriptan evoked contractions of human isolated middle meningeal arteries. GR127,935 (10 nm) acted as a non-competitive antagonist reducing the maximum response by 73% (see Figure 1, n = 4), whereas, GR125 743 (10 nm) competitively inhibited the responses giving an apparent pA2 of 9.1 (see Figure 1, n = 4). Vehicle control studies using sumatriptan in human middle meningeal arteries demonstrated there was no difference in first and second concentration-effect curves (see Figure 1, n = 5).
Figure 1.
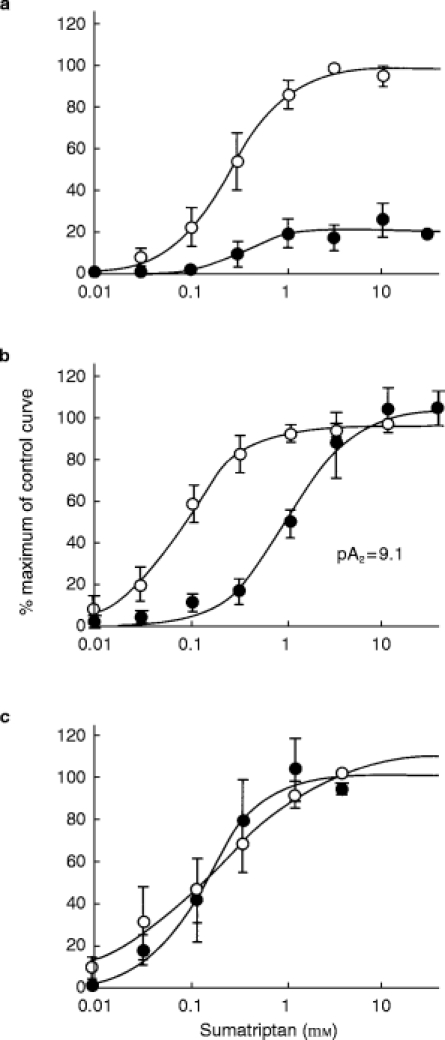
Effect of sumatriptan in human isolated middle meningeal arteries. a) concentration-effect curves to sumatriptan in the absence (open circles) and presence of GR127,935 (10 nm, filled circles). b) concentration-effect curves to sumatriptan in the absence (open circles) and presence of GR125,743 (10 nm, filled circles). c) concentration-effect curves to sumatriptan performed consecutively in the absence of antagonist (first concentration-effect curve—open circles, second concentration-effect curve—closed circles). Each point (mean value, n = 4–5) is expressed as a percentage of the maximum contraction obtained in the control curve and vertical bars signify±s.e.mean. Curves were fitted to a model using weighted least squares non-linear regression analysis.
Cell based assays
For the cloned human 5-HT1B- and 5-HT1D-receptors the IC50 values, for all seven agonists, were within a narrow range. For 5-HT1B-receptors this range was 1.4 nm to 9.3 nm and for 5-HT1D-receptors the range was 0.3 nm to 7.3 nm (Table 1) and none of the compounds showed greater than 5-fold selectivity for 5-HT1D-receptors over 5-HT1B-receptors. However, a greater range (7.6–2884 nm) of affinities for the cloned 5-HT1F-receptors was observed giving a rank order of affinity at 5-HT1F-receptors of 5-HT>sumatriptan > zolmitriptan > L-741,519 > L-741,604 > 5-CT > CP122,288 (see Table 1). Emax values for these compounds at 5-HT1B-and 5-HT1D-receptors were similar, displaying full agonism (relative to 5-HT) at both receptor subtypes (see Table 2).
Correlation between vasoconstrictor potency in human middle meningeal arteries and affinity at cloned 5-HT1-receptors
For the series of agonists used, there was a significant correlation between vasoconstrictor potency in human middle meningeal artery and IC50 values obtained at human cloned 5-HT1B-receptors (r = 0.93, P = 0.002, see Figure 2). The relationship between vasoconstrictor potency and IC50 values for 5-HT1D-receptor cell line was weaker and did not achieve statistical significance (r = 0.74, P = 0.06, see Figure 2). There was no relationship between vasoconstrictor potency and IC50 values for 5-HT1F-receptor cell line (r = 0.31, P = 0.49, see Figure 2).
