Abstract
Aims
To investigate the absorption profile and estimate the bioavailability of three doses of recombinant human growth hormone (rhGH) smaller than 2 IU in females with GH deficiency (GHD). A second aim of the study was to compare the mean 24 h GH concentrations after s.c. injection of rhGH with the physiological mean 24 h GH concentration of healthy females of comparable age, height, and BMI.
Methods
Fourteen female patients with substituted GHD, and 14 healthy females of comparable age, height, and BMI were studied. All GHD patients underwent 24 h GH sampling after s.c. injection of rhGH in doses of 0.6, 1.2, or 1.8 IU. In addition, these patients underwent a 4 h GH sampling after i.v. injection of rhGH (1 IU). In healthy subjects, blood was withdrawn every 10 min for 24 h to determine the physiological GH profile.
Results
A s.c. dose of 0.6 IU resulted in a mean and maximum GH concentration of 0.95±0.04 mU l−1 and 2.62±0.09 mU l−1. A doubling (or tripling) of the rhGH dose resulted in a doubling (or tripling) of the mean and maximum GH concentration. The time of maximum GH concentration was reached on average after 261±27 min. Mean GH concentration in healthy females was comparable with the mean GH concentration after a s.c. dose of 1.2 IU. Mean availability of the s.c. injected dose was 63%±4%.
Conclusions
A dose of 1.2 IU resulted in a mean GH concentration comparable with the mean physiological GH concentration in healthy females of comparable age, height, and BMI.
Keywords: GH, GH deficiency, somatotropin, adults, absorption
Introduction
Since the introduction of recombinant human growth hormone (rhGH), several clinical trials were designed to examine the effect of growth hormone (GH) replacement therapy in adults with GH deficiency (GHD). It has become apparent that GH treatment does have beneficial effects on body composition, quality of life, and bone mass in adults with GHD [1–4]. Initial studies of GH replacement in adults with GHD used high daily GH doses (about 5 IU day−1) based on experience in children [5]. The high incidence of side-effects (mainly related to fluid retention) and supranormal serum IGF-I levels, suggest that this dose may have been too large in adults with GHD. Reduced doses (about 2.5 IU day−1) were used in subsequent studies, resulting in a lesser increase in serum levels of IGF-I (although supranormal levels were still reported), and a decrease in the incidence of side-effects [6]. Based on results of our group and those of others, the current advice is to start at a dose of±0.6 IU day−1 and individualize this dose after 3–4 weeks until normal serum IGF-I concentrations are obtained. It appears that doses below 2 IU day−1 are often capable of normalizing serum IGF-I concentrations [7–9].
Although several investigators studied the absorption profile of subcutaneous (s.c.) injected rhGH in adults with GH deficiency (GHD), no data are available on the absorption profile of doses below 2 IU day−1 [10–13]. In this study we investigate the absorption profile of three different doses of rhGH all under 2 IU (0.6, 1.2 and 1.8 IU). In addition, mean GH concentrations after s.c. injection in females with GHD are compared with the physiological mean 24 h GH concentration in healthy females of comparable age, height, and BMI.
Data on the availability of s.c. administered rhGH are scarce. Based on infusion studies, Jørgensen et al. [14] reported a lower steady state concentration of GH with the s.c. infusion compared with the i.v. infusion, which suggests local degradation of s.c. administered GH. The estimation of the bioavailability of rhGH in a relatively high dose was 50–70% [11, 15]. An additional aim of this study was therefore to estimate the availability of three doses of s.c. administered rhGH all within the range of the substitution dose in adults with GHD.
Subjects
Fourteen female patients with GH deficiency (GHD) and 14 healthy females were studied. All patients had a peak serum GH response less than 1.3 mU l−1 during insulin-induced hypoglycaemia. One patient had childhood-onset GHD caused by a germinoma and 13 patients had adult-onset GHD (11 patients due to a pituitary adenoma, 1 patient due to a tumour in the pituitary region and 1 patient due to Sheehan’s syndrome). In addition to GHD, 1 patient had LH/FSH deficiency, 1 patient had LH/FSH and TSH deficiency, 3 patients had LH/FSH and ACTH deficiency, 7 patients had full anterior pituitary gland failure, and 2 patients had total pituitary gland failure. All patients were treated with conventional substitution when indicated, except for two older patients regarding oestrogen replacement therapy.
