Abstract
Aims
Neuropeptide Y (NPY) is a sympathetic neurotransmitter released with noradrenaline during sympathetic stimulation. Ageing has been shown to be associated with a reduction in α2 and ß-adrenoceptor mediated responses in veins, but it is not known whether NPY responsiveness is also altered with increasing age.
Methods
Using a dorsal hand vein technique, we examined NPY receptor responsiveness in 24 normal, healthy subjects (20–72 years; 10 males, 14 females). Graded infusions of NPY (25–2000 pmol min−1) were administered (5 min at each dose) into a dorsal hand vein. Venous distension at 45 mmHg was measured at 3–5 min of each infusion. Dose-response curves to NPY were constructed and the peak venoconstriction was calculated.
Results
Dose-dependent venoconstriction was seen in all but one subject. The peak venoconstriction observed with NPY was significantly and negatively correlated with the age of the normal subjects (r = −0.63, P<0.01). When subjects were ranked from youngest to oldest and divided into tertiles, (20–40 years, n = 8; 41–55 years, n = 8; 56–72 years, n = 8), mean dose-response curves were different with the oldest tertile being significantly less responsive (P<0.05). The peak venoconstriction observed (% of control) was 65.1±7.0, 46.5±9.4, and 24.4±4.8%, respectively. The oldest tertile had a significantly decreased peak venoconstriction compared with the youngest tertile (P<0.01). Infusion of NPY into a dorsal hand vein had no systemic effects on heart rate or blood pressure in any of the subjects studied.
Conclusions
Hand vein responsiveness to exogenously infused NPY in normal subjects is decreased as age increases. The reduction of NPY-receptor-mediated responses with age may influence sympathetic nervous system control of the venous system with advancing age.
Keywords: NPY receptors, venoconstriction, ageing, human veins
Introduction
Ageing brings many changes in all organs from the central nervous system to the cardiovascular system. Major structural changes in the heart and peripheral vasculature associated with ageing play an important role in the development of cardiovascular disorders such as hypertension, atherosclerosis, and heart failure [1, 2]. However, in addition to the structural changes, functional alterations that occur with ageing are also of importance. For example, arterial and venous smooth muscle relaxation and chronotropic cardiac responsiveness to the ß-adrenoceptor agonist isoprenaline are reduced with age in humans [3–5]. The effect of ageing on α-adrenoceptor function has been less well studied and the studies that have been done show conflicting results [6–8]. Hyland & Docherty [9] have demonstrated in vitro a decrease with age in the responsiveness of human saphenous vein postjunctional α2-adrenoceptors.
Upon sympathetic nerve stimulation, neuropeptide Y (NPY), a 36 amino acid peptide, is co-released with noradrenaline (NA) [10, 11] and has been implicated in the autonomic control of the circulation [12]. Postjunctional Y1 receptors and prejunctional Y2 receptors have been documented at the adrenergic neuroeffector junctions [13]. Activation of the vascular postjunctional Y1 receptor by NPY has been shown to produce potent vasoconstriction by a mechanism dependent on extracellular calcium in both in vitro and in vivo experiments [14, 15]. NPY acts at the postjunctional Y1 receptor where it activates a G protein coupled mechanism [16, 17], specifically via an inhibitory G protein (Gi) to adenylyl cyclase, which, upon receptor activation, results in a decrease in adenylyl cyclase activity and a consequent decrease in the production of the second messenger cAMP. Activation of the prejunctional Y2 receptor has been shown to inhibit the presynaptic release of both NA and NPY [14, 15].
To date, the in vivo vascular effects of NPY in humans have not been thoroughly investigated. Evidence exists showing that NPY is a potent and long-lasting vasoconstrictor in the arterial bed of the forearm [15] and the nasal mucosa [18] and it has recently been demonstrated that NPY has venoconstrictor effects in human dorsal hand veins in vivo [19]. Thus, we used the hand vein tonometry technique [20, 21] to determine whether the venous responsiveness to exogenously infused NPY in the human dorsal hand vein is altered with age.
Methods
Subjects
This study was reviewed and approved by the University Review Board for Health Sciences Research of the University of Western Ontario. Informed written consent was obtained from all volunteers. Intravenous NPY was manufactured under Good Manufacturing Practices.
