Abstract
Aims
Recent reports, largely in animal models, have suggested that either inhibition of nitric oxide (NO) synthase or endothelium removal in arteries inhibits the response to isoprenaline, a β-adrenoceptor agonist, and also enhances the response to sodium nitroprusside, a nitrovasodilator. This in vivo study was designed to determine whether NG-monomethyl-l-arginine (l-NMMA), an inhibitor of NO synthesis, influences relaxation of human hand veins mediated by isoprenaline or by sodium nitroprusside.
Methods
Using the dorsal hand vein technique, full dose-response curves to bradykinin (0.27–278 ng min−1, n=6), isoprenaline (2.12–271 ng min−1, n=8) and sodium nitroprusside (0.01–634 ng min−1, n=7) were generated on separate occasions before and after l-NMMA co-infusion (50 μg min−1).
Results
In veins preconstricted with the α1-adrenoceptor-selective agonist phenylephrine, the three vasodilators induced maximal responses (Emax) of 119±35, 72±18 and 103±17%, respectively. l-NMMA inhibited relaxation to bradykinin by 64% (P=0.014) but did not influence relaxation induced by isoprenaline. The sensitivity to sodium nitroprusside was significantly enhanced by l-NMMA co-infusion (concentration shift of 2.3, P=0.031).
Conclusions
We conclude that in human veins, spontaneously released NO does not play a major role in isoprenaline-induced relaxation. Our results also suggest that the effects of sodium nitroprusside in this vascular bed may be attenuated by endothelium-derived NO.
Keywords: isoprenaline, sodium nitroprusside, nitric oxide, endothelium, vein
Introduction
Nitric oxide (NO) is known to be a major physiologic regulator of vascular tone [1]. This potent vasodilator is formed in endothelial cells from l-arginine after activation of constitutive endothelial NO synthase (ecNOS) through a Ca2+/calmodulin pathway. NO rapidly diffuses into the vascular smooth muscle cells, where it activates guanylyl cyclase, leading to production of guanosine 3′:5′ cyclic monophosphate (cGMP) and vascular relaxation [2]. Various vasodilators such as acetylcholine, substance P and bradykinin have been shown to induce vascular relaxation by activation of ecNOS [1]. These compounds have been classified as endothelium-dependent agonists as they relax vascular smooth muscle only in the presence of endothelial cells. On the other hand, nitrovasodilators such as sodium nitroprusside, sydmonimine (SIN-1) or glyceryltrinitrate are NO donors [3]. They induce a direct activation of the smooth muscle-soluble guanylyl cyclase and are therefore considered as endothelium-independent vasodilators.
β-adrenoceptor-mediated relaxation has been conventionally proposed to involve increased intracellular concentrations of cyclic adenosine 3′:5′ monophosphate (cAMP) through the activation of adenylyl cyclase and subsequent activation of cAMP-dependent protein kinase in vascular smooth muscle [4]. Some reports, however, have suggested that the endothelium and nitric oxide (NO) may also contribute to the dilation induced by β-adrenoceptor agonists. Some [5–11] but not all [12–16] animal studies in arteries have demonstrated that the endothelium plays a role in the relaxing effect of isoprenaline, a non-selective β-adrenoceptor agonist. Recently, NG-monomethyl-l-arginine (l-NMMA), an inhibitor of ecNOS, has been shown to inhibit the forearm blood flow response to brachial artery infusions of isoprenaline in healthy men suggesting that this response is dependent on NO synthesis in this arterial bed [17]. The possibility that β-adrenergic dilation of human veins in vivo involves an endothelium-dependent mechanism linked to the l-arginine/NO pathway has not been directly considered previously.
Sodium nitroprusside is a NO donor [18]. Therefore, it should be expected that this compound and endogenous NO would act together to relax vascular smooth muscle. Instead, several observations in isolated vascular preparations, using either endothelium removal or preadministration of inhibitors of ecNOS, suggest that the endothelium exerts an inhibitory effect on the vasodilation induced by sodium nitroprusside and other nitrovasodilators [19–23]. However, this issue remains controversial in humans [17, 24].
The present research was designed to analyze whether NO modulates in vivo relaxation of human veins mediated by isoprenaline or sodium nitroprusside. The dorsal hand vein compliance technique was used because agonists such as acetylcholine and bradykinin have been clearly shown to be endothelium-dependent dilators in this model [25–27]. Moreover this technique permits in vivo studies of venous constriction and relaxation without confounding reflex alteration while allowing the generation of complete dose-response curves [28].