Verbal informed consent was obtained from all subjects, and the study was approved by the ethics committee of Leiden University Medical Center.
Design of the study
All females with GHD were randomized to one of the three dose groups: 0.6, 1.2 or 1.8 IU day−1, the latter two attained in one and two steps of 0.6 IU, respectively. Since these patients were selected from a study population of 60 patients, the distribution of these 14 patients among the dose groups was not equal: 5, 6, and 3 patients in the 0.6, 1.2 and 1.8 IU day−1 group, respectively.
After 6 months of treatment, all patients underwent frequent GH sampling after both an intravenous (i.v.) bolus injection and a s.c. injection of rhGH. In addition, measurement of body composition was performed.
Fourteen healthy females with comparable age and BMI were selected out of a larger (n = 19) female control population, which had all undergone a 24 h GH profile.
24 h absorption study
An intravenous catheter was placed in a forearm vein at 20.15 h, after the measurement of body composition (see below). Blood sampling at 30 min intervals for 24 h started at 21.00 h. At 21.15 h rhGH was injected subcutaneously in a skinfold at midthigh level by means of a 13 mm 29G needle. The dose of rhGH given was 0.6, 1.2 or 1.8 IU according to randomization at baseline (Genotropin 4 IU ml−1, Pharmacia & Upjohn, Peptide Hormones; International Reference Preparation (IRP) 88/624: 1 mg = 3 IU). During the study the patients were allowed to consume standard hospital meals and moderate physical activity was allowed.
The absorption pattern of the 24 h absorption study was described by several variables:
Cmax: the maximum observed GH concentration after the s.c. injection of rhGH.
tmax: the time point at Cmax.
AUC: the area under the curve as calculated by the linear trapezoidal method.
Mean concentration: mean 24 h GH concentration after s.c. injection of rhGH.
i.v. bolus
Patients were seen in the outpatient’s clinic after an overnight fast and omitting the standard s.c. GH dose from the evening before. An i.v. injection of 1 IU (Genotropin 4IU ml−1, Pharmacia & Upjohn, Peptide Hormones; IRP 88/624) of rhGH was given after taking a baseline blood sample. Blood was withdrawn every 4 min for 20 min, every 5 min for the following 40 min and every 15 min for another 3 h. The AUC was calculated by the trapezoidal method.
24 h profiles
Fourteen healthy subjects underwent blood-sampling at 10 min intervals for 24 h starting at 09.00 h. During the study the patients were allowed to consume standard hospital meals and moderate physical activity was allowed.
Estimation of the availability
The availability of rhGH (percentage of injected rhGH absorbed in the circulation) was estimated by comparing the individual AUC after the s.c. injection with the AUC after the i.v. bolus after correcting for the difference in the dose.
Body composition
With a minimum of clothes, weight was measured to the nearest 0.1 kg. Height was measured barefoot to the nearest 0.001 m. BMI was calculated as weight (kg)/height (m)2. Body surface was calculated by the formula developed by Du Bois [16]. Body impedance was measured with a Human-IM Scan impedance analyzer (Dietosystem, Milan, Italy). TBW was estimated based on body height and impedance at 100 kHz. For a detailed description see Janssen et al. [17]
Assays
GH was measured with a time-resolved immunofluorescent assay (Wallac, Turku, Finland), specific for the 22 kiloDalton GH protein. Standards were human biosynthetic GH (Pharmacia AB, Sweden) diluted in bovine calf serum, and calibrated against the WHO First IRP 80-505 (1 mg = 2.6 IU). The detection limit of the assay was 0.03 mU l−1 (= 0.012 μg l−1) and the intra-assay coefficient of variation was less than 8.4%. Three percent of the GH values were below the very low detection limit of 0.03 mU l−1 in one healthy control. These were set at the detection limit. All other subjects had detectable GH concentrations during the 24 h study.
Total serum IGF-I concentration was determined by RIA (Incstar, Stillwater, MN) after extraction and purification on ODS-silica columns. Inter-assay coefficient of variation was less than 11%. The detection limit was 1.5 nmol l−1.