Twenty-four normal subjects (10 males, 14 females) ranging in age from 20 to 72 years participated. All were healthy non-smokers with no significant past medical history, had normal electrocardiograms, and refrained from caffeine and alcohol containing beverages for at least 12 h prior to the study. Specifically, none had a history of hypertension or cardiovascular disease and none was on any vasoactive or nonsteroidal medications. Female subjects enrolled in the study taking oral contraceptives (n = 3) or conjugated oestrogens (n = 4) continued taking these medications during the study period.
Measurement of plasma noradrenaline and NPY
Thirty minutes after insertion of a 20 gauge Jelco winged catheter (Critikon, Inc., Tampa, Florida) into the contralateral arm, and prior to hand vein measurements, 8 ml of venous blood was drawn into a cooled syringe and transferred to an ice cold centrifuge tube containing glutathione. The blood sample was centrifuged (3000 g for 10 min at 4 °C) and the plasma was isolated. The catecholamines were extracted according the method of Anton & Sayre [22] and assessed by reverse phase high performance liquid chromatography with electrochemical detection [23]. The detection limit of NA was 25 pg ml−1. The intra-and inter-assay coefficients of variation were 3% and 8%, respectively.
Venous blood (7 ml) was also drawn into a centrifuge tube containing EDTA. The blood sample was centrifuged (3000 g for 10 min at 4 °C) and the plasma was isolated. Plasma concentrations of NPY-like immunoreactivity were measured by radioimmunoassay according to the method of Theodorsson-Norheim et al. [24]. The sensitivity of the method was around 10 pmol l−1 in a 0.5 ml plasma sample. Inter- and intra-assay coefficients of variation were 7% and 5%, respectively.
Hand vein measurements
Hand vein studies were carried out in a temperature controlled room (22–24 °C) with subjects resting quietly in the supine position with forearm and hand comfortably elevated above heart level by placing the subject’s hand on a standard inclined platform angled at 30° to the horizontal. This facilitated the emptying of the hand veins and allowed resting venous tone to be minimized.
A venous occlusion cuff was placed around the upper arm of the limb being studied and connected to a manually activated Hokanson Rapid Cuff Inflator (Issaqua, Washington). The occlusion cuff was inflated to a pressure of 45 mmHg to cause venous distension. A short (1.9 cm) 25 gauge butterfly needle (E–Z Infusion Set, Becton Dickinson Vascular Access, Utah) was inserted into a suitable dorsal superficial hand vein with a long, straight section with no immediate tributaries. A 0.9% saline solution was infused at a rate of 0.4 ml min−1 through the intravenous (i.v.) line using a Harvard Infusion Pump (Model 2400-003, Harvard Apparatus, Mass.).
A linear variable differential transformer (Schaevitz, Type 025 MHR), an electromechanical device consisting of primary and secondary coils with a lightweight movable ferromagnetic core, was placed vertically over the summit of that vein, 10 mm proximal to the tip of the needle [20, 21]. The transducer was connected to a physiograph that recorded hand vein distension which was measured as the difference between the position of the core prior to inflation of the venous occlusion cuff (baseline) and the height of the plateau during cuff inflation (45 mmHg for 2 min). The fingers were covered with a light cloth to avoid local cold-induced venoconstriction and the fingers were loosely taped to minimise small finger twitches. A small temperature probe (YSI 409B, VWR Scientific of Canada Ltd) was placed on the dorsum of the hand and was attached to a thermometer with digital readout display to monitor skin temperature. Blood pressure and heart rate were monitored in the contralateral arm by a semi-automated blood pressure recorder (Dinamap 846SX, Critikon, Tampa, Florida) throughout the experiment.
NPY (GMP, Peninsula Laboratories, CA) was initially diluted to 250 μg/5 ml with 0.5% albumin saline. The NPY was then passed through a low protein binding millipore filter (Millex GV, 0.22 μm) and was then further diluted to the required doses in glass bottles and infused into a dorsal hand vein through a glass syringe. Average loss of peptide during filtration was 40.6% in this experiment as assessed by the difference between the expected syringe concentrations and the actual concentrations (1 ml NPY sample post-filtration provided for assay) measured by radioimmunoassay as described above and represents an improvement from initial studies [15].
Study protocol
After 30 min of supine rest, three recordings of hand vein distension (45 mmHg) during saline infusion were obtained to ensure a stable baseline and the mean was taken as the control distension. Graded local infusions (0.4 ml min−1) of NPY (25, 50, 100, 200, 500, 1000, 2000 pmol min−1) diluted in 0.5% albumin saline were given (5 min at each dose level) and the cuff inflated (45 mmHg) at the third min and deflated at the fifth min of each 5 min interval during which venous distension at plateau was measured and expressed as a percent of the control distension.