Methods
Subjects
Studies were conducted in 17 healthy subjects (10 males and 7 females; 10 Whites, 4 Asians, 1 Asian-Indian, 1 Hispanic, 1 African-American) aged 20–49 years (mean±s.d.:29±8 years). Since the venodilatory response to isoprenaline has been shown to be impaired with ageing [29], subjects younger than 30 years were recruited for this component of the study. Written informed consent was obtained from each subject and the protocol was approved by the Administrative Panel on Human Subjects in Medical Research at Stanford University. Routine physical examination, standard twelve lead electrocardiogram, complete blood count and biochemical profile were normal in all subjects. All the subjects were non-smokers, were not taking any medication and were normotensive. They were asked to refrain from caffeine and alcohol for at least 12h before the study.
The dorsal hand vein technique
The dorsal hand vein technique, as modified by Aellig [30], was used as described in detail previously [29]. The subjects were supine at a room temperature of 21–23° C. One arm was placed on a support sloping upward at an angle of 30° from horizontal to ensure complete emptying of the superficial hand veins. A 21-gauge butterfly needle was inserted into a suitable dorsal hand vein and a continuous infusion of physiological saline (rate 0.31 ml min−1) was started. After 30 min, a linear variable differential transducer (LVDT model 100 MHR, Schaevitz Engineering, Pennsauken, NJ, USA) was mounted onto the back of the hand by means of a tripod. The LVDT’s freely moveable core was placed over the centre of the vein under study approximately 10 mm downstream from the tip of the needle. When the core was properly centred within the transformer and placed on the top of the vein, there was a linear relationship over the range employed between the vertical movement of the core and voltage output, which was recorded on a strip chart recorder. Recordings of the position of the core were made both before and after inflation of a sphygmomanometer cuff on the same arm to 45 mmHg. Results are presented as normalized dose-response curves, in which the diameter of the vein during saline infusion with the cuff inflated was defined as 100% relaxation. The vein was constricted to 20% of the baseline size, by infusing increasing doses of phenylephrine. This degree of preconstriction was defined as 0% dilation. The vasodilation produced by bradykinin, isoprenaline and sodium nitroprusside alone or in the presence of l-NMMA was calculated as a percentage of the range between maximum and 0% vasodilation.
Study design
Effect of l-NMMA on bradykinin dose-response curves (n=6)
After preconstriction of the vein with phenylephrine, a complete dose-response curve to bradykinin was constructed (doses ranging from 0.27 to 278 ng min−1). Phenylephrine alone, at the same dose, was then infused for 45 min; l-NMMA (50 μg min−1) was added to the phenylephrine infusion for the last 20 min of this wash-out period and a second dose-response curve to bradykinin was repeated in presence of l-NMMA. A wash-out period of 45 min has been shown to avoid desensitization of bradykinin-mediated response in the dorsal hand vein [26].
Effect of l-NMMA on isoprenaline dose-response curves (n=8, 6 Whites, 2 Asians)
On a different occasion, a similar design was used to evaluate the effects of l-NMMA (50 μg min−1) on isoprenaline-induced venodilation. Isoprenaline was infused at a rate of 2.12–271 ng min−1.
Effect of l-NMMA on nitroprusside dose-response curves (n=7)
On a different occasion, the same procedure was used to examine the effect of l-NMMA (50 μg min−1) on nitroprusside-induced venodilation in doses ranging from 0.01–634 ng min−1.
Drugs
All drugs were diluted in normal saline. The following drugs were used : phenylephrine hydrochloride (1% injection) (Winthrop Laboratories, New York, NY, USA); bradykinin (Sigma F & D Division, St Louis, MO, USA) used under IND#32261; l-NMMA (Calbiochem, San Diego, CA, USA) under IND#40595; isoprenaline and sodium nitroprusside (Elkins-Sinn Inc, Cherry Hill, NJ, USA).
