Abstract
Aims
To assess CYP3A enzyme activity in human neonates by measuring the urinary 6β-hydroxycortisol/cortisol (6β-OHF/C) ratio.
Methods
Fifty-six mature male neonates with normal delivery, seventeen of their mothers and twenty-four healthy non-pregnant young women participated in this study. Urinary 6β-OHF/C ratio was determined on the day of birth in neonates and their mothers. In addition, changes in the ratio after birth were determined in neonates.
Results
On the day of birth, the urinary 6β-OHF/C ratio of neonates was significantly higher than that of their mothers (20.5 vs 6.9). In contrast, no significant difference was observed in the mean ratio of urinary 6β-OHF/C between women with and without pregnancy (6.9 vs 9.0). The urinary 6β-OHF/C ratio after birth was decreased day by day in neonates.
Conclusion
These results indicate that the high urinary 6β-OHF/C ratio in mature neonates on the day of birth is independent of the activity of CYP3A enzyme in their mothers.
Keywords: neonates, mothers, CYP3A, 6β-hydroxycortisol, urine
Introduction
In the hepatic cytochrome P450 supergene family, CYP3A isoforms play an important role in the metabolism of many exogenous and endogenous substrates [1]. In addition, the human CYP3A enzymes consist of at least three closely related haemoproteins [2]. It has been demonstrated that CYP3A4 is a major form of CYP3A enzymes in adult human livers, but CYP3A5 is polymorphically regulated and is expressed only in about 20% of adult human livers [3–6]. In contrast, CYP3A7, but not CYP3A4, has been shown to be a major form of CYP3A enzymes expressed in human fetal livers [7, 8]. These findings lead us to believe that the quantitative and/or qualitative changes in the forms of CYP3A enzymes occur in human livers after birth. However, there is, very little, if any, information on the capacities of drug metabolizing enzymes in human neonates mainly for ethical reasons.
The ratio of urinary 6β-OHF/C has been widely used as a noninvasive maker to investigate an inhibitory or stimulatory effect of drugs and chemical compounds on CYP3A enzymes [10–14]. We recently reported that the mean ratio of 6β-OHF/C was higher in mature neonates (gestational age >37 weeks) than those observed in adults and premature neonates whose gestational ages were less than 37 weeks [9]. In addition, we demonstrated that the mean ratio of 6β-OHF/C in mature neonates rapidly decreased during the early stage after birth whereas the ratio observed in premature neonates remained virtually unchanged after birth. However, the influence of 6β-OHF/C ratio in the mother on the ratio in the neonate on the day of their birth, is not clear at present. Therefore, in this study, we have investigated the urinary 6β-OHF/C ratio of mature neonates and their mothers on the day of birth to clarify whether CYP3A activity of mother affects the ratio of urinary 6β-OHF/C in neonate.
Methods
Materials
Cortisol and 6β-hydroxycortisol were purchased from Wako Chemical Co. (Osaka, Japan), and Steraloid Co. (Wilton, NH, USA), respectively. Other chemicals and reagents were of the highest grade commercially available.
Subjects
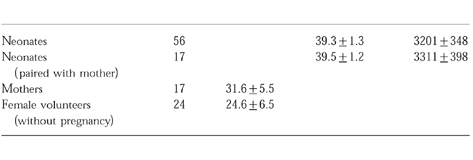
Fifty-six mature male neonates with normal delivery, and their mothers participated in this study to estimate the urinary 6β-OHF/C ratio. In addition, twenty-four healthy young female volunteers without pregnancy also participated. Subjects were not receiving any regular medication or suffering from any disease. Informed consent was obtained from the mothers and the volunteer women. The study was approved by the ethics committee of the School of Medicine, Chiba University. Table 1 shows details of subjects.
Table 1.
Details of subjects. Data are shown as mean±s.d.
Protocol
The first spot urine sample was collected in each neonate within 24 h of birth to obtain the urinary 6β-OHF/C ratio. Then, to estimate postnatal changes in the urinary 6β-OHF/C ratio, a morning spot urine sample from neonates was collected on days 1, 3, 5 and 7 after birth. In addition, urine samples of mothers were collected at 2 h after delivery. In the case of healthy young female volunteers, a morning spot urine sample was collected. The urine samples were stored at −20° C until use.
Analytical methods
The concentration of cortisol and 6β-hydroxycortisol were measured by h.p.l.c., and the concentration ratio of 6β-hydroxycortisol to free cortisol was calculated. The detailed procedure was carried out according to the method described previously [9].
Statistical methods
Statistical analysis was performed using a paired t-test for comparison of urinary 6β-OHF/C ratio on the birth day between neonates and their mothers. An unpaired t-test was performed for comparison of urinary 6β-OHF/C ratio between healthy young female volunteers and mothers on the day of delivery, and between the day of birth and 1, 3, 5, 7-days after birth in neonates. Data were expressed as the mean±s.d., and P < 0.05 was considered significant.