Data analysis and statistics
Dose-response curves (semi-logarithmic) were constructed for NPY using a non-linear curve fitting programme (GraphPad Inplot 4.0 software package, H.J. Motulsky, San Diego, CA). Peak venoconstriction occurring at the highest dose of NPY was assessed and was calculated as a percent of the control distension. Linear regression analysis was performed on the data to compare venous responsiveness to NPY with increasing age and with plasma NPY levels. The subjects were ranked according to age from youngest to oldest and divided into tertiles. MANOVA (BMDP,5V) was used to compare the differences in dose-response curves to NPY among the groups. The peak venoconstriction for each tertile was expressed as mean±s.e.mean. One way analysis of variance (ANOVA) followed by post-hoc analysis using Tukey’s procedure on significant main effects was used to compare the differences in plasma NA and NPY levels, basal hand vein diameter, MAP, and heart rates between the groups of subjects. A two-tailed P value less than 0.05 was considered to be of statistical significance.
Results
Skin temperature remained constant during each study and did not vary among subjects (mean temperature range, 31.0±0.3–31.3±0.3 °C). Resting arterial pressure and heart rate did not change significantly over the duration of the study. Plasma NA levels were elevated in the older tertile of subjects when compared with younger subjects (P<0.05) but plasma NPY levels were not significantly different among the groups (Table 1). Basal vein diameter at 45 mmHg venous occlusion pressure during 0.9% saline infusion was not significantly different among the tertiles (Table 1).
Table 1.
Basal plasma noradrenaline and NPY levels, mean arterial pressure, heart rate, basal vein diameter and NPY peak constriction.

Figure 1 demonstrates a typical tracing of dorsal hand vein distension during intravenous administration NPY in a young normal subject. Graded local infusions of NPY induced dose-dependent venoconstriction in all subjects studied, except for one female (50 years), with an average peak venoconstriction of 45.3±5.3% (Figure 2). The study was repeated, with fresh solution, in this non-responding subject with similar results. The average dose-response curves were plotted for each tertile demonstrating that the response to NPY for the oldest tertile was significantly decreased (P<0.05) (Figure 3). The peak venoconstriction to NPY expressed as the geometric mean (95% confidence interval) for each tertile of age was 65.1 (63.7, 78.8; 20–40 years), 46.5 (28.1, 64.9; 41–55 years), and 24.4 (15.0, 33.8; 56–72 years) (Table 1) with the oldest tertile significantly (P<0.01) decreased from the youngest tertile. Peak venoconstriction to NPY was significantly and negatively correlated with the age of the normal subjects (Figure 4, r = −0.63, P<0.01). Peak venoconstriction to NPY was also significantly and negatively correlated with the plasma NPY levels (Figure 5, r = −0.56, P<0.01). In addition, plasma NA levels were significantly correlated with plasma NPY levels (Figure 6, r = 0.59, P<0.01). Plasma NPY levels showed a strong trend to increase with subject age (r = 0.41, P = 0.06). No correlation was found between basal vein diameter and circulating NA or NPY, between basal vein diameter and peak NPY responsiveness.
Figure 1.
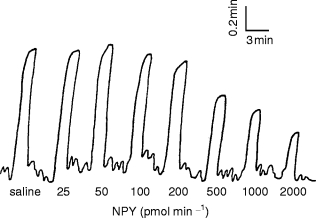
Sample original tracing of dorsal hand vein distension during intravenous administration of NPY in a 20 year old normal subject.
Figure 2.
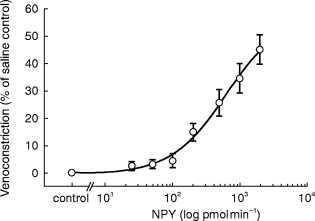
Average superficial hand vein response to graded infusions of NPY in all subjects (n = 24). Results are given as means±s.e. mean.
Figure 3.
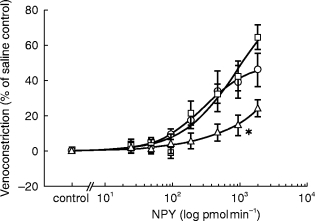
Average superficial vein response to graded infusions of NPY in younger (□, n = 8), middle (○, n = 8), and older (▵, n = 8) subjects. Results are given as means±s.e. mean. * Significantly different from younger and middle-aged subjects (P<0.05).