Data analysis
Results are expressed as means±s.d. Individual dose-response curves to bradykinin, isoprenaline and sodium nitroprusside, before and after l-NMMA infusion, were fitted to a four parameter logistic equation [31]. This iterative curve-fitting program provides an estimate of the maximal response (Emax) and of the dose producing half maximal response (ED50); ED50 values were log transformed and the geometric means were calculated as the antilogs of the means of log values. A Student’s t-test for paired observations was used to compare data obtained from subjects before and during l-NMMA coadministration. The Wilcoxon signed-rank test for pairwise comparisons was performed for data that were not normally distributed. Differences were considered significant at P<0.05.
Results
The data for each subject corresponding to the three protocols are presented in Table 1.
Table 1.
Effect of l-NMMA (50 μg min−1) co-infusion on the venodilation induced by bradykinin, isoprenaline and sodium nitroprusside:individual data.
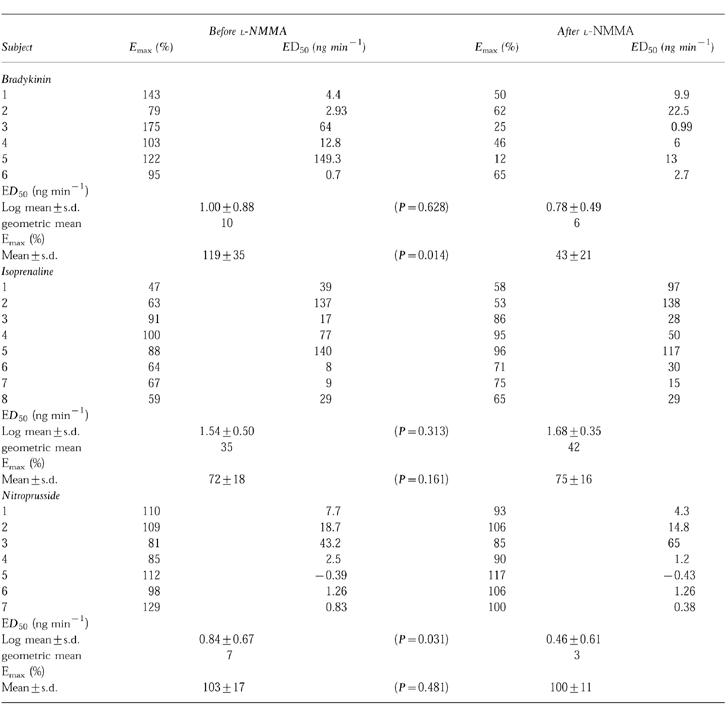
Effect of l-NMMA on bradykinin-induced venodilation
Bradykinin induced concentration-dependent relaxations in human veins. These relaxing effects of bradykinin were significantly decreased by concomitant infusion of l-NMMA (Figure 1). The mean maximal response to bradykinin was 119±35% (95% Confidence Intervals (CI): 83, 156) and decreased to 43.3±20.8% (95% CI: 21, 65) after l-NMMA coinfusion (95% CI for the difference: 23, 129; P=0.014). The mean log ED50 values for bradykinin were not significantly different before (1.00±0.88, geometric mean 10 ng min−1, 95% CI: 0.08, 1.92) and after l-NMMA (0.78±0.49; geometric mean 6 ng min−1, 95% CI: 0.27, 1.29; 95% CI for the difference: −0.88, 1.32; P=0.628).
Figure 1.
Effect of l-NMMA infusion (50 μg min−1) on bradykinin-induced venodilation in the dorsal hand vein preconstricted with phenylephrine. Dose-response curves for bradykinin alone (•) and for bradykinin with concurrent infusion of l-NMMA (○), were generated as described in the Methods section. Venodilation is expressed as a percentage of baseline (phenylephrine) vein diameter. The data shown are the results in a typical subject.
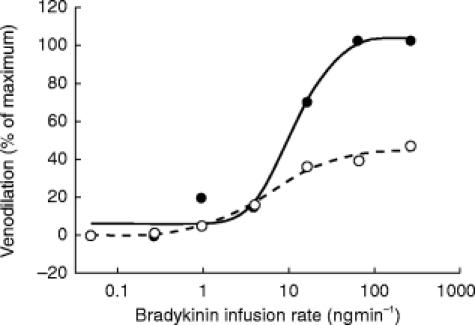
Effect of l-NMMA on isoprenaline-induced venodilation
Isoprenaline caused concentration-dependent relaxations in veins preconstricted with phenylephrine. l-NMMA did not affect this venodilation (Figure 2). Pre-treatment values were 72±18% for Emax (95% CI: 57, 87) and 1.54±0.50 for log ED50 (geometric mean 35 ng min−1, 95% CI: 1.14, 1.94). After coinfusion with l-NMMA, no change in Emax (75±16%, 95% CI: 62, 89, 95% CI for the ratio of means: 0.87, 1.07; P=0.313) or log ED50 (1.68±0.35, geometric mean 42 ng min−1, 95% CI: 1.40, 1.96, 95% CI for the difference: −0.35, 0.07; P=0.161) was detected.
