Abstract
Aims
Cysteamine, the only drug available for the treatment of cystinosis in paediatric patients, is available as the hydrochloride, the bitartrate and as sodium phosphocysteamine salts. It has been suggested that cysteamine bitartrate and phosphocysteamine are better tolerated and may have a better bioavailability than cysteamine hydrochloride. This has, however, never been demonstrated.
Methods
We compared the pharmacokinetics and tolerance of these three formulations of cysteamine in 18 healthy adult male volunteers in a double-blind, latin-square, three-period, single oral dose cross-over relative bioavailability study.
Results
No statistical difference was found between relative bioavailabilities, AUC (0, ∞) (geometric mean and s.d. in μmol l−1h: 169±51, 158±46, 173±49 with cysteamine hydrochloride, phosphocysteamine and cysteamine bitartrate respectively), Cmax (geometric mean and s.d. in μmol l−1: 66±25.5, 59±12, 63±20) and tmax (median and range in h: 0.88 (0.25–2), 1.25 (0.25–2), 0.88 (0.25–2)) with each of the three forms of cysteamine tested. Bioequivalence statistics (90% confidence intervals) showed non equivalence of Cmax of cysteamine base as the only non equivalence of pharmacokinetics between the three formulations: 90% CI for Cmax relative ratios to cysteamine hydrochloride were [75.6–105.8] for phosphocysteamine and [74.2–124.2] for cysteamine bitartrate. The only significant adverse event was vomiting whose frequency was inversely correlated with body weight (Spearman’s r =−0.76, P<0.001). The nature of the salt tested did not influence vomiting.
Conclusions
While none of the three forms of cysteamine tested has a clear advantage over the others in terms of pharmacokinetics and tolerance profile, this should now however be addressed in patients treated for cystinosis during repeat administrations.
Keywords: cysteamine, phosphocysteamine, cysteamine salts, pharmacokinetics, bioequivalence, adverse event, cystinosis
Introduction
Cystinosis generally leads to death in infancy before 10 years of age unless dialysis or renal transplantation is performed. No current therapy can definitively cure the disease. Due to its relatively low prevalence, the pharmaceutical industry has little interest in developing drugs for its treatment and cystinosis can therefore be considered as an orphan disease.
Cysteamine (β-mercapto-ethylamine) has been used as a cystine depleting agent under its hydrochloride formulation for more than 20 years [1] (50 mg kg−1 day−1) in paediatric patients. Previous studies showed that its absolute bioavailability could be less than 10% [2] and it has been suggested that it could be improved by administration of sodium phosphocysteamine [3] (cysteamine phosphothioester) which has been approved for clinical use since 1980 in the USA [4] (32 mg kg−1 daily). Another cysteamine salt, cysteamine bitartrate with an allegedly improved bioavailability relative to cysteamine hydrochloride [5] has recently been approved by the FDA.
However, the optimal dosing regimen and relative bioavailability of the various cysteamine formulations remain unknown. The present study compares the relative bioavailability and pharmacokinetics of cysteamine base following administration of three cysteamine formulations and compares their gastro-intestinal tolerance.
Methods
Mean age of the 20 subjects included in this study was 26±5 years (range: 20 to 36 years) and mean body weight was 71±10 kg (range: 52 to 92 kg). Two subjects (body weight 59 kg and 66 kg) withdrew after the first study period due to intolerable vomiting. Eighteen healthy male adults were given single oral administrations of 15.55 mmol (1200 mg) of cysteamine base (1768 mg cysteamine hydrochloride (C2H7NS.HCl), 2750 mg phosphocysteamine (C2H7NS.NaPO3) or 3534 mg cysteamine bitartrate (C2H7NS.C4H6O6)) conditioned into 11 gelatin capsules, in a double-blind, randomized cross-over balanced latin-square trial. All subjects were studied after an overnight fast and a light standardized breakfast on three occasions separated by a drug-free interval of 3 to 5 days. The study was approved by the Committee for the Protection of Human Subjects in Biomedical Research of Cochin Hospital (Paris).
Drugs were administered at 08.30 h and subjects stayed in a sitting position for 1 h thereafter. Standardized meals were given at 13.00 h and 19.00 h. During each study period, a 7 ml blood sample was taken before and 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 4, 6, 8 and 12 h after cysteamine administration. Cysteamine was quantified using a sensitive and specific h.p.l.c. assay, with fluorescence detection of cysteamine base as described previously [6]. The limit of quantification was 2 nmol ml−1 and a limit of detection (signal-to-noise ratio of 3) of 0.5 nmol ml−1 was obtained. The CV (%) was 10.4 and 11.5 (respectively within and between day) for a concentration of 2 μmol l−1 and 0.7 and 0.8 for 150 μmol l−1.