Figure 4.
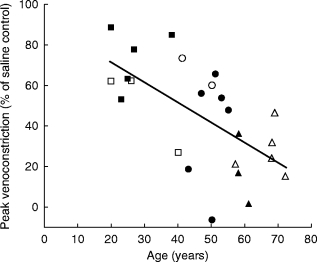
Correlation between peak venoconstriction to NPY and age in all subjects, r = −0.63, P<0.01. (female closed symbols, male open symbols). ▪, □ young, n = 8 •, ○ middle, n = 8 and ▴, ▵ older, n = 8 subjects. r = −0.56, P<0.01. Note: Two older subjects excluded due to non-detectable plasma NPY levels in the blood sample.
Figure 5.
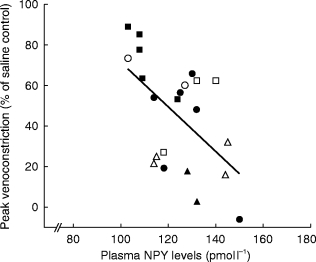
Correlation between peak venoconstriction to NPY and plasma NPY levels in all subjects (female closed symbols, male open symbols). ▪, □ young, n = 8 •, ○ middle, n = 8 and ▴, ▵ older, n = 8 subjects. r = −0.56, P<0.01. Note: Two older subjects excluded due to non-detectable plasma NPY levels in the blood sample.
Figure 6.
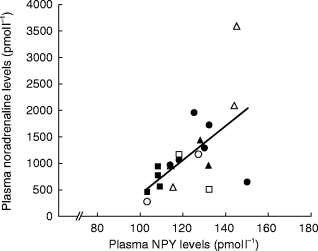
Correlation between plasma noradrenaline and plasma NPY in all subjects. (female closed symbols, male open symbols). ▪, □ young, n = 8, •, ○ middle, n = 8 and ▴, ▵ older, n = 8 subjects. r = 0.59, P<0.01. Note: Two young subjects excluded due to laboratory contamination of supplied blood sample for plasma NA levels. Two older subjects (same as in Figure 5) excluded for non-detectable plasma NPY levels.
Discussion
In the present study, we have demonstrated in vivo for the first time that venous vascular NPY receptor function in humans is decreased with increasing age. This complements previous findings which demonstrated that β- and α2-adrenoceptor mediated responses also decrease with increasing age [3, 4, 5, 9]. For example, Pan et al. [3], in healthy subjects aged 19 to 79 years, found a decrease to the β-adrenoceptor agonist isoprenaline but no differences in human hand vein responsiveness to local infusions of the α1-adrenoceptor agonist, phenylephrine, or the smooth muscle relaxant, nitroglycerin. Phenylephrine causes venoconstriction via the Gq protein which activates phospholipase C specific for the hydrolysis of phosphoinositides [25], while relaxation caused by nitroglycerin is a consequence of activation of the cGMP system [26].
Stimulation of the β-receptor activates the Gs protein to stimulate the activation of adenylyl cyclase and the production of cAMP. Stimulation of the α2-receptor acts via the same intracellular pathway as NPY receptors by activating the Gi protein to decrease the activation of adenylyl cyclase and the production of cAMP. Therefore, the β, α2, and NPY receptors all function through the adenylyl cyclase/cAMP pathway and studies in animals have demonstrated that cAMP production is blunted in vessels from older animals [27]. Bedarida et al. [28] found that the histamine H2-receptor-mediated pathway, which is also dependent on cAMP, appears to be blunted with ageing whereas the H1-receptor-mediated pathway, which is dependent on the endothelium, appears to be preserved in human hand veins. This is in agreement with a possible impairment of the adenylyl cyclase/cAMP pathway in ageing. However, responses to some other cAMP-dependent venodilators, namely prostaglandin E1 and adenosine, are preserved with increasing age in human veins in vivo [29, 30]. Thus, the age-related decline in vascular response is highly specific to certain agonists and does not reflect a generalized age-associated loss in responsiveness to adenylyl cyclase coupled receptors. Since NPY, β, and α2-mediated venoconstriction show similar changes with ageing, this supports intracellular rather than receptor changes since they are known to act via different receptors. Studies using human lymphocytes also demonstrated an age related decline in affinity of the β-adrenoceptor agonists with reduced isoprenaline-stimulated adenylyl cyclase activity but without a change in receptor number [31, 32]. Thus, while our studies did not address the molecular mechanism for the loss in response to NPY, and while receptor downregulation cannot be ruled out, it is probable that ageing affects the intracellular NPY receptor-adenylyl cyclase coupling pathway.