Figure 2.
Effect of l-NMMA infusion (50 μg min−1) on isoprenaline-induced venodilation in the dorsal hand vein preconstricted with phenylephrine. Dose-response curves for isoprenaline alone (•) and for isoprenaline with concurrent infusion of l-NMMA (○), were generated as described in the Methods section. Venodilation is expressed as a percentage of baseline (phenylephrine) vein diameter. The data shown are the results in a typical subject.
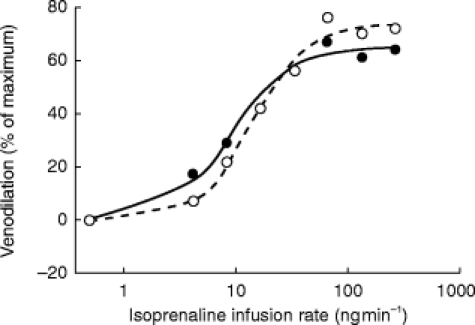
Effect of l-NMMA on nitroprusside-induced venodilation
Nitroprusside caused concentration-dependent relaxations in veins precontracted with phenylephrine (Figure 3). The maximal response to the nitrovasodilator was not affected by concomitant infusion of l-NMMA. Pre- and post-treatment values for Emax were 103±17 (95% CI: 88, 118) and 100±11 (95% CI: 90, 110, 95% CI for the difference: −9, 17; P=0.481), respectively. The sensitivity to nitroprusside was significantly enhanced after l-NMMA co-infusion. The log ED50 of nitroprusside were 0.84±0.67 (geometric mean: 7 ng ml−1, 95% CI: 0.23, 1.45) and 0.46±0.61 (geometric mean: 3 ng ml−1, 95%CI: −0.1, 1.02, 95% CI for the difference: −0.09, 0.86; P=0.031) before and after l-NMMA co-infusion.
Figure 3.
Effect of l-NMMA infusion (50 μg min−1) on nitroprusside-induced venodilation in the dorsal hand vein preconstricted with phenylephrine. Dose-response curves for nitroprusside alone (•) and for nitroprusside with concurrent infusion of l-NMMA (○), were generated as described in the Methods section. Venodilation is expressed as a percentage of baseline (phenylephrine) vein diameter. The data shown are the results in a typical subject.
Discussion
In the present in vivo experiments, l-NMMA, a powerful inhibitor of NO synthesis from l-arginine [32], decreased bradykinin-induced relaxation in human hand veins confirming previous findings showing that increased NO synthesis plays an important role in the venodilation induced by this peptide [25–27]. The response not blocked by l-NMMA has previously been shown to be attenuated by a cyclooxygenase inhibitor [26]. In the same experimental model, l-NMMA failed to alter significantly the venodilatory effects of isoprenaline but was associated with a slight increase in the sensitivity to nitroprusside.
β-adrenoceptor agonists are thought to produce vasodilation via activation of adenylyl cyclase and the consequent stimulation of cAMP formation in vascular smooth muscle cells [4]. However, recent data suggest that NO may also play a role in β-adrenoceptor-mediated dilation. Conflicting results are found in the literature about the contribution of endothelium and the potential involvement of NO in this vasorelaxation. A brief review of available experimental and animal studies suggests that the mechanism of relaxation to isoprenaline may vary, not only from species to species, but also from one vascular bed to another. Isoprenaline-induced relaxation in rat aorta and mesenteric arteries is attenuated after removal of the endothelium, or by pretreatment of the vessels with methylene blue, an inhibitor of the soluble guanylyl cyclase, or by pretreatment with the ecNOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME) [6–9, 11]. On the other hand, it has also been observed that isoprenaline-induced relaxation of rat arteries was unchanged by endothelium removal or by inhibitors of ecNOS [13, 14]. The role of endothelium in β-adrenergic relaxation has been also extensively investigated in canine coronary arteries. In large coronary arteries, an in vitro study [5] has suggested that β-adrenoceptors may produce an endothelium-dependent vasorelaxation but the direct involvement of NO was not investigated in that study. More recently, in vitro [15] and in vivo [16] studies of large epicardial coronary arteries have clearly demonstrated that β-adrenoceptor-mediated relaxation is endothelium-independent. Another in vivo study, considering the role of endothelium at the level of resistance coronary arteries in dogs, found that the release of NO was involved in isoprenaline-mediated dilation [10]. Recently, the role of endothelium in isoprenaline-induced relaxation has been investigated in human forearm vasculature [17]. In this vascular bed, l-NMMA coinfusion inhibited the response to isoprenaline by 59%, suggesting that β-adrenoceptor-mediated vasodilation is dependent on NO synthesis in that model.
