Abstract
Aims
To determine the molecular basis of the intermediate extensive metaboliser (EM) CYP2D6 phenotype in healthy Gabonese subjects.
Methods
The CYP2D6 phenotype of 154 healthy Gabonese subjects was assessed by giving the subject a single dose of 30 mg dextromethorphan, and collecting their urine for the next 8 h. The CYP2D6 genotype was determined for 50 individuals of the EM phenotypic group by Southern blotting and various PCR-based procedures aimed at identifying different CYP2D6 alleles.
Results
We found that in the studied Gabonese population, as compared with a French population, there is significantly higher frequency of intermediate EM phenotype having lower frequency of CYP2D6 PM alleles. To clarify this discrepancy phenotype-genotype relationship was studied. We found that the CYP2D6*17 and CYP2D6*2 alleles, prevalent in this black population, are characterised by their low capacity for dextromethorphan demethylation. Our data also show that the CYP2D6*1 allele is associated with the highest in vivo activity followed by the CYP2D6*2 allele and then the CYP2D6*17 allele.
Conclusions
The higher frequencies of the CYP2D6*2 and CYP2D6*17 alleles than the CYP2D6*1 allele account for the high frequency of the intermediate EM phenotype in this black population. The polymorphism of the CYP2D6 enzyme activity in African populations could have important implications for use of drugs that are substrates for CYP2D6 and have a narrow therapeutic window.
Keywords: CYP2D6 polymorphism, Bantu population, genotype, phenotype
Introduction
The debrisoquine/sparteine type polymorphism is a well-characterised genetic variation in drug metabolism associated with cytochrome P450 CYP2D6 [1]. People with active CYP2D6 are termed extensive metaboliser (EM). The enzyme activity in this group shows wide variation and individuals can be classified as ultrarapid, intermediate and slow EMs. Between 1 and 7% of Caucasians and 29% of Ethiopians are ultrarapid metabolisers with multiple copies of functional CYP2D6 genes [2–4]. The genetic basis of the poor metaboliser (PM) group is now well understood, but much less is known about the molecular basis of the heterogeneity within the EM group. EMs with intermediate activity are either heterozygotes for one of the poor metaboliser alleles or carry at least one of the functionally active but less efficient CYP2D6 alleles (CYP2D6*2 for Caucasians, CYP2D6*10 for Chinese, CYP2D*17 for Zimbabweans [5, 6, 7, 8, 9, 10]).
We investigated the relationship between metabolic phenotype and CYP2D6 genotype in a Bantu-speaking black population in Gabon. We also explored the molecular basis of interethnic differences in CYP2D6 activity by comparing data obtained from the Gabonese population with those previously obtained from a French population [11].
Methods
One hundred and fifty-four unrelated, healthy Gabonese volunteers (102 men and 52 women) gave their informed consent to participate in the phenotype studies performed at the ‘Centre International de Recherches Médicales de Franceville’ (Gabon). CYP2D6 phenotype was assessed by giving the subject a single dose of 30 mg dextromethorphan, and collecting their urine for the next 8 h. The amount of dextromethorphan (DEM) and dextrorphan (DOR) in the urine was determined by high performance liquid chromatography (h.p.l.c.) [11] and the urinary metabolic ratio (MR) DEM/DOR was calculated. Subjects with a log DEM/DOR ratio higher than −0.5 were assigned to the PM phenotype.
Blood samples for genotyping were obtained from 50 individuals of the EM group. These DNA samples were analysed by Southern blotting (XbaI polymorphism) and using various published PCR-based procedures aimed at identifying the following CYP2D6 alleles: CYP2D6*1, *2, *3, *4, *10 and*17 [7, 9, 12, 13] except for the CYP2D6*2 allele. The CYP2D*2 allele frequency was obtained by substracting the frequency of the CYP2D6*17 allele (exon 2 variation [9]) from the overall frequency of the exon 6 mutation [7].
The analysis of variance test was used for statistical evaluation of the differences between mean MR in individual genotypes. Where statistically significant differences were found groups were compared using the Student’s t-test with a Bonferroni correction. Chi-square analysis was used to test the differences in genotype and allelic variations between different ethnic groups.
Results
The distribution profile of the log MR was bimodal (Figure 1a) with a PM phenotype frequency of 0.02 (n =3 subjects). The distribution of the data for this black population was right-skewed as compared with that obtained for a French Caucasian population (Figure 1b) [11], suggesting a higher frequency of intermediate EM phenotype.
Figure 1.
