Abstract
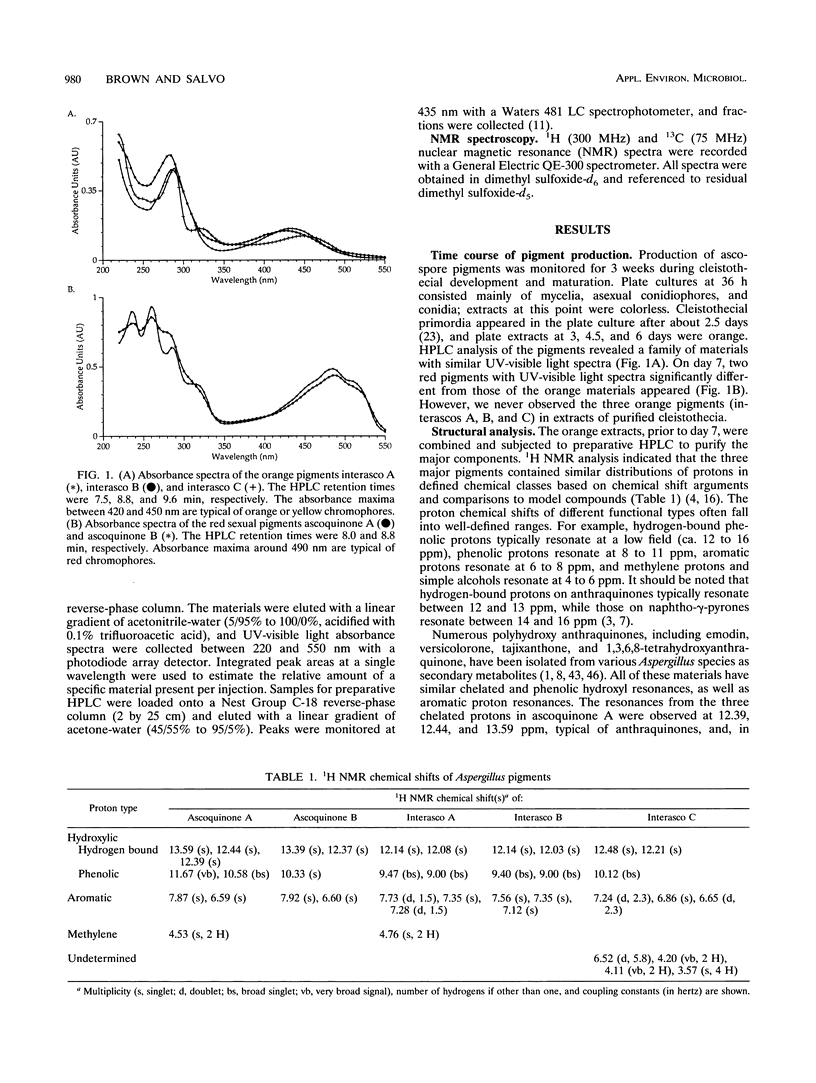
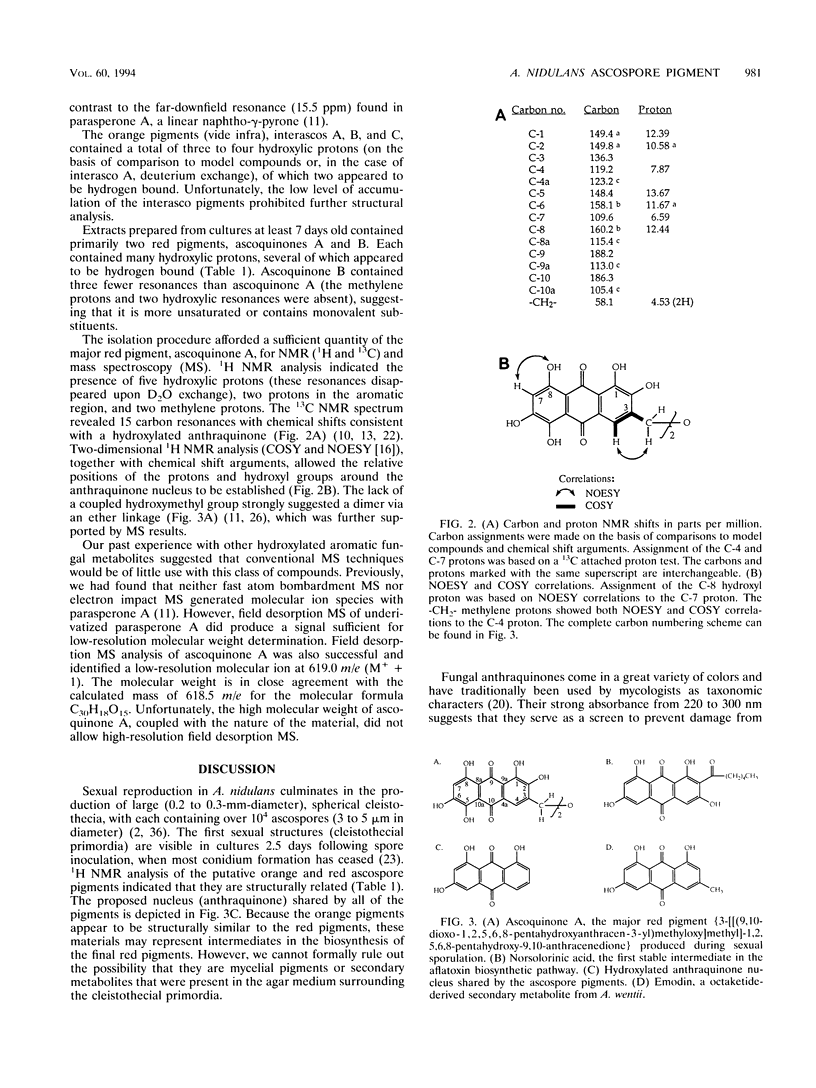
The homothallic ascomycete Aspergillus nidulans produces two types of pigmented spores: conidia and ascospores. The synthesis and localization of the spore pigments is developmentally regulated and occurs in specialized cell types. On the basis of spectroscopic evidence, we propose that the major ascospore pigment of A. nidulans (ascoquinone A) is a novel dimeric hydroxylated anthraquinone. The structure of ascoquinone A, as well as a comparison to model compounds, suggests that it is the product of a polyketide synthase. Previous studies have revealed that the conidial pigments from A. nidulans and a related Aspergillus species (A. parasiticus) also appear to be produced via polymerization of polyketide precursors (D. W. Brown, F. M. Hauser, R. Tommasi, S. Corlett, and J. J. Salvo, Tetrahedron Lett. 34:419-422, 1993; M. E. Mayorga and W. E. Timberlake, Mol. Gen. Genet. 235:205-212, 1992). The structural similarity between the ascospore pigment and the toxic anthraquinone norsolorinic acid, the first stable intermediate in the aflatoxin pathway, suggests an evolutionary relationship between the respective polyketide synthase systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends P., Helboe P. Xanthone studies. IV. Hydroxyl proton chemical shifts in the structural investigation of xanthones. Acta Chem Scand. 1972;26(10):4180–4182. doi: 10.3891/acta.chem.scand.26-4180. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Henderberg A., Grossman K. Sterigmatocystin production on complex and defined substrates. Mycopathologia. 1989 Jan;105(1):35–38. doi: 10.1007/BF00443827. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Lee L. S., Shoss S. M., Boudreaux G. H. Identification of averantin as an aflatoxin B1 precursor: placement in the biosynthetic pathway. Appl Environ Microbiol. 1980 Apr;39(4):835–839. doi: 10.1128/aem.39.4.835-839.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. K., Chater K. F. Spore colour in Streptomyces coelicolor A3(2) involves the developmentally regulated synthesis of a compound biosynthetically related to polyketide antibiotics. Mol Microbiol. 1990 Oct;4(10):1679–1691. doi: 10.1111/j.1365-2958.1990.tb00545.x. [DOI] [PubMed] [Google Scholar]