Pharmacokinetic parameters were calculated with use of a non-compartmental model using data from eighteen subjects who received the three forms of cysteamine and completed the whole study. After log transformation, AUC(0,t) AUC(0,∞) and Cmax were compared by ANOVA and Dunnett-test. Parameters expressed as 90% confidence interval ratio had to be within the 80–125% range to declare bioequivalence [7]. Experimental tmax data were compared by using the Kruskall-Wallis and Wilcoxon non parametric tests for paired samples. Tolerance parameters (number of vomitings episodes and ease for digesting cysteamine capsules assessed on a 10 cm visual analogue scale, VAS) were analyzed, 3 and 10 h after drug administration, with use of non parametric tests using data of all subjects (n =20).
Results
Pharmacokinetics
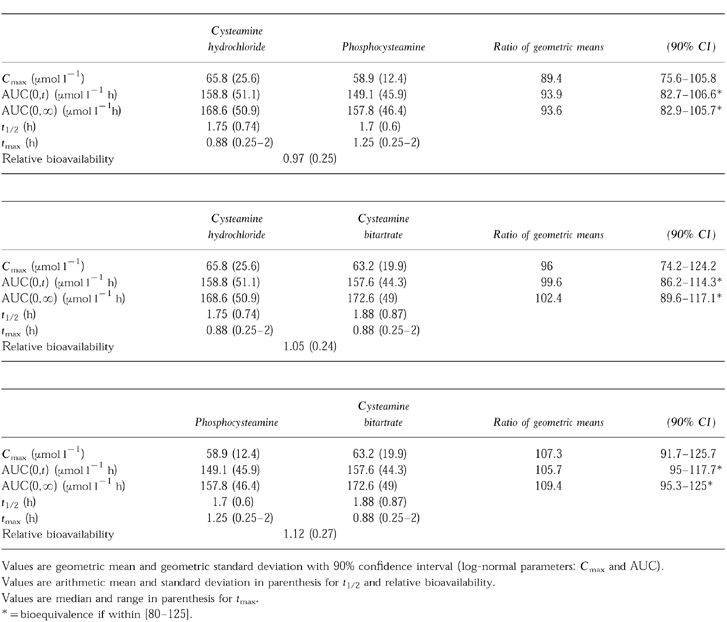
There was no significant difference in AUC(0,t) and AUC(0,∞) among the various formulations of cysteamine (Table 1). The 90% confidence interval ratios were within the 80–125% interval. Thus, all three forms were bioequivalent with respect to AUC. There was no statistically significant difference in the relative bioavailability or in t½ for cysteamine among the various formulations. Although there was no statistically significant differences among values of observed Cmax, the values did not strictly reflect bioequivalence (Table 1) according to the 80–125% criterion. Referring to the 70–143%Cmax bioequivalence interval proposed by Steinijans et al. [8] and the FDA’s Guidelines, the formulations would be declared bioequivalent on the basis of values for Cmax.
Table 1.
Plasma pharmacokinetic parameters of cysteamine hydrochloride, phosphocysteamine and cysteamine bitartrate for 18 subjects.
Digestive tolerance
Eleven subjects (55%) never vomited during the study. Their mean (±s.d.) body weight was 78±8 kg (range 72 to 92 kg). Nine subjects (45%) vomited at least once during their participation in the study (median and extreme times from drug intake: 2.5 h (0.7 to 3.4 h)). AUC(0,∞), Cmax, tmax and t½ did not differ significantly in subjects who vomited and in those who did not. In the first study period, the mean body weight was 63±7 kg (range 52 to 74 kg) in subjects who vomited and was lower than the body weight of subjects who did not (P<0.05). There was an inverse correlation (Spearman’s r =−0.76; P<0.001) between the total number of vomiting episodes experienced during the first study period and body weight in the 20 subjects who were included in the study.
All subjects who vomited at least once did so during the first study period, whichever form was administered first in the randomization schedule. However, this statistically-significant (P<0.03) study-period effect was not associated with a treatment effect, i.e. the nature of the cysteamine formulation administered did not influence the occurrence of vomiting. There was no significant difference in the gastro-intestinal tolerance, measured with the VAS, for each formulation of cysteamine (Table 2). Digestive tolerance significantly improved over time (between 3 h and 10 h post drug intake) following administration of cysteamine hydrochloride and phosphocysteamine but not of cysteamine bitartrate.
Table 2.
Visual scale assessment of cysteamine gastro-intestinal tolerance.