Statistics
Statistical analysis was performed using SPSS for Windows (release 7.0, SPSS, Chicago, IL). Results are expressed as the mean±s.e.mean, unless specified otherwise. Pearson’s correlation coefficient was used to calculate correlations. ANOVA was used to test the influence of the dose on the kinetic characteristics. Student’s t-test was used to compare results of the s.c. study with the results of the 24 h profile in healthy subjects. Differences were considered significant for P<0.05.
Results
Age, height, and BMI of the females with GHD were not significantly different from those in healthy controls (age: 44±3 and 44±3 years; height 166.7±2.1 and 164.4±2.6 cm; BMI: 25.5±1.0 and 23.3±0.9 kg m−2, in GHD females and healthy controls, respectively). The mean 24 h GH profile of 14 healthy females is shown in Figure 1a. Figure 1b shows the 24 h profile after a s.c. injection of rhGH in adults with GHD. Mean GH concentrations after s.c. injection of rhGH were within the range of the mean GH concentration in healthy females, except in one patient (range 0.85–4.00 and 0.75–3.40 mU l−1, in GHD patients and healthy controls respectively). In females with GHD the mean GH concentration after a s.c. injection of 1.2 IU (95% CI: 1.54, 2.41 mU l−1) was not statistically significantly different from that in healthy females (95% CI: 1.28, 2.06 mU l−1) whereas the mean concentration after 0.6 and 1.8 IU of rhGH, respectively, was significantly lower (95% CI: 0.87, 1.03 mU l−1; P = 0.004) and higher (95% CI: 1.92, 3.98 mU l−1; P = 0.019) than the mean GH concentration in healthy females.
Figure 1.
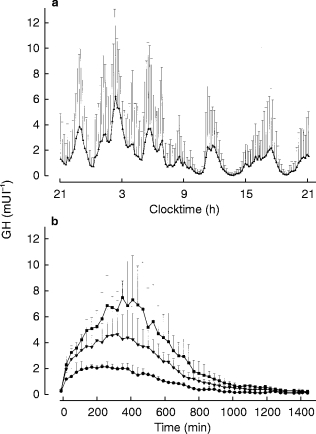
Mean (+1 s.d.) physiological 24 h GH profile of 14 healthy subjects (a) and mean (+1 s.d.) 24 h GH profile after three doses (•: 0.6 IU; ▾: 1.2 IU; ▪: 1.8 IU) of subcutaneous injected rhGH in females with GH deficiency (b).
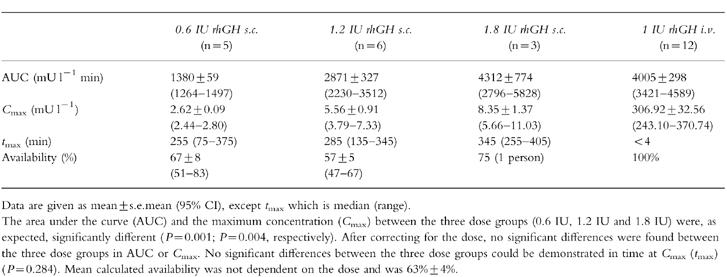
Characteristics of the s.c. profiles are given in Table 1. The AUC and the Cmax after the s.c. injection were significantly different between the three dose groups (P = 0.001 and P = 0.004, respectively). No significant differences were found after correcting for dose (95% CI: AUC/s.c. dose, 2.1–2.5, 1.9–2.9, 1.6–3.2: P = 0.958; Cmax/s.c. dose, 4.1–4.7, 3.2–.6.1, 3.2–6.1: P = 0.942 for low, middle and high dose, respectively), indicating that a doubling (or tripling) of a dose doubles (or triples) the AUC and the maximum GH concentration after s.c. injection. tmax was 261±27 min after the s.c. injection. No significant differences in tmax could be demonstrated between dose groups (P = 0.284).
Table 1.
Characteristics of GH absorption after a s.c. injection of rhGH in three doses.