- Ellis W. O., Smith J. P., Simpson B. K., Oldham J. H. Aflatoxins in food: occurrence, biosynthesis, effects on organisms, detection, and methods of control. Crit Rev Food Sci Nutr. 1991;30(4):403–439. doi: 10.1080/10408399109527551. [DOI] [PubMed] [Google Scholar]
- Gill M., Steglich W. Pigments of fungi (Macromycetes). Fortschr Chem Org Naturst. 1987;51:1–317. doi: 10.1007/978-3-7091-6971-1_1. [DOI] [PubMed] [Google Scholar]
- HOWARD B. H., RAISTRICK H. Studies in the biochemistry of micro-organisms. 94. The colouring matters of species in the Aspergillus nidulans group. I. Asperthecin, a crystalline colouring matter of Aspergillus quadrilineatus Thom & Raper. Biochem J. 1955 Mar;59(3):475–484. doi: 10.1042/bj0590475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann T. E., Kurtz M. B., Champe S. P. Laccase localized in hulle cells and cleistothecial primordia of Aspergillus nidulans. J Bacteriol. 1983 May;154(2):955–964. doi: 10.1128/jb.154.2.955-964.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K., Morikawa K., Fujita M., Natori S. Inhibitory effects of plant secondary metabolites on cytotoxic activity of polymorphonuclear leucocytes. Planta Med. 1992 Apr;58(2):137–145. doi: 10.1055/s-2006-961415. [DOI] [PubMed] [Google Scholar]
- Krivobok S., Seigle-Murandi F., Steiman R., Marzin D. R., Betina V. Mutagenicity of substituted anthraquinones in the Ames/Salmonella microsome system. Mutat Res. 1992 May 1;279(1):1–8. doi: 10.1016/0165-1218(92)90259-3. [DOI] [PubMed] [Google Scholar]
- Leighton T. J., Stock J. J., Herring F. G. Electron spin resonance evaluation of pigment accumulation during asexual sporulation in Aspergillus flavus. Biochim Biophys Acta. 1971 Apr 20;237(1):128–131. doi: 10.1016/0304-4165(71)90039-0. [DOI] [PubMed] [Google Scholar]