Figure 2.
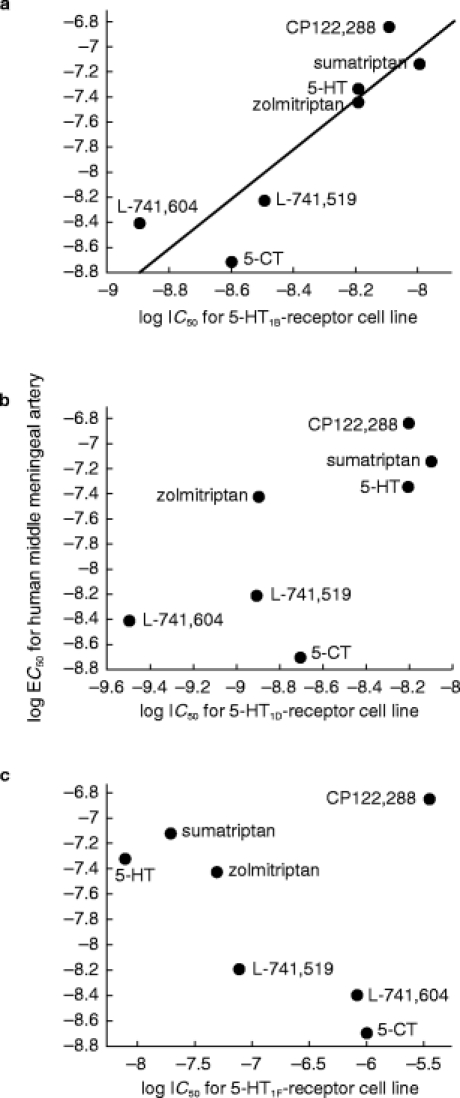
Correlations between binding affinity (IC50) at human cloned 5-HT1B, 5-HT1D and 5-HT1F-receptors expressed in CHO cell lines and vasoconstrictor potency (EC50) in human isolated middle meningeal arteries. a) 5-HT1B-receptor affinity vs vasoconstrictor potency, r = 0.93 (P = 0.002). b) 5-HT1D-receptor affinity vs vasoconstrictor potency, r = 0.74 (P = 0.06). c) 5-HT1F-receptor affinity vs vasoconstrictor potency, r = 0.31 (P = 0.49).
Efficacy values for human middle meningeal arteries and 5-HT1B-and 5-HT1D-receptor cell lines
The Emax values obtained in human middle meningeal arteries and the Emax values obtained for 5-HT1B-and 5-HT1D-receptors in the cell lines (see Table 2) for the selective 5-HT1B/1D-receptor agonists, which have no affinity at 5-HT2-receptors, were similar. However, 5-HT was more efficacious producing a bigger contractile response in human middle meningeal arteries than the selective agonists.
Discussion
GR127,935 and GR125,743 have been shown to be selective 5-HT1B/1D-receptor antagonists with pKi values of 8.5 at both of these receptor subtypes [18, 19] but have relatively low affinity (micromolar) for 5-HT1F-receptors (unpublished in-house data). In the present study, GR125,743 inhibited sumatriptan evoked contractile responses in human isolated middle meningeal artery and since this antagonist acted in a competitive manner it was possible to calculate an apparent pA2 value of 9.1. GR127,935 (used at a concentration expected to produce substantial 5-HT1B/1D-receptor blockade but not to interact with 5-HT1F-receptors) non-competitively antagonised contractile responses to sumatriptan, producing a marked depression of the maximal response by 73%. Non-competitive antagonistic effects of GR127,935 have also been reported in other blood vessels and have been attributed to slow dissociation kinetics of this compound [18, 22]. The use of GR127,935 and GR125,743 as selective 5-HT1B/1D-receptor antagonists indicates that the majority of the sumatriptan vasoconstrictor response in human middle meningeal artery is mediated by 5-HT1B/1D-receptor activation with little contribution from 5-HT1F-receptors.