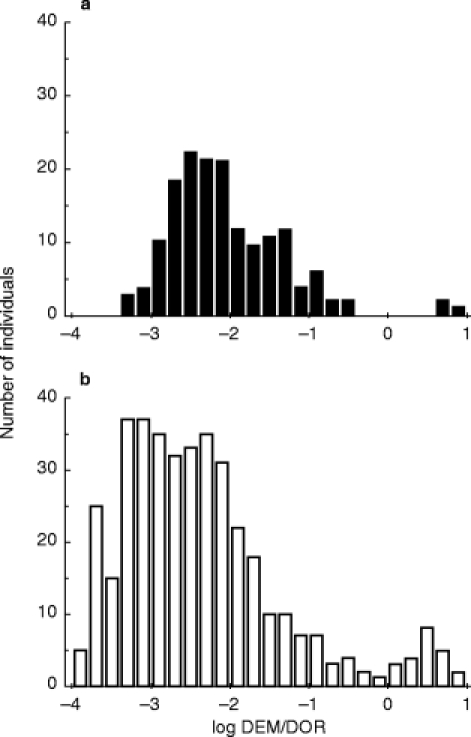
a) Frequency distribution of the log dextromethorphan (DEM) and dextrorphan (DOR) urinary metabolic ratio (MR) in 154 healthy Gabonese individuals. EM and PM individuals were defined as those with a log DEM/DOR MR lower (EM) and higher (PM) than—0.5. b) Frequency distribution of the log DEM/DOR MR in 422 healthy French Caucasians. The data are from [11].
The CYP2D6 genotype and phenotype observed in the Gabonese subjects are shown in Table 1a. Analysis of allele segregation in family members indicated that the 16+9 kb-XbaI allele was associated with the CYP2D6*4 poor metaboliser (PM) allele whereas the 42 kb-XbaI fragment was associated with the CYP2D6*2X2 extensive metaboliser (EM) allele. As with other black populations [14–16], the frequency of the CYP2D6 PM alleles in the Gabonese EM group was significantly lower (0.09) than that in Caucasians (0.21) (P<0.02). The most prevalent mutant allele in the black population was the CYP2D6*5 allele (frequency 0.07). The heterozygotes (EM/PM) had a lower mean CYP2D6 activity, as shown by their low MR values (−1.4±0.9 (n =9) vs−2.1±0.9 for the homozygotes XbaI 29 kb/29 kb EM/EM (n =39)): (P<0.05). Within the homozygous EM XbaI 29/29kb group (n =39 individuals), the observed frequencies were 0.32 for CYP2D6*1, 0.44 for CYP2D6*2, and 0.24 for CYP2D6*17.
Table 1.
CYP2D genotype and phenotype in the Gabonese.
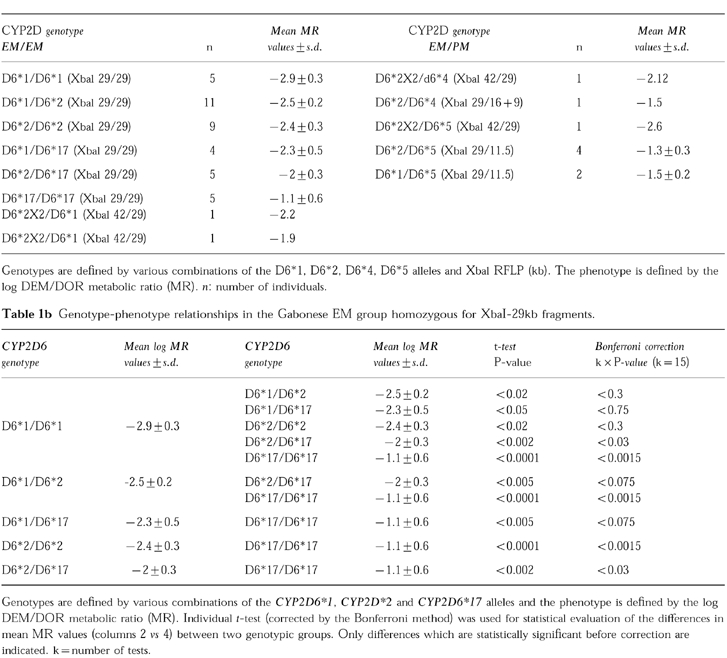
The relationship between MR values and six genotypes defined by the CYP2D6*1, CYP2D6*2, CYP2D6*17 alleles was analysed in the Gabonese EM group (Table 1b)(only subjects homozygous for 29 kb fragments were analysed to avoid a bias due to differences in CYP2D gene copy numbers). These results show a non random distribution of the CYP2D genotypes within the EM phenotypes. Finally, there was no significant difference in mean MR values between the French Caucasian [11] and Gabonese individuals with CYP2D6*1/CYP2D6*1, CYP2D6*2/CYP2D6*1 and CYP2D6*2/CYP2D6*2 genotypes (data not shown).