GH dose was significantly correlated with mean GH concentration (r = 0.842; P<0.0005). A higher correlation coefficient was found when the dose was corrected for total body water (as estimated by multifrequency BIA), body weight, or body surface (r = 0.944; r = 0.936, r = 0.924, respectively).
Due to technical problems during the i.v. bolus study, the results of 2 of the 14 patients, both randomized to 1.8 IU rhGH, were excluded from the estimation of the availability of the s.c. injected dose. Mean availability of the s.c. injected dose was 63% (95% CI: 55, 71%).
After 6 months of rhGH treatment serum IGF-I was 11.9±3.6, 15.8±4.2, and 20.0±2.6 nmol l−1 in the low, middle, and high dose group, respectively. No correlation was found between mean serum GH concentration and serum IGF-I concentration (r = 0.383: P = 0.177). Mean serum IGF-I in healthy controls was 20.2±2.0 nmol l−1.
Discussion
In this study we investigated the absorption pattern of a subcutaneously administered dose of rhGH in the thigh, which is in agreement with the recommendations regarding rhGH therapy in both children and adults. The three doses compared in this study were 0.6, 1.2, and 1.8 IU, which is lower than that administered in other pharmacokinetic studies and more in accordance with the currently recommended dose in adults with GHD, at least at the start of rhGH therapy.
We recently investigated the effects of 0.6, 1.2 or 1.8 IU of rhGH day−1 for 3 months in adults with GHD [7]. The applied doses were based on the physiological data on mean 24 h GH concentration in healthy subjects with an assumption of an availability of 60%. In the present study, the range in mean GH concentrations with these three doses was comparable with the range in mean physiological GH concentration in healthy females of comparable age, height, and BMI. No significant difference was found between the mean 24 h GH concentration after a s.c. injection of 1.2 IU and the mean physiological GH concentration. In females with GHD using 1.2 IU of rhGH day−1 for 6 months mean serum IGF-I concentration was however slightly lower (although not significantly) compared with that in the healthy control group, implying that higher doses of rhGH and thus higher mean GH concentrations are necessary to normalize serum IGF-I concentrations. Recent results indeed suggest that mean GH doses of 1.6 IU day−1 are needed to normalize serum IGF-I levels after long-term (2 years) rhGH treatment in adults with GHD, and that these doses are relatively higher in females compared with males (1.8 and 1.4 IU day−1 respectively) [8].
It is not known whether the pulsatility of the GH secretion is of physiological relevance in healthy controls. In rats, pulsatile GH administration has been shown to be more effective than continuous infusion in stimulating serum IGF-I mRNA, longitudinal bone growth and body weight gain [18–20]. In adults with GHD, short-term (44h) serum IGF-I responses were higher after frequent (n = 8) i.v. bolus injections compared to less frequent (n = 2) i.v. injections of the same dose [21]. Serum IGF-I responses after continuous infusion were however as large as those after 8 i.v. boluses, suggesting that duration of elevated GH levels could be as essential as pulsatility at least in the short term [21]. This suggestion is however not in agreement with the results of the present study since the percentage of plasma samples below a GH concentration of 0.1 mU l−1 is larger in healthy females (11.8%) than in substituted GHD patients (0.3% with a s.c. dose of 1.2) (data not shown) whereas serum IGF-I concentrations were, although not significant, slighthly higher in healthy females.
The mean estimated availability of rhGH in this study was 63% and was based on the ratio of the AUC after s.c. injection (in the thigh in the evening) to the AUC after i.v. injection (in the morning) after correcting for differences in the GH dose. The estimation must be considered as the maximum availability, since several factors, for which no correction can be made, influence the calculated availability. First, the method of entry of GH into the bloodstream influences the plasma half-life of GH [22]. The half-life with an i.v. bolus is shorter than that following steady state infusion of GH. It is therefore likely that the metabolic clearance rate of GH is relatively smaller after s.c. injection compared with that after an i.v. bolus, which results in an overestimation of the availability. Second, the half-life of exogenous GH has been reported to be shorter following i.v. injection of GH in the morning than in the evening [23]. Third, although in the present study only patients with severe GHD (maximum GH during insulin-induced hypoglycaemia below 1.3 mU l−1) were included, we cannot totally exclude that some endogenous GH production may have been present. This implies a relative overestimation of the 24 h AUC (s.c. injection) compared with the 4 h AUC (i.v. injection), resulting in a possible slight overestimation of availability.