In the second part of this study, we used a series of 5-HT-receptor agonists and correlated their vasoconstrictor potency in human middle meningeal artery with measurements of affinity obtained in cell lines expressing either human 5-HT1B-, 5-HT1D-or 5-HT1F-receptors. These studies showed that vasoconstriction in human middle meningeal artery is 5-HT1B-receptor mediated. There was a significant correlation (r = 0.93, P = 0.002) between vasoconstrictor potency in human middle meningeal artery and binding affinity at 5-HT1B-receptors. In contrast, the relationship between vasoconstrictor potency and binding affinity at 5-HT1D-receptors was weaker and not statistically significant (r = 0.74, P = 0.06). Finally, there was no relationship between vasoconstrictor potency and 5-HT1F-receptor affinity (r = 0.33, P = 0.46). These findings are consistent with previous reports showing positive correlations using both full and partial agonists in causing vasoconstriction in human isolated cerebral and coronary arteries and affinity at cloned 5-HT1B-receptors [16, 23].
The present results demonstrate that there was little difference in binding affinity between the agonists for 5-HT1D-receptors (i.e. range between 0.3–7.3 nm) or for 5-HT1B-receptors (i.e. range 1.4–9.3 nm), however, the range of affinities for 5-HT1F-receptors was much greater (i.e. range 7.6–2884 nm). Furthermore, although some compounds (e.g. 5-CT, CP122,288) showed marked differences in affinity for either 5-HT1B or 5-HT1D-receptors over 5-HT1F-receptors (i.e. >356-fold), there was little difference in affinity (i.e. <3-fold) for 5-HT1B-over 5-HT1D-receptors. Nevertheless, it was possible to detect a significant correlation between vasoconstrictor potency and 5-HT1B-receptor affinity but not 5-HT1D- or 5-HT1F-receptor affinity. The present study shows that the agonists retain high affinity for 5-HT1D-receptors and the EC50 values from functional experiments in the cell lines matched the IC50 values obtained in binding experiments, as previously shown for cloned human 5-HT1D-and 5-HT1F-receptors expressed in cell lines [6, 24, 25]. In contrast, whilst the same agonists retain high affinity for human cloned 5-HT1B-receptors, their functional potency at this receptor is greatly reduced compared with their binding affinity (see also [24, 25]). Interestingly, this functional selectivity is also retained for dog cloned 5-HT1D-receptors over 5-HT1B-receptors [26] and may reflect a property of the cloned receptors and the cell expression system. Alternatively, it may reflect differences in the intracellular coupling mechanisms of the receptors per se. It is not known whether this functional selectivity is maintained in native systems for although it can be shown that EC50 values for vasoconstrictor potency in middle meningeal artery match closely the EC50 values obtained in functional assays in the 5-HT1B-receptor cell line, this comparison cannot be done for 5-HT1D-receptors since there is no functional bioassay for native 5-HT1D-receptors.
In the present study there is a weak relationship between vasoconstrictor potency and 5-HT1D-receptor affinity and this is consistant with previous reports using other human arteries [16, 27]. The possibility that 5-HT1D-receptor activation contributes to vasoconstriction can be excluded since molecular and immunohistochemical studies have failed to detect the presence of 5-HT1D-receptor protein or its mRNA in vascular smooth muscle [16, 28]. Expression of mRNA coding for 5-HT1D-receptors has been detected in human cultured endothelial cells [29]. The physiological role of this receptor is unclear (assuming that the mRNA is translated into functional receptor protein). Indeed, in the case of 5-HT1F-receptors, although mRNA has been detected in vascular smooth muscle cells [8], this receptor clearly does not mediate vasoconstriction in human middle meningeal and furthermore, Johnson et al. [7] reported that the 5-HT1F-receptor selective agonist, LY334370, did not cause vasoconstriction of rabbit saphenous vein.