Discussion
Our results show that the CYP2D6*17 allele was associated with the lowest capacity for dextromethorphan demethylation in the Gabonese population (Table 1b). Although the CYP2D6*2 allele has been reported to have similar catalytic activity (for debrisoquine) to that of CYP2D6*1 [2, 9], we found that the CYP2D6*2 allele was associated with lower levels of CYP2D6 activity, consistent with previous in vivo observations in Caucasians [7, 10]. We do not know the reason for this difference but the association of the CYP2D6*2 allele with lower CYP2D6 activity might apply only to dextromethorphan and not to debrisoquine, as these substrates belong to two different chemical classes [17]. Recently, Oscarson et al. [18] reported in vitro studies showing that the CYP2D6*2 protein exhibits lower activity with bufuralol than CYP2D6*1, which is in agreement with our data. Further studies using different substrates are required to determine the molecular basis for the lower CYP2D6 activity of the CYP2D6*2 enzyme. Nevertheless, the CYP2D6*2 allele in vivo exhibited higher CYP2D6 activity than the CYP2D6*17 allele (Table 1b) (also in agreement with in vitro studies [18]). The CYP2D6*17 allele was relatively frequent in the Gabonese population studied consistent with results for another black population [9]. However, the frequency of the CYP2D6*2 allele was lower in Zimbabwean [9] than in Gabonese (0.13 vs 0.40). This difference may be due to i) genuine ethnic differences between these two population groups, or ii) a difference in the CYP2D6*2 allele detection procedure. Concerning the latter possibility, the CYP2D6*2 allele was detected by analysis of sequence variation in intron 1 [9] rather than by detection of CYP2D6 coding sequence variations (in exons 3, 6 and 9) (see Method section).
This analysis in a Bantu population from Gabon shows that the CYP2D6*1 allele is associated with the highest activity in vivo followed by the CYP2D6*2 allele and then the CYP2D6*17 allele. In conclusion, the higher frequencies of the CYP2D6*2 and CYP2D6*17 alleles than the CYP2D6*1 allele may account for the high incidence of the intermediate EM phenotype in this black population. The polymorphism of the CYP2D6 enzyme activity in African populations could have important implications for use of drugs that are substrates for CYP2D6 and have a narrow therapeutic window [19, 20].
Acknowledgments
This work was supported by grants from ‘Association Française contre les Myopathies’, ‘La Ligue Nationale Française contre le Cancer’ and ‘La Caisse Nationale d’Assurance Maladie des Travailleurs Salariés’. The ‘Association pour la Recherche sur le Cancer’ provided a fellowship to SP.
References
- 1.Daly AK, Brockmoller J, Eichelbaum M, et al. Nomenclature for human CYP2D6 alleles. Pharmacogenetics. 1996;6:193–201. doi: 10.1097/00008571-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Johansson I, Lundqvist E, Bertilsson L, Dahl ML, Sjöqvist F, Ingelman-Sundberg M. Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc Natl Acad Sci USA. 1993;90:11825–11829. doi: 10.1073/pnas.90.24.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agundez J, Ledesma MC, Ladero JM, Benitez J. Prevalence of CYP2D6 gene duplication and its repercussion on the oxidative phenotype in a white population. Clin Pharmacol Ther. 1995;57:265–269. doi: 10.1016/0009-9236(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 4.Aklillu E, Persson I, Bertilsson L, Johansson I, Rodrigues F, Ingelman-Sundberg M. Frequent distribution of ultrarapid metabolisers of debrisoquine in an Ethiopian population carrying duplicated and multiplicated functionnal CYP2D6 alleles. J Pharmacol Exp Ther. 1996;278:441–446. [PubMed] [Google Scholar]
- 5.Broly F, Gaedigk A, Heim M, Eichelbaum M, Morike K, Meyer UA. Debrisoquine/sparteine hydroxylation genotype and phenotype: analysis of common mutations and alleles of CYP2D6 in a European population. DNA Cell Biol. 1991;10:545–558. doi: 10.1089/dna.1991.10.545. [DOI] [PubMed] [Google Scholar]