Results
As shown in Figure 1, the urinary 6β-OHF/C ratio decreased day by day after birth in neonates with normal delivery. The urinary 6β-OHF/C ratios on day 3 (10.9±7.1, P < 0.01), day 5 (8.7±5.5, P < 0.01), and day 7 (6.8±3.5, P < 0.01) were significantly lower than that obtained on the day of birth (17.6±7.8). Figure 2 shows a comparison of urinary 6β-OHF/C ratios between mature neonates, their mothers and female volunteers without pregnancy. On the day of birth, the urinary 6β-OHF/C ratio in all of the mature neonates studied was significantly higher than that in their mothers (6.9±3.7 vs 20.5±7.0, P < 0.01). On the other hand, no significant difference in urinary 6β-OHF/C ratio was observed between mothers and female volunteers without pregnancy (6.9±3.7 vs 9.0±5.7).
Figure 1.
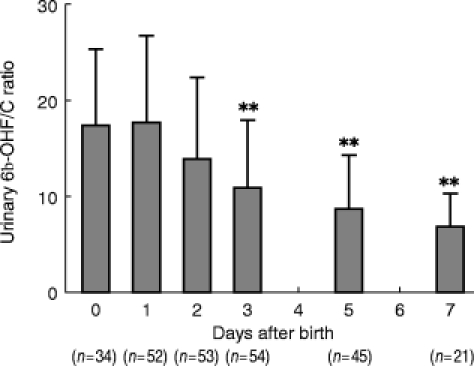
Changes in the urinary 6β-OHF/C ratio after birth in mature male neonates. Data are shown as mean±s.d. **P<0.01 compared with day of birth.
Figure 2.
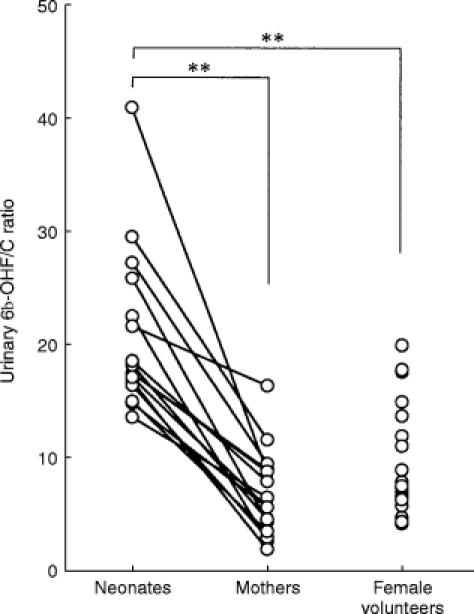
Comparison of urinary 6β-OHF/C ratio between mature male neonates with normal delivery (n = 17), their mothers (n = 17) and healthy young female volunteers without pregnancy (n = 24). Urine was collected within 24 h after birth in neonates, 2 h after delivery in their mothers, and in the morning in female volunteers. **P<0.01 compared with neonates.
Discussion
In this study using the large number of mature neonates with normal delivery, the urinary 6β-OHF/C ratio on the day of birth was found to be at a high level, and rapidly decreased during the post-partum period. These results were in agreement with our observations previously reported [9]. As one of the reasons why neonates on the day of birth showed a high urinary 6β-OHF/C ratio, the apparent metabolic ratio of cortisol in neonates is considered to be modulated by their mother’s CYP3A activity. That is, the possibility that the part of 6β-OHF measured in urine of neonates might be attributed to 6β-OHF transplacentally distributed from their mother, cannot be excluded. However, as shown in Figure 2, urinary 6β-OHF/C ratio of neonates on the day of birth were significantly higher than that of their mothers, indicating that CYP3A enzyme activity of the mother may not be contributing to a high urinary 6β-OHF/C ratio in neonates on the day of birth.
CYP3A7 and CYP3A4 have been clarified as major forms of CYP3A enzyme in fetal and adult livers, respectively, although CYP3A4 and CYP3A7 mRNAs can be detected both in foetal and adult livers [15]. Furthermore, it is well known that testosterone and dehydroepiandrosterone are probe substrates for CYP3A4 and CYP3A7, respectively. Lacroix et al. [16] have recently demonstrated, using liver microsomes of neonate, that 6β-hydroxylation of testosterone was at a low level in the early stages of birth, and began to rise after birth, whereas the 16α-hydroxylation of dehydroepiandrosterone was at a high level at the early stage of birth, and began to decrease after birth. In addition, in our preliminary investigation using CYP3A enzymes expressed in COS-7 cells, CYP3A7 as well as CYP3A4 was found to be capable of metabolizing 6β-hydroxylation of cortisol (unpublished results).