Since the present results indicate that vasoconstriction in human middle meningeal arteries is 5-HT1B-receptor mediated, comparisons of agonist efficacies were made with efficacy measurements obtained in the human 5-HT1B-receptor cell line. In middle meningeal arteries there was little difference in the maximal contractile responses elicited by the 5-HT1B/1D-receptor selective agonists in human isolated middle meningeal artery. The Emax values for zolmitriptan, L-741,519, L-741,604 and 5-CT were similar in magnitude to those produced by sumatriptan, whereas, CP122,288 produced the lowest maximal contractile response. These data were comparable with the Emax values obtained for these compounds in the 5-HT1B-receptor cell line where CP122,288 produced the lowest stimulation of [35S]GTPγS turnover. The size of the vasoconstrictor response to 5-HT was greater than those of the selective 5-HT1B/1D-receptor agonists and most likely reflects that the effect of 5-HT is mediated through the activation of both 5-HT1B- and 5-HT2-receptors. Indeed, in other human isolated blood vessels such as saphenous vein, coronary and temporal arteries both 5-HT1B- and 5-HT2-receptors can contribute to the vasoconstrictor responses to 5-HT [23, 30, 31]. Compared with the vessel types mentioned above the 5-HT2-receptor component in middle meningeal blood vessels, is somewhat smaller and therefore, the response to 5-HT is predominantly mediated via 5-HT1B-receptor activation with a small 5-HT2-receptor component.
In terms of treating migraine, it is not clear whether vasoconstriction and/or inhibition of neuropeptide release from sensory nerve fibres are important for the therapeutic action. LY334370, the selective 5-HT1F-receptor agonist which inhibits dural neurogenic inflammation in guinea-pigs [7, 12] is now being tested in clinical trials as an anti-migraine agent. If successful, this would imply that the neuronal mechanism is important. However, unequivocal evidence for the occurrence of dural neurogenic inflammation in man is not available and recent clinical trials using a substance P NK1-receptor antagonist, lanepitant, LY303870 (a class of compound that is effective in blocking neurogenic extravasation in rats and guinea-pigs, [32, 33], has been found to be ineffective in alleviating migraine headache [34]. Hence, if vasoconstriction is an important factor for migraine headache relief then selective 5-HT1F-receptor agonists, which appear to be devoid of vasoconstrictor properties, may not prove to be successful anti-migraine treatments.
Acknowledgments
Thank you to the neurosurgeons at Addenbrookes Hospital and of course the patients who donated their tissues.
References
- 1.Connor HE, Beattie DT. 5-Hydroxytryptamine receptor subtypes and migraine. In: Sandler M, Ferrari M, Harnett S, editors. Migraine: Pharmacology and Genetics. Chapman and Hall; 1996. pp. 18–31. [Google Scholar]
- 2.Weinshank RL, Zgombick JM, Macchi M, Branchek TA, Hartig PR. Human serotonin1D receptor is encoded by a subfamily of two distinct genes: 5-HT1Dα and 5-HT1Dβ. Proc Natl Acad Sci. 1992;89:3630–3634. doi: 10.1073/pnas.89.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friberg L, Olesen J, Iversen HK, Sperling B. Migraine pain associated with middle cerebral artery dilatation: reversal by sumatriptan. Lancet. 1991;338:13–17. doi: 10.1016/0140-6736(91)90005-a. [DOI] [PubMed] [Google Scholar]
- 4.Humphrey PPA, Feniuk W. Mode of action of the anti-migraine drug sumatriptan. Trends Pharmacol Sci. 1991;12:444–446. doi: 10.1016/0165-6147(91)90630-b. [DOI] [PubMed] [Google Scholar]
- 5.Moskowitz MA. Neurogenic versus vascular mechanisms of sumatriptan and ergot alkaloids in migraine. Trends Pharmacol Sci. 1992;13:307–311. doi: 10.1016/0165-6147(92)90097-p. [DOI] [PubMed] [Google Scholar]