- 6.Yokota H, Tamura S, Furuya H, et al. Evidence for a new variant CYP2D6 allele CYP2D6J in a Japanese population associated with lower in vivo rates of sparteine metabolism. Pharmacogenetics. 1993;3:256–263. doi: 10.1097/00008571-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Panserat S, Mura C, Gérard N, et al. DNA haplotype-dependent differences in the amino sequence of debrisoquine 4-hydroxylase (CYP2D6): evidence for two major allozymes in extensive metabolisers. Hum Genet. 1994;94:401–406. doi: 10.1007/BF00201601. [DOI] [PubMed] [Google Scholar]
- 8.Johansson I, Oscarson M, Yue QY, Bertilsson L, Sjöqvist F, Ingelman-Sundberg M. Genetic analysis of the Chinese cytochrome P4502D locus: characterization of variant CYP2D6 genes present in subjects with diminished capacity for debrisoquine hydroxylation. Mol Pharmacol. 1994;46:452–459. [PubMed] [Google Scholar]
- 9.Masimirembwa CM, Person I, Bertilsson L, Hasler JA, Ingelman-Sundberg M. A novel mutant variant of the CYP2D6 gene (CYP2D*17) common in a black African population: association with diminished debrisoquine hydroxylase activity. Br J Clin Pharmacol. 1996;42:713–719. doi: 10.1046/j.1365-2125.1996.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sachse C, Brockmoller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997;60:284–295. [PMC free article] [PubMed] [Google Scholar]
- 11.Mura C, Panserat S, Vincent-Viry M, Galteau MM, Jacqz-Aigrain E, Krishnamoorthy R. DNA haplotype dependency of debrisoquine 4-hydroxylase (CYP2D6) expression among extensive metabolizers. Hum Genet. 1993;92:367–372. doi: 10.1007/BF01247337. [DOI] [PubMed] [Google Scholar]
- 12.Daly AK, Steen VM, Idle JR. CYP2D6 multiallelism. Methods in Enzymology. 1996;272:199–210. doi: 10.1016/s0076-6879(96)72024-4. [DOI] [PubMed] [Google Scholar]
- 13.Wang SL, Huang JD, Lai MD, Liu BH, Lai ML. Molecular basis of genetic variation in debrisoquine hydroxylation in Chinese subjects: polymorphism in RFLP and DNA sequence of CYP2D6. Clin Pharmacol Ther. 1993;53:410–418. doi: 10.1038/clpt.1993.44. [DOI] [PubMed] [Google Scholar]
- 14.Relling MV, Cherrie J, Schell MJ, Petros WP, Meyer WH, Evans WE. Lower prevalence of the debrisoquine oxidative poor metabolizer phenotype in American black versus white subjects. Clin Pharmacol Ther. 1991;50:308–313. doi: 10.1038/clpt.1991.141. [DOI] [PubMed] [Google Scholar]
- 15.Evans WE, Relling MV, Rahman A, McLeod HL, Scott EP, Li JS. Genetic basis for a lower prevalence of deficient CYP2D6 oxidative drug metabolism phenotypes in black Americans. J Clin Invest. 1993;91:2150–2154. doi: 10.1172/JCI116441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masimirembwa CM, Johansson I, Hasler JA, Ingelman-Sundberg M. Genetic polymorphism of cytochrome P450 CYP2D6 in zimbabwean population. Pharmacogenetics. 1993;3:275–280. doi: 10.1097/00008571-199312000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Koymans LM, Vermeulen NPE, van Acker S, et al. A predictive model for substrates of cytochrome P450-debrisoquine (2D6) Chem Res Toxicol. 1992;5:211–219. doi: 10.1021/tx00026a010. [DOI] [PubMed] [Google Scholar]
- 18.Oscarson M, Hidestrand M, Johansson I, Ingelman-Sundberg M. A combination of mutations in the CYP2D6*17 (CYP2D6Z) allele causes alterations in enzyme function. Mol Pharmacol. 1997;52:1034–1040. doi: 10.1124/mol.52.6.1034. [DOI] [PubMed] [Google Scholar]
- 19.Masimirembwa CM, Hasler JA. Genetic polymorphism of drug metabolising enzymes in African populations: implications for the use of neuroleptics and antidepressants. Brain Res Bull. 1997;44:641–571. doi: 10.1016/s0361-9230(97)00307-9. [DOI] [PubMed] [Google Scholar]
- 20.Simooya OO, Sijumbil G, Lennard MS, Tucker GT. Halofantrine and chloroquine inhibit CYP2D6 activity in healthy Zambians. Br J Clin Pharmacol. 1998;45:315–317. doi: 10.1046/j.1365-2125.1998.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