In the study subjects, plasma NPY levels were not significantly different between the older and younger groups as has been described previously by Hetland et al. [33]. However, there was a strong trend of increased NPY levels over a narrow range with increased age in this study. In addition, there was a significant, negative correlation between plasma NPY and peak venoconstriction to NPY. In contrast with this, plasma NA levels were significantly increased in the oldest tertile of subjects compared with the youngest tertile consistent with previous findings which demonstrated that plasma NA concentrations are increased with advancing age in resting healthy subjects [34]. Furthermore, plasma NPY levels were significantly correlated with plasma NA levels consistent with the concept of co-release of NPY in conjunction with NA from sympathetic nerve endings.
The hand vein technique was chosen as it measures responses to very small amounts of vasoactive drugs without systemic effects. Systemic haemodynamic effects could stimulate baroreflexes, though these are known to be impaired with ageing [35, 36], and alter the direct vasoconstrictor effect. No change in arterial pressure or heart rate occurred during local NPY infusions in this study. Also, veins undergo fewer structural modifications during ageing than arteries [36]; differences in vasoconstrictor responses therefore reflect functional rather than structural changes. The dose-response curves generated were cumulative but a complete sigmoidal curve was not achieved. We infused the highest concentration of NPY approved by our Ethics board, and this was similar or greater than those reported previously in the literature of hand vein responses. Such a difference may reflect a potential loss of peptide during filtration or known interindividual variability in the NPY mediated venoconstrictive response (as evident in the non-responding subject in our experiment which is consistent with results of previous studies [19]).
We have demonstrated that the venous responsiveness to exogenously infused NPY is decreased in vivo with increasing age in humans. This may be the result of improper coupling of the receptor/G protein complex to adenylyl cyclase or a decreased functioning of the adenylyl cyclase since similar changes are seen with β, α2, and H2-receptor agonists. This decreased responsiveness may play a beneficial role by counterbalancing the increased sympathetic nerve activity observed in many of the cardiovascular disorders associated with increased age. Alternatively, the increased sympathetic activity may be partly in response to decreased end organ responsiveness. It will be important to study NPY responsiveness in diseases such as hypertension and heart failure which have an increased prevalence in the elderly. The current study emphasizes the importance of comparing these patients to age-matched normals.
Acknowledgments
The authors wish to thank Dr Lars Edvinsson, MD (University Hospital of Lund, Sweden) and Dr Don Mills, PhD (Pharmacy Department, London Health Sciences Centre, Canada) for their respective expertise in the measurement of plasma NPY samples and the NPY filtration process.
The study was supported by an operating grant from Medical Research Council of Canada. MLL was supported by a UWO Scholarship for Graduate Studies. IDC was supported by Victoria Hospital Research Development Fund. QPF was supported by a Research Fellowship Award from the Heart and Stroke Foundation of Ontario. JMOA was supported by a Career Health Scientist Award from the Pharmaceutical Manufacturers Association of Canada.