- 6.Adham N, Borden LA, Schechter LE, et al. Cell-specific coupling of the human 5-HT1F receptor to multiple signal transduction pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1993;348:566–575. doi: 10.1007/BF00167231. [DOI] [PubMed] [Google Scholar]
- 7.Johnson KW, Schaus JM, Durkin MM, et al. 5-HT1F- receptor agonists inhibit neurogenic dural inflammation in guinea pigs. Neuroreport. 1997;8:2237–2240. doi: 10.1097/00001756-199707070-00029. [DOI] [PubMed] [Google Scholar]
- 8.Bouchelet I, Cohen Z, Case B, Seguela P, Hamel E. Differential expression of sumatriptan-sensitive 5-hydroxytryptamine receptors in human trigeminal ganglia and cerebral blood vessels. Mol Pharmacol. 1996;60:219–223. [PubMed] [Google Scholar]
- 9.Buzzi MG, Moskowitz MA. The anti-migraine drug, sumatriptan (GR43175), selectively blocks neurogenic plasma extravasation from blood vessels in the dura mater. Br J Pharmacol. 1990;99:202–206. doi: 10.1111/j.1476-5381.1990.tb14679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin GR. Pre-clinical profile of the novel 5-HT1D receptor agonist 311C90. In: Clifford Rose F, editor. New Advances in Headache Research. Vol. 4. Nishimura: Smith Gordon; 1994. pp. 3–4. [Google Scholar]
- 11.Williamson DJ, Hargreaves RJ, Hill RG, Shepheard SL. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat—intravital microscope studies. Cephalalgia. 1997;17:525–531. doi: 10.1046/j.1468-2982.1997.1704525.x. [DOI] [PubMed] [Google Scholar]
- 12.Phebus LA, Johnson KW, Audia JE, et al. Characterisation of LY334370, a potent and selective 5-HT1F receptor agonist, in the neurogenic dural inflammation model of migraine pain. Proc Soc Neurosci (USA) 1996;22:1331. [Google Scholar]
- 13.Pascual J, Del-arco C, Romon T, Del-olmo E, Pazos A. [3H]Sumatriptan binding sites in human brain: regional dependent labelling of 5 HT1D and 5-HT1F receptors. Eur J Pharmacol. 1996;295:271–274. doi: 10.1016/0014-2999(95)00748-2. [DOI] [PubMed] [Google Scholar]
- 14.Waeber C, Moskowitz MA. [3H]sumatriptan labels both 5-HT1D and 5-HT1F receptor binding sites in the guinea-pig brain: an autoradiographic study. Naunyn-Schmiedebergs Arch Pharmacol. 1995;352:263–275. doi: 10.1007/BF00168556. [DOI] [PubMed] [Google Scholar]
- 15.Goadsby PJ, Knight YE. Direct evidence for central sites of action of zolmitriptan (311C90): an autoradiographic study in cat. Cephalalgia. 1997;17:153–158. doi: 10.1046/j.1468-2982.1997.1703153.x. [DOI] [PubMed] [Google Scholar]
- 16.Hamel E, Fan E, Linvill D, et al. Expression of mRNA for the serotonin 5-hydroxytryptamine1Dβ receptor subtye in human and bovine cerebral arteries. Mol Pharmacol. 1993;44:242–246. [PubMed] [Google Scholar]
- 17.Ullmer C, Schmuck K, Kalkman HO, Lubbert H. Expression of serotonin receptor mRNAs in blood vessels. FEBS Letts. 1995;370:215–221. doi: 10.1016/0014-5793(95)00828-w. [DOI] [PubMed] [Google Scholar]
- 18.Clitherow JW, Scopes D, Skingle M, et al. Evolution of a novel series of [(N,N-dimethylamino)propyl]- and piperazinylbenzanilides as the first selective 5-HT1D antagonists. J Med Chem. 1994;37:2253–2257. doi: 10.1021/jm00041a001. [DOI] [PubMed] [Google Scholar]
- 19.Scopes DIC, Clitherow JW, Mitchell WL, et al. Selective 5HT1D antagonists: novel series of biaryl anilides. Pap Amer Chem Soc. 1994;207:164. [Google Scholar]