- Mayorga M. E., Timberlake W. E. The developmentally regulated Aspergillus nidulans wA gene encodes a polypeptide homologous to polyketide and fatty acid synthases. Mol Gen Genet. 1992 Nov;235(2-3):205–212. doi: 10.1007/BF00279362. [DOI] [PubMed] [Google Scholar]
- Moreno M. A., Ramos M. C., González A., Suárez G. Effect of ultraviolet light irradiation on viability and aflatoxin production by Aspergillus parasiticus. Can J Microbiol. 1987 Oct;33(10):927–929. doi: 10.1139/m87-162. [DOI] [PubMed] [Google Scholar]
- NEELAKANTAN S., POCKER A., RAISTRICK H. Studies in the biochemistry of micro-organisms. 101. The colouring matters of species in the Aspergillus nidulans group. 2. Further observations on the structure of asperthecin. Biochem J. 1957 Jun;66(2):234–237. doi: 10.1042/bj0660234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak A. Melanin as a virulence factor in pathogenic fungi. Mycoses. 1990 May;33(5):215–224. doi: 10.1111/myc.1990.33.5.215. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Kelton W. H. Production of sterigmatocystin by some species of the genus Aspergillus and its toxicity to chicken embryos. Appl Microbiol. 1975 Oct;30(4):589–591. doi: 10.1128/am.30.4.589-591.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Egashira T., Takahama U. Inactivation of Neurospora crassa conidia by singlet molecular oxygen generated by a photosensitized reaction. J Bacteriol. 1979 May;138(2):293–296. doi: 10.1128/jb.138.2.293-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. M., Cole R. J., Kirksey J. W. Emodin, a toxic metabolite of Aspergillus wentii isolated from weevil-damaged chestnuts. Appl Microbiol. 1975 Jul;30(1):26–28. doi: 10.1128/am.30.1.26-28.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M. H., Bell A. A. Melanins and their importance in pathogenic fungi. Curr Top Med Mycol. 1988;2:338–387. doi: 10.1007/978-1-4612-3730-3_10. [DOI] [PubMed] [Google Scholar]