References
- 1.Raven PB, Mitchell J. The effect of aging on the cardiovascular response to dynamic and static exercise. In: Weisfeldt ML, editor. The aging heart (Aging vol 12) New York: Raven Press; 1980. pp. 269–296. [Google Scholar]
- 2.Wei JY. Age and the cardiovascular system. N Engl J Med. 1992;327:1735–1739. doi: 10.1056/NEJM199212103272408. [DOI] [PubMed] [Google Scholar]
- 3.Pan H, Hoffman BB, Pershe RA, Blaschke TF. Decline in beta adrenergic receptor-mediated vascular relaxation with aging in man. J Pharmacol Exp Ther. 1986;239:802–807. [PubMed] [Google Scholar]
- 4.Van Brummelen P, Buhler FR, Kiowski W, Amann FW. Age-related decrease in cardiac and peripheral vascular responsiveness to isoprenaline: studies in normal subjects. Clin Sci. 1981;60:571–577. doi: 10.1042/cs0600571. [DOI] [PubMed] [Google Scholar]
- 5.Vestal RE, Wood AJJ, Shand DG. Reduced β-adrenoceptor sensitivity in the elderly. Clin Pharmacol Ther. 1979;26:181–186. doi: 10.1002/cpt1979262181. [DOI] [PubMed] [Google Scholar]
- 6.Klein C, Gerber JG, Payne NA, Nies AS. The effect of age on the sensitivity of the α1-adrenoceptor to phenylephrine and prazosin. Clin Pharmacol Ther. 1990;47:535–539. doi: 10.1038/clpt.1990.68. [DOI] [PubMed] [Google Scholar]
- 7.Martin SA, Alexieva S, Carruthers SG. The influence of age on dorsal hand vein responsiveness to norepinephrine. Clin Pharmacol Ther. 1986;40:257–260. doi: 10.1038/clpt.1986.172. [DOI] [PubMed] [Google Scholar]
- 8.O’Malley K, Docherty JR, Kelly JG. Adrenoceptor status and cardiovascular function in ageing. J Hypertension. 1988;6(Suppl 1):S59–62. [PubMed] [Google Scholar]
- 9.Hylund L, Docherty JR. An investigation of age-related changes in pre- and postjunctional α-adrenoceptors in human saphenous vein. Eur J Pharmacol. 1985;114:361–364. doi: 10.1016/0014-2999(85)90381-4. [DOI] [PubMed] [Google Scholar]
- 10.Ekblad E, Edvinsson L, Wahlestedt C, Uddman R, Hakanson R, Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Peptides. 1984;8:225–235. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- 11.Lundberg JM, Fried G, Pernow J Theodorsson-Norheim E. Co-release of neuropeptide Y and catecholamines upon adrenal activation in the cat. Acta Physiol Scand. 1986;126:231–238. doi: 10.1111/j.1748-1716.1986.tb07810.x. [DOI] [PubMed] [Google Scholar]
- 12.Lundberg JM, Terenius L, Hokfelt T, Goldstein M. High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neuroscience Letters. 1983;42:172. doi: 10.1016/0304-3940(83)90401-9. [DOI] [PubMed] [Google Scholar]
- 13.Wahlestedt C, Yanaihara N, Hakanson R. Evidence for different pre- and post-junctional receptors for neuropeptide Y and related peptides. Regul Peptides. 1986;13:307–318. doi: 10.1016/0167-0115(86)90048-0. [DOI] [PubMed] [Google Scholar]
- 14.Dahlof C, Dahlof P, Lundberg JM. Neuropeptide Y (NPY) reduces field stimulation-evoked release of noradrenaline and enhances force of contraction in the rat portal vein. Naunyn Schmiedeberg’s Arch Pharmacol. 1985;328:327–330. doi: 10.1007/BF00515562. [DOI] [PubMed] [Google Scholar]
- 15.Pernow J, Lundberg JM, Kaijser L. Vasoconstrictor effects in vivo and plasma disappearance rate of neuropeptide Y in man. Life Sci. 1987;40:54. doi: 10.1016/0024-3205(87)90251-7. [DOI] [PubMed] [Google Scholar]
- 16.Aakerlund L, Gether U, Fuhlendorff J, Schwartz TW, Thastrup O. Y1 receptors for neuropeptide Y are coupled to mobilization of intracellular calcium and inhibition of adenylate cyclase. FEBS Letters. 1990;260:73–78. doi: 10.1016/0014-5793(90)80069-u. [DOI] [PubMed] [Google Scholar]
- 17.Herzog H, Hort HJ, Ball HJ, Hayes G, Shine J, Selbie LA. Cloned human neuropeptide Y receptor couples to two different second messenger systems. Proc Natl Acad Sci USA. 1992;89:5794–5798. doi: 10.1073/pnas.89.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baraniuk JN, Silver PB, Kaliner MA, Barnes PJ. Neuropeptide Y is a vasoconstrictor in human nasal mucosa. J Appl Physiol. 1992;73:1867–1872. doi: 10.1152/jappl.1992.73.5.1867. [DOI] [PubMed] [Google Scholar]