The dorsal hand vein technique [30] has allowed us to investigate the potential endothelial modulation of isoprenaline-induced venodilation. Our selection of the dose of l-NMMA was guided by earlier hand vein studies [27]. With this approach, the arginine analogue provides a stable blockade of venous response to local infusion of bradykinin, an endothelium-dependent agonist which induces the release of NO. We did not detect any significant alteration in isoprenaline-induced relaxation after l-NMMA coinfusion and the 95% confidence intervals exclude an effect of greater than 15% in magnitude. We found a tendency for the log ED50 to be higher (ratio of the geometric means=1.4) after l-NMMA coinfusion. This result could suggest a small loss in sensitivity for isoprenaline-induced venodilation but 95% confidence intervals suggest that the effect is unlikely to be as great as 20%. However, from a mechanistic point of view, we feel that this difference, if there is one at all, has little relevance in the hand vein model where 3–10 fold shifts are commonly observed in pharmacological investigations [28]. Therefore, it seems reasonable to conclude that the endothelium does not provide a major contribution to isoprenaline-induced relaxation in veins of healthy humans. This observation is in agreement with an experimental study in the dog saphenous vein suggesting that the endothelium is not involved in β-adrenoceptor agonist-induced relaxation in the venous circulation [12] but in contrast with recent in vivo findings in the human brachial artery [17].
Several interesting possibilities are suggested to explain this potential differences between arterial and venous circulations. β-adrenoceptor-mediated smooth muscle relaxation and vasodilation have been extensively studied in the forearm vasculature [17, 33, 34] and in the dorsal hand vein [28, 35, 36]. In human brachial arteries, relaxations to several β-adrenoceptor agonists, including isoprenaline, are mediated predominantly through β2-adrenoceptors and are partially dependent on NO synthesis [17]. Studies on human isolated saphenous veins have clearly shown that β2-adrenoceptors are present on these vessels and that these receptors mediate relaxation after stimulation by isoprenaline [34]. Similarly, the effects of isoprenaline in human dorsal hand veins are likely mediated largely via β2-adrenoceptors [35, 36]. However, no correlation was found in a recent study [37] between measures of sensitivity to isoprenaline in the dorsal hand vein and in the forearm, suggesting that venous and arterial β2-adrenoceptor responses are regulated differently. Interestingly, autoradiographic studies have shown a high density of β2-adrenoceptors localized on the endothelium of the internal mammary artery but not of the saphenous veins, whereas in both vessels, relaxation to isoprenaline is mediated via β2-adrenoceptors located on smooth muscle cells [38]. Such endothelial β2-adrenoceptors have been suggested to be linked directly to ecNOS in human brachial arteries [17] and their absence on venous endothelium may explain the lack of effect of l-NMMA on isoprenaline-induced venodilation. This hypothesis merits further investigation.
Since increased arterial flow increases shear stress, non-specific haemodynamic changes in response to isoprenaline-induced vasodilation in arteries may increase NO biosynthesis in this vascular bed. This phenomenon has been clearly demonstrated in vivo for large canine coronary arteries where the endothelium reinforces the dilatory response to isoprenaline through an indirect, flow-dependent mechanism [16]. Failure of l-NMMA to inhibit similar responses to other vasodilators such as verapamil in the brachial artery [17] suggests that these non-specific changes are not involved in the human forearm vasculature. Recently, it has been observed that there is cross-talk between cAMP and cGMP intracellular mechanisms [39–41]. There is considerable evidence that NO, acting through cGMP can potentiate cAMP responses through inhibition of phosphodiesterase activity [41]. However, under basal conditions, veins release less NO than do arteries [25]. Consequently, inhibition of basal endothelial NO synthesis by l-NMMA and the consequent decrease in cGMP in underlying smooth muscle cells in arteries but not in veins could explain this apparent involvement of NO in β-adrenoceptor responses. This hypothesis also merits further investigation.