The results shown in this study suggest that the high urinary 6β-OHF/C ratio in mature neonates on the day of birth was independent of the activity of CYP3A enzyme in their mothers, and that the ratio rapidly decreased during the early stage after birth. At this time, we do not have a completely satisfying explanation for the mechanism by which urinary 6β-OHF/C ratio in mature neonates on the day of birth was higher than that observed in their mothers, and was decreased during the early stage after birth. The possibilities that other metabolic pathways of cortisol and/or cortisol metabolism in extrahepatic organs affects the ratio of 6β-OHF/C in neonates, cannot be excluded. It is, however, likely that the high level of urinary 6β-OHF/C ratio on the day of birth, and the change of the ratio in early stage after birth may be due to alteration of CYP3A7 level expressed in neonatal livers. For safe use of drugs in the neonatal period, more precise investigations to understand the changes in CYP3A enzymes after birth are important.
Acknowledgments
This work was partly supported by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture, Japan.
References
- 1.Shimada T, Yamazaki H, Miura M, Inui Y, Guengerich FP. Interindivisual variation in human liver cytochrome P450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 2.Wrighton SA, Ring BJ, Watkins PB, Vandenbranden M. Identification of a polymorphically expressed member of the human cytochrome P450III family. Mol Pharmacol. 1990;38:207–213. [PubMed] [Google Scholar]
- 3.Beaune PH, Umbenhauer DR, Bork RS Guengerich FP. Isolation and sequence determination of a cDNA clone related to human cytochrome P-450 nifedipine-oxidese. Proc Natl Acad Sci USA. 1986;83:8064–8068. doi: 10.1073/pnas.83.21.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bork RW, Muto T, Beaune PH, Srivastava PK, Lloyd RS, Guengerich FP. Characterization of mRNA species related to human liver cytochrome P-450 nifedipine oxidase and regulation of catalytic activity. J Biol Chem. 1989;246:910–919. [PubMed] [Google Scholar]
- 5.Aoyama T, Yamano S, Waxman DJ, et al. Cytochrome P450hCN3, anovel cytochrome P450IIIA gene product that is differentially expressed in adult human liver. J Biol Chem. 1989;264:10388–10395. [PubMed] [Google Scholar]
- 6.Wrighton SA, Brian WR, Sari MA, et al. Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5(HLp3) Mol Pharmacol. 1989;36:97–105. [PubMed] [Google Scholar]
- 7.Komori M, Nishino K, Fujitani T, et al. Isolation of a new human fetal liver cytochrome P450 cDNA in human fetal liver. Arch Biochem Biophys. 1989;272:219–225. doi: 10.1016/0003-9861(89)90213-0. [DOI] [PubMed] [Google Scholar]
- 8.Komori M, Nishino K, Ohi H, Kitada M, Kamataki T. Molecular cloning and sequence analysis of cDNA containing the entire coding region for human fetal liver cytochrome P450. J Biochem. 1989;105:161–163. doi: 10.1093/oxfordjournals.jbchem.a122632. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura H, Hirai M, Ohmori S, et al. Changes in urinary 6β-hydroxycortisol/cortisol ratio after birth in human neonates. Eur J Clin Pharmacol. 1998;53:343–346. doi: 10.1007/s002280050390. [DOI] [PubMed] [Google Scholar]
- 10.Zhiri A, Wellman-Bednawska M, Siest G. Elisa of 6β-hydroxycortisol in human urine: diurnal variations and effects of antiepileptic therapy. Clin Chem Acta. 1986;157:267–276. doi: 10.1016/0009-8981(86)90302-5. [DOI] [PubMed] [Google Scholar]
- 11.Desager JP, Dumont E, Harvengt C. The urinary 6β-hydroxycortisol excretion in man on inducers and inhibitors of the hepatic mixed function oxidase. Pharmacol Ther. 1987;33:197–199. doi: 10.1016/0163-7258(87)90051-9. [DOI] [PubMed] [Google Scholar]
- 12.Ohnhaus EE, Breckenridge AM, Park BK. Urinary excretion of 6β-hydroxycortisol and time course measurement of enzyme induction in man. Eur J Clin Pharmacol. 1989;36:39–46. doi: 10.1007/BF00561021. [DOI] [PubMed] [Google Scholar]
- 13.Tomlinson B, Young RP, Ng MCY, Anderson PJ, Kay R, Critchley JAJP. Selective liver enzyme induction by carbamazepine and phenytoin in Chinese epileptics. Eur J Clin Pharmacol. 1996;50:411–415. doi: 10.1007/s002280050132. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura H, Yamagata S, Sawai M, Nakamura H, Ohmori S, Kitada M. Effect of grapefruit juice on urinary 6β-OHF/C ratio. Jpn J Hosp Pharmac. 1997;23:134–139. [Google Scholar]
- 15.Hakkola J, Pasanen M, Purkunen R, et al. Expression of xenobiotic-metabolizing cytochrome P450 forms in human adult and fetal liver. Biochem Pharmacol. 1994;48:59–64. doi: 10.1016/0006-2952(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 16.Lacroix D, Sonnier M, Moncion A, Cheron G, Cresteil T. Expression of CYP3A in the human liver: Evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem. 1997;247:625–634. doi: 10.1111/j.1432-1033.1997.00625.x. [DOI] [PubMed] [Google Scholar]