- 19.Peduzzi P, Simper D, Linder L, Strobel W, Haefeli WE. Neuropeptide Y in human hand veins: Pharmacologic characterization and interaction with cyclic guanosine monophosphate-dependent venodilators in vivo. Clin Pharmacol Ther. 1995;58:675–683. doi: 10.1016/0009-9236(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 20.Aellig WH. Clinical pharmacology, physiology and pathophysiology of superficial veins -1. Br J Clin Pharmacol. 1994;38:181–196. doi: 10.1111/j.1365-2125.1994.tb04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aellig WH. Clinical pharmacology, physiology and pathophysiology of superficial veins -2. Br J Clin Pharmacol. 1994;38:289–305. doi: 10.1111/j.1365-2125.1994.tb04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anton AH, Sayre DF. A study of the factors affecting the aluminum oxidetrihydrooxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962;138:360–374. [PubMed] [Google Scholar]
- 23.Hjemdahl P, Daleskog M, Kahan T. Determination of plasma catecholamines by high performance liquid chromatography with electrochemical detection: Comparison with a radioenzymatic method. Life Sci. 1979;25:131–138. doi: 10.1016/0024-3205(79)90384-9. [DOI] [PubMed] [Google Scholar]
- 24.Theodorsson-Norheim E, Hemsen A, Lundberg JM. Radioimmunoassay for neuropeptide Y (NPY); chromatographic characterization of immunoreactivity in plasma and tissue extracts. Scand J Clin Lab Invest. 1985;45:355–365. doi: 10.3109/00365518509161019. [DOI] [PubMed] [Google Scholar]
- 25.Caron MG, Lefkowitz RJ. Catecholamine receptors: structure, function, and regulation. Recent Prog Horm Res. 1993;48:277–290. doi: 10.1016/b978-0-12-571148-7.50014-2. [DOI] [PubMed] [Google Scholar]
- 26.Kukovetz WR, Holzmann S. Mechanism of nitrate-induced vasodilation and tolerance. Z Kardiol. 1983;72(suppl 3):14–19. [PubMed] [Google Scholar]
- 27.Cohen ML, Berkowitz BA. Age-related changes in vascular responsiveness to cyclic nucleotides and contractile agonists. J Pharmacol Exp Ther. 1974;191:147–155. [PubMed] [Google Scholar]
- 28.Bedarida G, Bushell E, Blaschke TF, Hoffman BB. 1-and H2-histamine receptor-mediated vasodilation varies with aging in humans. Clin Pharmacol Ther. 1995;58:73–80. doi: 10.1016/0009-9236(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 29.Hiremath AN, Pershe RA, Hoffman BB, Blaschke TF. Comparison of age-related changes in prostaglandin E1 and beta-adrenergic responsiveness of vascular smooth muscle in adult males. J Gerontology. 1989;44:M13–17. doi: 10.1093/geronj/44.1.m13. [DOI] [PubMed] [Google Scholar]
- 30.Ford GA, Hoffman BB, Vestal RE, Blaschke TF. Age-related changes in adenosine and β-adrenoceptor responsiveness of vascular smooth muscle in man. Br J Clin Pharmacol. 1992;33:83–87. doi: 10.1111/j.1365-2125.1992.tb04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abrass IB, Scarpace PJ. Human lymphocyte β-adrenergic receptors are unaltered with age. J Gerontology. 1981;36:298–301. doi: 10.1093/geronj/36.3.298. [DOI] [PubMed] [Google Scholar]
- 32.Feldman RD, Limbird LE, Nadeau J, Robertson D, Wood AJJ. Alteration in leukocyte β-adrenoceptor affinity with ageing. N Engl J Med. 1984;310:815–819. doi: 10.1056/NEJM198403293101303. [DOI] [PubMed] [Google Scholar]
- 33.Hetland ML, Eldrup E, Bratholm P, Christensen NJ. The relationship between age and venous plasma concentrations of noradrenaline, catecholamine metabolites, DOPA and neuropeptide Y-like immunoreactivity in normal human subjects. Scand J Clin Lab Invest. 1991;51:219–224. doi: 10.3109/00365519109091608. [DOI] [PubMed] [Google Scholar]
- 34.Christensen NJ. Is plasma NA an index of biological age? In: Christensen NJ, Henriksen O, Lassen NA, editors. The sympathoadrenal system: Physiology and pathophysiology. Copenhagen: Munksgaard; 1993. pp. 266–272. [Google Scholar]
- 35.Mancia G, Grass G, Ferrari A, Zanchetti RA. Reflex cardiovascular regulation in humans. J Cardiovasc Pharmacol. 1985;7:5152–5159. doi: 10.1097/00005344-198500073-00018. [DOI] [PubMed] [Google Scholar]
- 36.Folkow B, Svanborg A. Physiology of vascular aging. Physiol Rev. 1993;73:725–764. doi: 10.1152/physrev.1993.73.4.725. [DOI] [PubMed] [Google Scholar]