Sodium nitroprusside and related nitrovasodilators are NO donors. Their vasodilator effects have been suggested to be unaffected by the endothelium [5, 42]. Some in vitro studies have shown that the endothelium might exert an inhibitory effect on the vasodilation induced by nitrovasodilators [19–23]. The precise mechanism which may contribute to this phenomenon in vitro remains unclear. In human arteries, the response to sodium nitroprusside [17] does not seem to be influenced by l-NMMA in vivo. However, only two or three doses of nitrovasodilator were tested in this brachial artery study. In our in vivo study, in which complete dose-response curves were generated, local co-infusion of l-NMMA had no influence on the maximal response to the nitrovasodilator. We found, however, that inhibition of NO synthesis slightly increased the sensitivity (2.3 fold) to sodium nitroprusside. This observation is consistent with previous in vitro findings in human saphenous veins [23]. Compared with the 7 fold shift observed in arterial preparations [20], the less pronounced effect in veins would be consistent with a discrete basal release of NO in endothelial cells of human veins. From a clinical point of view, this finding may have interesting implications in heart failure, a disease associated with a reduced production of NO [44]. Indeed, the enhanced sensitivity to sodium nitroprusside, a drug widely used in this condition, may help to reduce the preload of the heart.
We conclude from these studies that in vivo, spontaneously released NO does not play a major role in isoprenaline-induced relaxation but seems to attenuate the effects of sodium nitropruside in human veins. Differences with previous findings in arteries might be explained by the lower functional significance of NO in veins.
Acknowledgments
We would like to acknowledge the valuable asssitance of the nursing staff of the Geriatric Research Education and Clinical Research Center of the VA Hospital, Palo Alto. This work was supported by NIH grant AG05627. Dr Chalon and Dr Moreno Jr were supported by Merck Sharp and Dohme International Fellowships in Clinical Pharmacology.
References
- 1.Moncada S, Higgs EA. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 2.Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986;78:1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter U, Waldmann R, Nieberding M. Intracellular mechanism of action of vasodilators. Eur Heart J. 1988;9(suppl H):1–6. doi: 10.1093/eurheartj/9.suppl_h.1. [DOI] [PubMed] [Google Scholar]
- 4.Kukovetz WR, Poch C, Holtzmann S. Cyclic nucleotides and relaxation of vascular smooth muscle. In: Vanhoutte PM, Leusen I, editors. Vasodilation. New York: Raven; 1981. pp. 339–353. [Google Scholar]
- 5.Rubanyi G, Vanhoutte PM. Endothelium-removal decreases relaxations of canine coronary arteries caused by β-adrenergic agonists and adenosine. J Cardiovasc Pharmacol. 1985;7:139–144. doi: 10.1097/00005344-198501000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Grace GC, MacDonald PS, Dusting GJ. Cyclic nucleotide interactions involved in endothelium-dependent dilatation in rat aortic rings. Eur J Pharmacol. 1988;148:17–24. doi: 10.1016/0014-2999(88)90449-9. [DOI] [PubMed] [Google Scholar]
- 7.Kamata K, Miyata N, Kasuya Y. Involvement of endothelial cells in relaxation and contraction responses to isoprenaline in naive and streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 1989;249:890–894. [PubMed] [Google Scholar]
- 8.Gray DW, Marshall I. Novel signal transduction pathway mediating endothelium-dependent β-adrenoceptor vasorelaxation in rat thoracic aorta. Br J Pharmacol. 1992;107:684–690. doi: 10.1111/j.1476-5381.1992.tb14507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graves J, Poston L. β-Adrenoceptor agonist mediated relaxation of rat isolated resistance arteries: a role for the endothelium and nitric oxide. Br J Pharmacol. 1993;108:631–637. doi: 10.1111/j.1476-5381.1993.tb12853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parent R, Al-Obaidi M, Lavallee M. Nitric oxide formation contributes to β-adrenergic dilation of resistance coronary vessels in conscious dogs. Circ Res. 1993;73:241–251. doi: 10.1161/01.res.73.2.241. [DOI] [PubMed] [Google Scholar]