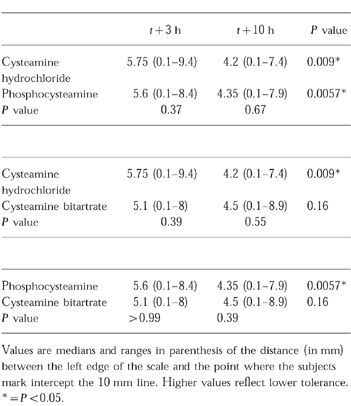
There was an inverse correlation between body weight and ease of digestion of the three cysteamine forms at 3 h (Spearman’s r =−0.35, P =0.008) and at 10 h (Spearman’s r =−0.59, P<0.001).
Discussion
No significant pharmacokinetic difference was found in our study among the three formulations of cysteamine. Digestive tolerance was equally poor and was significantly associated with a low body weight. Data indicated the three formulations to be bioequivalent except for Cmax which tended to be higher after administration of cysteamine hydrochloride. However, using a wider confidence interval, as has recently been proposed [8], the values of this parameter also suggest bioequivalence. Our results do not support the preferential use of one of these forms of cysteamine over the others based on a clear advantage in terms of pharmacokinetic profile or gastro-intestinal tolerance.
We performed this study in healthy volunteers and it is questionable whether our findings can be extrapolated to children treated for cystinosis and to repeat administration. However, there is no evidence that age influences the pharmacokinetics of cysteamine. Additionally, in view of its short half-life, it is unlikely that cysteamine will accumulate in plasma on repeat administration.
Gastro-intestinal intolerance to cysteamine is a major source of non-compliance to treatment which was reported to be as high as 14% in a study of 93 children receiving cysteamine hydrochloride orally [9]. However, gastro-intestinal intolerance is also observed after intravenous administration suggesting that cysteamine may have a centrally-mediated emetic action [10].
Acknowledgments
This study was supported by grants in aid from the GERMED, the INSERM and the ‘Assistance Publique—Hôpitaux de Paris’ at the Clinical Investigation Center of Saint-Antoine University Hospital. We thank S. Deraedt, M. Stachowicz, F. Gloaguen, J.L. Demolis and D. Costagliola for their contribution to the conduct of the study.
References
- 1.Thoene JG, Oshima RG, Crawhall JC, Olson DL, Schneider JA. Intracellular cystine depletion by aminothiols in vitro and in vivo. J Clin Invest. 1976;58:180–189. doi: 10.1172/JCI108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolin LA, Clark KF, Thoene JG, Gahl WA, Schneider JA. A comparison of the effectiveness of cysteamine and phosphocysteamine in elevating plasma cysteamine concentration and decreasing leukocyte free in nephropathic cystinosis. Ped Res. 1988;23:616–620. doi: 10.1203/00006450-198806000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Smolin LA, Schneider JA. Measurement of total plasma cysteamine using high-performance liquid chromatography with electrochemical detection. Anal Biochem. 1988;168:374–379. doi: 10.1016/0003-2697(88)90332-6. [DOI] [PubMed] [Google Scholar]
- 4.Thoene JG, Lemons R. Cystine depletion of cystinotic tissues by phosphocysteamine. J Pediatr. 1980;96:1043–1044. doi: 10.1016/s0022-3476(80)80637-8. [DOI] [PubMed] [Google Scholar]
- 5.Schneider JA, Clark KF, Greene AA, et al. Recent advances in the treatment of cystinosis. J Inher Metab Dis. 1995;18:387–397. doi: 10.1007/BF00710051. [DOI] [PubMed] [Google Scholar]
- 6.Stachowicz M, Lehmann B, Tibi A, Prognon P, Daurat V, Pradeau D. Determination of total cysteamine in human serum by a suitable high-performance liquid chromatography with fluorescence detection. J Pharm Biomed Anal. 1998;17:767–773. doi: 10.1016/s0731-7085(97)00248-3. [DOI] [PubMed] [Google Scholar]
- 7.Westlake WJ. Bioavailability and bioequivalence of pharmaceutical formulations. In: Peace KE, editor. Biopharmaceutical statistics for drug development. New York: Marcel Dekker, Inc; 1988. pp. 329–352. [Google Scholar]
- 8.Steinijans VW, Hauschke D, Jonkman JHG. Controversies in bioequivalence studies. Clin Pharmacokinet. 1992;22:247–253. doi: 10.2165/00003088-199222040-00001. [DOI] [PubMed] [Google Scholar]
- 9.Gahl WA, Reed GF, Thoene JG, et al. Cysteamine therapy for children with nephropathic cystinosis. N Engl J Med. 1987;316:971–977. doi: 10.1056/NEJM198704163161602. [DOI] [PubMed] [Google Scholar]
- 10.Gahl WA, Ingelfinger J, Mohan P, Bernardini I, Hyman PE, Tangerman A. Intravenous cysteamine therapy for nephropathic cystinosis. Ped Res. 1995;38:579–584. doi: 10.1203/00006450-199510000-00018. [DOI] [PubMed] [Google Scholar]