In this study, the absorption profile of doses of rhGH within the currently recommended dose in adults with GHD is described. Not all injected GH was absorbed: about 63% of the injected dose was absorbed in the circulating volume. A dose of 1.2 IU resulted in a mean GH concentration comparable with the mean physiological GH concentration in healthy females of comparable age, height, and BMI. Mean serum IGF-I levels were however slighlty lower (although non significantly) suggesting that higher doses of rhGH, and thus higher mean serum GH concentrations, are needed to normalize serum IGF-I concentrations.
Acknowledgments
This work was supported by a grant from Pharmacia & Upjohn BV, Peptide Hormones.
References
- 1.Jørgensen JO, Pedersen SA, Thuesen L, et al. Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet. 1989;i:1221–1225. doi: 10.1016/s0140-6736(89)92328-3. [DOI] [PubMed] [Google Scholar]
- 2.Cuneo RC, Salomon F, McGauley GA, Sönksen PH. The growth hormone deficiency syndrome in adults. Clin Endocrinol. 1992;37:387–397. doi: 10.1111/j.1365-2265.1992.tb02347.x. Oxf. [DOI] [PubMed] [Google Scholar]
- 3.Bengtsson BÅ, Eden S, Lonn L, et al. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab. 1993;76:309–317. doi: 10.1210/jcem.76.2.8432773. [DOI] [PubMed] [Google Scholar]
- 4.De Boer H, Blok GJ, van der Veen EA. Clinical aspects of growth hormone deficiency in adults. Endocr Rev. 1995;16:63–86. doi: 10.1210/edrv-16-1-63. [DOI] [PubMed] [Google Scholar]
- 5.Salomon F, Cuneo RC, Hesp R, Sönksen PH. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med. 1989;321:1797–1803. doi: 10.1056/NEJM198912283212605. [DOI] [PubMed] [Google Scholar]
- 6.Mårdh G, Lindeberg A on behalf of the investigators. Growth hormone replacement therapy in adult hypopituitary patients with growth hormone deficiency: combined clinical safety data from clinical trials in 665 patients. Endocrinology and Metabolism. 1995;2:11–16. [Google Scholar]
- 7.Janssen YJH, Frölich M, Roelfsema F. A low starting dose of genotropin in growth hormone-deficient adults. J Clin Endocrinol Metab. 1997;82:129–135. doi: 10.1210/jcem.82.1.3669. [DOI] [PubMed] [Google Scholar]
- 8.Janssen YJH, Hamdy NA, Frölich M, Roelfsema F. Skeletal effects of two years of treatment with low physiological doses of recombinant human growth hormone (GH) in patients with adult-onset GH deficiency. J Clin Endocrinol Metab. 1998;83:2143–2148. doi: 10.1210/jcem.83.6.4851. [DOI] [PubMed] [Google Scholar]
- 9.Johannsson G, Rosen T, Bengtsson BÅ. Individualized dose titration of growth hormone (GH) during GH replacement in hypopituitary adults. Clin Endocrinol. 1997;47:571–581. doi: 10.1046/j.1365-2265.1997.3271123.x. Oxf. [DOI] [PubMed] [Google Scholar]
- 10.Laursen T, Jørgensen JO, Christiansen JS. Pharmacokinetics and metabolic effects of growth hormone injected subcutaneously in growth hormone deficient patients: thigh versus abdomen [published erratum appears in Clin Endocrinol (Oxf) 1995 Jan;42(1):109] Clin Endocrinol. 1994;40:373–378. doi: 10.1111/j.1365-2265.1994.tb03934.x. Oxf. [DOI] [PubMed] [Google Scholar]
- 11.Laursen T, Grandjean B, Jørgensen JO, Christiansen JS. Bioavailability and bioactivity of three different doses of nasal growth hormone (GH) administered to GH-deficient patients: comparison with intravenous and subcutaneous administration. Eur J Endocrinol. 1996;135:309–315. doi: 10.1530/eje.0.1350309. [DOI] [PubMed] [Google Scholar]