- 11.Satake N, Shibata M, Shibata S. Endothelium- and cytochrome P-450-dependent relaxation induced by isoprenaline in rat aortic rings. Eur J Pharmacol. 1997;319:37–41. doi: 10.1016/s0014-2999(96)00822-9. [DOI] [PubMed] [Google Scholar]
- 12.DeMey JG, Vanhoutte PM. Heterogeneous behavior of the canine arterial and venous wall: importance of the endothelium. Circ Res. 1982;51:439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- 13.Konishi M, Su C. Role of endothelium in dilator responses of spontaneously hypertensive rat arteries. Hypertension. 1983;5:881–886. doi: 10.1161/01.hyp.5.6.881. [DOI] [PubMed] [Google Scholar]
- 14.Moncada S, Rees DD, Schultz R, Palmer RMA. Development and mechanisms of a specific supersensitivity to nitro-vasodilators after inhibition of vascular nitric oxide synthase in vivo. Proc Natl Acad Sci USA. 1991;88:2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bea ML, Ghaleh B, Giudicelli JF, Berdeaux A. Lack of importance of NO in β-adrenoceptor-mediated relaxation of large epicardial canine coronary arteries. Br J Pharmacol. 1994;111:981–982. doi: 10.1111/j.1476-5381.1994.tb14839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghaleh B, Bea ML, Dubois-Rande JL, Giudicelli JF, Hittinger L, Berdeaux A. Endothelial modulation of β-adrenergic dilation of large coronary arteries in conscious dogs. Circulation. 1995;92:2627–2635. doi: 10.1161/01.cir.92.9.2627. [DOI] [PubMed] [Google Scholar]
- 17.Dawes M, Chowienczyck PJ, Ritter JM. Effects of inhibition of the l-arginine/nitric oxide pathway on vasodilation caused by β-adrenergic agonists in human forearm. Circulation. 1997;95:2293–2297. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- 18.Feelisch M, Stamler JS. Donors of nitrogen oxides. In: Feelisch M, Stamler JS, editors. Methods in Nitric Oxide Research. New-York: Wiley and Sons Ltd.; 1996. pp. 71–115. [Google Scholar]
- 19.Shirasaki Y, Su C. Endothelium removal augments vasodilation by sodium nitroprusside and sodium nitrite. Eur J Pharmacol. 1985;114:93–96. doi: 10.1016/0014-2999(85)90527-8. [DOI] [PubMed] [Google Scholar]
- 20.Pohl U, Busse R. Endothelium-derived relaxant factor inhibits effects of nitrocompounds in isolated arteries. Am J Physiol. 1987;252:H307–H313. doi: 10.1152/ajpheart.1987.252.2.H307. [DOI] [PubMed] [Google Scholar]
- 21.Ralevic V, Mathie RT, Alexander B, Burnstock G. NG-Nitro-l-arginine methyl ester attenuates vasodilator responses to acetylcholine but enhances those to sodium nitroprusside. J Pharm Pharmacol. 1991;43:871–874. doi: 10.1111/j.2042-7158.1991.tb03199.x. [DOI] [PubMed] [Google Scholar]
- 22.Busse R, Pohl U, Mulsch A, Bassenge E. Modulation of the vasodilator action of SIN-1 by the endothelium. J Cardiovasc Pharmacol. 1989;14(Suppl 11):S81–S85. doi: 10.1097/00005344-198906152-00015. [DOI] [PubMed] [Google Scholar]
- 23.Luscher TF, Richard V, Yang Z. Interaction between endothelium-derived nitric oxide and SIN-1 in human and porcine blood vessels. J Cardiovasc Pharmacol. 1989;14(suppl 11):S76–S80. [PubMed] [Google Scholar]
- 24.Joannides R, Richard V, Haefeli WE, Linder L, Luscher TF, Thuillez C. Role of basal and stimulated release of nitric oxide in the regulation of radial artery caliber in humans. Hypertension. 1995;26:327–331. doi: 10.1161/01.hyp.26.2.327. [DOI] [PubMed] [Google Scholar]
- 25.Vallance P, Collier J, Moncada S. Nitric oxide synthesized from l-arginine mediates endothelium-dependent dilations in human veins in vivo. Cardiovasc Res. 1989;23:1053–1057. doi: 10.1093/cvr/23.12.1053. [DOI] [PubMed] [Google Scholar]