- 20.Razzaque Z, Shaw D, Smith D, et al. Pharmacological analysis of 5-HT-receptor mediated vasoconstriction of human isolated middle meningeal arteries: determining the contribution of 5-HT1B- and 5-HT1F-receptor activation. Br J Pharmacol. 1996;120 doi: 10.1046/j.1365-2125.1999.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazareno S, Birdsall NJM. Pharmacological characterisation of acetylcholine-stimulated [35S]-GTPgammaS binding mediated by human muscarinic m1-m4 receptors: antagonist studies. Br J Pharmacol. 1993;109:1120–1127. doi: 10.1111/j.1476-5381.1993.tb13738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razzaque Z, Longmore J, Hill RG. Differences in the effects of ketanserin and GR127935 on 5-HT-receptor mediated responses in rabbit saphenous vein and guinea-pig jugular vein. Eur J Pharmacol. 1995;283:199–206. doi: 10.1016/0014-2999(95)00349-p. [DOI] [PubMed] [Google Scholar]
- 23.Kaumann AJ, Frenken M, Posival H, Brown AM. Variable participation of 5-HT1-like receptors and 5-HT2 receptors in serotonin-induced contraction of human isolated coronary arteries. Circulation. 1994;90:1141–1153. doi: 10.1161/01.cir.90.3.1141. [DOI] [PubMed] [Google Scholar]
- 24.Zgombick JM, Borden LA, Cochran TA, et al. Dual coupling of cloned human 5-hydroxytryptamine1Dα and 5-hydroxytryptamine1Dβ receptors stably expressed in murine fibroblasts: inhibition of adenylate cyclase and elevation of intracellular calcium concentrations via pertussis toxin-sensitive G proteins. Mol Pharmacol. 1993;44:575–582. [PubMed] [Google Scholar]
- 25.Pauwels PJ, Colpaert FC. Selective antagonism of human 5-HT1D and 5-HT1B receptor mediated responses in stably transfected C6-glial cells by ketanserin and GR127,935. Eur J Pharmacol. 1996;300:141–145. doi: 10.1016/0014-2999(96)00011-8. [DOI] [PubMed] [Google Scholar]
- 26.Shaw D, Stanton JA, Beer MS, et al. In vitro assessment of the vascular effects of 5-HT1D-receptor agonists: relationships with 5-HT1Dα- or 5-HT1Dβ-receptor binding affinity. Br J Pharmacol. 1996;119 [Google Scholar]
- 27.Kaumann AJ, Parsons AA, Brown AM. Human arterial constrictor serotonin receptors. Cardiovas Res. 1993;27:2094–2103. doi: 10.1093/cvr/27.12.2094. [DOI] [PubMed] [Google Scholar]
- 28.Longmore J, Razzaque Z, Shaw D, et al. Comparison of the middle meningeal versus coronary artery selectivity of the 5HT1B/1D-receptor agonists rizatriptan and sumatriptan using human isolated blood vessels. Br J Clin Pharmacol. 1999 (in press) [Google Scholar]
- 29.Cohen Z, Bouchelet I, Yong VW, et al. Differential expression of serotonin receptors 5-HT1Dα and 5-HT2A mRNA in human brain vessels, vascular cells an astrocytes in culture. Society for Neuroscience Abstracts. 1995;21:1853. (727.10) [Google Scholar]
- 30.Bax WA, Van Nolsen-heuven D, Tadipatri S, et al. Sumatriptan contracts human isolated saphenous vein via a 5-HT1-like receptor resembling the 5-HT1D receptor subtype. In: Olesen J, Saxena PR, editors. 5-Hydroxytryptamine Mechanisms in Primary Headache. New York: Raven Press; 1993. pp. 178–182. [Google Scholar]
- 31.Verheggen R, Freudenthaler S, Meyer-Dulheuer F, Kaumann AJ. Participation of 5-HT1-like and 5-HT2A receptors in the contraction of human temporal artery by 5-hydroxytryptamine and related drugs. Br J Pharmacol. 1996;117:283–292. doi: 10.1111/j.1476-5381.1996.tb15188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shepheard SL, Williamson DJ, Hill RG, Hargreaves RJ. The non-peptide neurokinin1 receptor antagonist, RP67580, blocks neurogenic plasma extravasation in the dura mater of rats. Br J Pharmacol. 1993;108:11–12. doi: 10.1111/j.1476-5381.1993.tb13432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phebus LA, Johnson KW, Stengel PW, et al. The non-peptide NK-1 receptor antagonist LY303870 inhibits neurogenic dural inflammation in guinea-pigs. Life Sci. 1997;60:1553–1561. doi: 10.1016/s0024-3205(97)00121-5. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein D. Tachykinins and their antagonists. London: William Harvey Research Conferences; 1996. Update on clinical trials with lanepitant ( LY303870) [Google Scholar]