- 12.Vahl N, Jensen SB, Rasmussen MH, et al. Bioavailability of recombinant human growth hormone in different concentrations and formulations. Pharmacol Toxicol. 1996;79:144–149. doi: 10.1111/j.1600-0773.1996.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 13.Jørgensen JO, Flyvbjerg A, Lauritzen T, Alberti KG, Ørskov H, Christiansen JS. Dose-response studies with biosynthetic human growth hormone (GH) in GH-deficient patients. J Clin Endocrinol Metab. 1988;67:36–40. doi: 10.1210/jcem-67-1-36. [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen JO, Flyvbjerg A, Lauritzen T, Ørskov H, Christiansen JS. Subcutaneous degradation of biosynthetic human growth hormone in growth hormone deficient patients. Acta Endocrinol. 1988;118:154–158. doi: 10.1530/acta.0.1180154. Copenh. [DOI] [PubMed] [Google Scholar]
- 15.Laursen T, Møller J, Jørgensen JO, Orskov H, Christiansen JS. Bioavailability and bioactivity of intravenous vs subcutaneous infusion of growth hormone in GH-deficient patients. Clin Endocrinol. 1996;45:333–339. doi: 10.1046/j.1365-2265.1996.00814.x. Oxf. [DOI] [PubMed] [Google Scholar]
- 16.Du Bois D, Du Bois EF. Clinical calorimeter. A formula to estimate the approximate surface if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 17.Janssen YJH, Deurenberg P, Roelfsema F. Using dilution techniques and multifrequency bioelectrical impedance to assess both total body water and extracellular water at baseline and during recombinant human growth hormone (GH) treatment in GH-deficient adults. J Clin Endocrinol Metab. 1997;82:3349–3355. doi: 10.1210/jcem.82.10.4272. [DOI] [PubMed] [Google Scholar]
- 18.Jansson JO, Albertsson-Wikland K, Eden S, Thorngren KG, Isaksson O. Effect of frequency of growth hormone administration on longitudinal bone growth and body weight in hypophysectomized rats. Acta Physiol Scand. 1982;114:261–265. doi: 10.1111/j.1748-1716.1982.tb06980.x. [DOI] [PubMed] [Google Scholar]
- 19.Clark RG, Jansson JO, Isaksson O, Robinson IC. Intravenous growth hormone: growth responses to patterned infusions in hypophysectomized rats. J Endocrinol. 1985;104:53–61. doi: 10.1677/joe.0.1040053. [DOI] [PubMed] [Google Scholar]
- 20.Isgaard J, Carlsson L, Isaksson OG, Jansson JO. Pulsatile intravenous growth hormone (GH) infusion to hypophysectomized rats increases insulin-like growth factor I messenger ribonucleic acid in skeletal tissues more effectively than continuous GH infusion. Endocrinology. 1988;123:2605–2610. doi: 10.1210/endo-123-6-2605. [DOI] [PubMed] [Google Scholar]
- 21.Jørgensen JO, Møller N, Lauritzen T, Christiansen JS. Pulsatile versus continuous intravenous administration of growth hormone (GH) in GH-deficient patients: effects on circulating insulin-like growth factor-I and metabolic indices. J Clin Endocrinol Metab. 1990;70:1616–1623. doi: 10.1210/jcem-70-6-1616. [DOI] [PubMed] [Google Scholar]
- 22.Shah N, Evans WS, Veldhuis JD. Mode of GH entry into the bloodstream, rather than gender or sex-steroid hormones, determines GH half-life in the human. Annual Meeting of the Endocrine Society. 1997;P02 Abstract. [Google Scholar]
- 23.Holl RW, Schwarz U, Schauwecker P, Benz R, Veldhuis JD, Heinze E. Diurnal variation in the elimination rate of human growth hormone (GH): the half-life of serum GH is prolonged in the evening, and affected by the source of the hormone, as well as by body size and serum estradiol. J Clin Endocrinol Metab. 1993;77:216–220. doi: 10.1210/jcem.77.1.8325945. [DOI] [PubMed] [Google Scholar]