- 26.Dachman WD, Ford GA, Blaschke TF, Hoffman BB. Mechanism of bradykinin-induced venodilation in humans. J Cardiovasc Pharmacol. 1993;21:241–248. doi: 10.1097/00005344-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Bedarida GV, Kim D, Blaschke TF, Hoffman BB. Characterization of an inhibitor of nitric oxide synthase in human-hand veins. Horm Metab Res. 1994;26:109–112. doi: 10.1055/s-2007-1000784. [DOI] [PubMed] [Google Scholar]
- 28.Aellig WH. Clinical pharmacology, physiology and pathophysiology of superficial hand veins, 2. Br J Clin Pharmacol. 1994;38:289–305. doi: 10.1111/j.1365-2125.1994.tb04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan HYM, Hoffman BB, Pershe RA, Blaschke TF. Decline in beta-adrenergic receptor-mediated vascular relaxation with aging in man. J Pharmacol Exp Ther. 1986;239:802–807. [PubMed] [Google Scholar]
- 30.Aellig WH. A new technique for recording compliance of human hand veins. Br J Clin Pharmacol. 1981;11:237–243. doi: 10.1111/j.1365-2125.1981.tb00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay and physiological dose-response curvs. Am J Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- 32.Rees DD, Palmer RMJ, Moncada S. A specific inhibitor of nitric oxide formation from l-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989;96:418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamin N, Calver A, Collier J, Robinson B, Vallance P, Webb D. Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension. 1995;25:918–923. doi: 10.1161/01.hyp.25.5.918. [DOI] [PubMed] [Google Scholar]
- 34.Ikezono K, Zerkowski HR, Beckeringh JJ, Michel MC, Brodde OE. Beta-2 adrenoceptor-mediated relaxation of the isolated human saphenous vein. J Pharmacol Exp Ther. 1987;241:294–299. [PubMed] [Google Scholar]
- 35.Stein M, Deegan R, Wood AJJ. Long-term exposure to β2-receptor agonist specifically desensitizes β-receptor-mediated venodilation. Clin Pharmacol Ther. 1993;54:187–193. doi: 10.1038/clpt.1993.130. [DOI] [PubMed] [Google Scholar]
- 36.Kapoor C, Singarajah C, Zafar H, et al. Impaired β2-adrenergic agonist-induced venodilation in Indians of Asian origin. Clin Pharmacol Ther. 1996;59:569–576. doi: 10.1016/S0009-9236(96)90185-X. [DOI] [PubMed] [Google Scholar]
- 37.Stein M, Deegan R, Wood AJJ. Lack of correlation between arterial and venous β-adrenergic receptor sensitivity. Hypertension. 1997;29:1273–1277. doi: 10.1161/01.hyp.29.6.1273. [DOI] [PubMed] [Google Scholar]
- 38.Molenaar P, Malta E, Jones CR, Buxton BF, Summers RJ. Autoradiographic localization and function of β-adrenoceptors on the human internal mammary artery and saphenous vein. Br J Pharmacol. 1988;95:225–233. doi: 10.1111/j.1476-5381.1988.tb16568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vigne P, Lund L, Frelin C. Cross-talk among cyclic AMP, cyclic GMP, and Ca2+-dependent intracellular signalling mechanisms in brain capillary endothelial cells. J Neurochem. 1994;62:2269–2274. doi: 10.1046/j.1471-4159.1994.62062269.x. [DOI] [PubMed] [Google Scholar]
- 40.Rebich S, Devine JO, Armstead WM. Role of nitric oxide and cAMP in β-adrenoceptor-induced pial artery vasodilation. Am J Physiol. 1995;268:H1071–H1076. doi: 10.1152/ajpheart.1995.268.3.H1071. [DOI] [PubMed] [Google Scholar]
- 41.Delpy E, Coste H, le Monnier de Gouville AC. Effects of cyclic GMP elevation on isoprenaline-induced increase in cyclic AMP and relaxation in rat aortic smooth muscle: role of phosphodiesterase. Br J Pharmacol. 1996;119:471–478. doi: 10.1111/j.1476-5381.1996.tb15696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rapoport RM, Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983;52:352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- 43.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;ii:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- 44.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifet SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]


